Page 1

APOLLO 4000
World’s first fully-integrated Free Radical Analyzer
INSTRUCTION MANUAL
Serial No._____________________
APOLLO 4000
WARNING
This instrument must not be connected to a local
network nor to the Internet. Do not attach any
peripheral device other than a USB printer. Any
change to the proprietary hard disk registry in this
device — whether by virus or by normally benign
hardware or software installations — may render the
drive or the Apollo software inoperable, requiring the
instrument’s return to the factory for reformatting.
File corruption or damage to applications or
operating system caused by such use will not be
covered by the Warranty.
www.wpiinc.com
061506
World Precision Instruments
Page 2

Page 3
APOLLO 4000
Contents
INTRODUCTION ............................................................................... A-1
Design Architecture ........................................................................................ A-2
Plug-and-Play Design ..................................................................................... A-2
Free Radical Sensor Technology .................................................................... A-2
Instrument Description ................................................................................... A-3
Unpacking ...................................................................................................... A-5
OPERATING INSTRUCTIONS ...........................................................B-1
Setting up the APOLLO 4000 ......................................................................... B-1
APOLLO.EXE Operating Software .................................................................. B-4
Example of a real signal application ............................................................ B-12
USING THE APOLLO 4000 TO DETECT NITRIC OXIDE ................... C-1
Initial Set-up .................................................................................................... C-1
Calibration of the NO Sensor .......................................................................... C-1
The Calibration Kit .......................................................................................... C-1
Calibration by the chemical generation of NO ............................................... C-2
Calibration of NO sensor by decomposition of SNAP .................................... C-7
Calibration of NO sensor using aqueous standards prepared with NO Gas C-15
Measurement of NO ..................................................................................... C-18
Maintenance of NO Sensors ........................................................................ C-20
USING THE APOLLO 4000 TO DETECT OXYGEN ............................ D-1
Initial Set-up .................................................................................................... D-1
Calibration and Use of Oxygen Sensors ........................................................ D-1
Calibration ...................................................................................................... D-2
Probe Structure and Assembly....................................................................... D-8
USING THE APOLLO 4000 TO DETECT HYDROGEN PEROXIDE..... E-1
Initial Set-up ..................................................................................................... E-1
The structure of the HPO sensor .....................................................................E-1
Calibration of the HPO Sensor ........................................................................ E-2
Calibration Procedure......................................................................................E-2
Interference ..................................................................................................... E-4
Maintenance of HPO Sensors ......................................................................... E-4
TROUBLESHOOTING FOR APOLLO 4000 ........................................ F-1
any language, in any form, without prior written permission of World Precision Instruments, Inc.
Copyright © 2003 by World Precision Instruments, Inc. All rights reserved. No part of this publication may be reproduced or translated into
WORLD PRECISION INSTRUMENTS 3
APPENDIX: BASIC GROUNDING & SHIELDING PRINCIPLES ........ G-1
Page 4

Page 5

INTRODUCTION
The APOLLO 4000 is the end result of an
extensive three-year research and
development program aimed at designing the
most advanced multifunctional free radical
detection system available. Building on
WPI’s worldwide-recognized expertise in
the field of nitric oxide detection and the
success of its popular NO detector (the
ISO-NO series), WPI’s scientists
embarked on an ambitious plan to
develop a state-of-the-art free radical
detection system incorporating the very
latest digital signal processing (DSP) technology.
APOLLO 4000
INTRODUCTION
The APOLLO 4000 is an optically isolated multi-channel electrode-based free
radical analyzer designed specifically for the detection of a variety of redoxreactive species of biomedical importance. The electrochemical (amperometric)
detection principle used is similar to that employed in WPI’s popular nitric oxide
detection system, the ISO-NO Mark II. However, the APOLLO 4000 incorporates
numerous highly advanced design features that enable it to detect a broad range
of redox-reactive species with unsurpassed accuracy and sensitivity. Using WPI’s
extensive range of free radical sensing electrodes the APOLLO 4000 is able to
detect nitric oxide, hydrogen peroxide, s-nitrosothiols and oxygen. On-going
research at WPI is focusing on expanding the range of detectable species.
NO sensors used with ISO-NO Mark II are completely compatible with the APOLLO
4000.
WORLD PRECISION INSTRUMENTS A-1
Page 6

APOLLO 4000
INTRODUCTION
Design Architecture
The APOLLO 4000 is based on an optically isolated 4-channel configuration (a 2-
channel version is also available). This design enables simultaneous real-time
measurement of NO (or other free radicals) to be performed using up to 4 different
electrodes. In addition, each free radical sensing channel also contains an
independent channel for temperature measurement.
APOLLO 4000 incorporates a powerful single board computer and proprietary
software (apollo.exe) that enables real-time display and data-acquisition of
individual channels or any combination of channels. An extensive graphical user
interface (GUI) based on a full color LCD monitor allows complete control and
programming of all detection and data-acquisition parameters to be made using
the standard keyboard and mouse included with the system.
The APOLLO 4000 consists of two functionally independent modules: Front End
Converter (FEC) and User Interface (UI). The FEC is an 8-channel data-acquisition
module based on a 24-bit A-to-D and 16-bit D-to-A conversion driven by a Digital
Signal Processor (DSP). The User Interface is built on a standard PC platform with
Windows 2000® operating system. A standard serial port (RS232) provides the
communication between the FEC and UI. The system is fully compatible with a
standard keyboard and mouse and can be readily interfaced with PC’s, computer
networks, printers, and any device that uses Ethernet Tbase-10/100, USB, Serial
Port or Parallel Port communications.
Plug-and-Play Design
The APOLLO 4000 is designed for use with WPI’s range of free radical sensors.
The user simply plugs the required sensor into any one of the input channels
located on the instruments main front panel and then selects the detection and
acquisition parameters using the integrated software control. Each channel is also
provided with an independent temperature input port that allows real-time
monitoring of temperature using WPI’s appropriate temperature sensors.
Free Radical Sensor Technology
The APOLLO 4000 and its associated free radical sensors can provide fast,
accurate, and stable measurements over a wide range of concentrations in both
aqueous solutions and in gas mixtures. Its features include a rapid response time,
WORLD PRECISION INSTRUMENTSA-2
Page 7
APOLLO 4000
INTRODUCTION
high sensitivity and selectivity, ease of use, and versatility unmatched by any other
similar instrument.
The detection principles are based on the electrochemical (amperometric)
response produced by the various compatible free radical sensors. In summary,
the free radical of interest diffuses through a selective membrane covering the
sensor and is oxidized at the working electrode, resulting in an electrical (redox)
current. The amount of redox current produced is proportional to the free radical
concentration in the sample. All of WPI’s free radical sensors are “combination
electrodes” in which the sensing and reference electrodes have been combined
within a high performance Faraday shield designed to minimize susceptibility to
environmental noise. The Apollo software can be programmed to display either
redox current (
can also be collected via BNC connectors on the rear panel of the instrument.
APOLLO 4000 is fully compatible with WPI’s extensive range of free radical
sensors. Currently this range of sensors includes electrodes for monitoring; nitric
oxide, oxygen and hydrogen peroxide. However, new sensors are currently in
development. For details on the complete list of compatible sensors please see the
latest WPI product catalog, or visit WPI’s website (www.wpiinc.com).
e.g.
, pA) or concentration (
e.g.
, nM). Output from the APOLLO 4000
Instrument Description
Parts List
The package should contain the following items:
Part No. Description
APOLLO4000 Free Radical Analyzer
800408 Standard Keyboard
800407 Standard Mouse
800406 17” LCD monitor
35209 Program CD
— Power cables
— Instruction Manual
WORLD PRECISION INSTRUMENTS A-3
Page 8
APOLLO 4000
INTRODUCTION
DVD / CD-RW
Drive
Temperature
LED (red)
Channels
Temperature
Input
}
Power
Mains
Power
Power
Supply for
Monitor
Sensor
(green)
110V/220V
Switch
Keyboard
LED
mouse
Ethernet
USB
Outputs
Serial
Analog
(BNC)
Video
Sensor Input
Parallel Joystick
LR
Audio
Audio
Output
Input
WORLD PRECISION INSTRUMENTSA-4
Page 9
APOLLO 4000
INTRODUCTION
Unpacking
Upon receipt of this product, make a thorough inspection of the contents and check
for possible damage. Missing cartons or obvious damage to cartons should be noted
on the delivery receipt before signing. Concealed loss or damage should be reported at once to the carrier and an inspection requested. Please read the section
entitled “Claims and Returns” on the Warranty page of this manual.
Returns: Do not return any goods to WPI without obtaining prior approval (RMA #
required) and instructions from our Returns Department. Goods returned (unauthorized) by collect freight may be refused. If a return shipment is necessary, use the
original container. If the original container is not available, use a suitable substitute
that is rigid and of adequate size. Wrap the instrument in paper or plastic surrounded
with at least 100 mm (four inches) of shock absorbing material. Please read the section entitled “Claims and Returns” on the Warranty page of this manual.
WORLD PRECISION INSTRUMENTS A-5
Page 10

APOLLO 4000
INTRODUCTION
WORLD PRECISION INSTRUMENTSA-6
Page 11
APOLLO 4000
OPERATING INSTRUCTIONS
OPERATING INSTRUCTIONS
To exploit the APOLLO 4000’s capabilities fully, it is very important that the user be
aware of the general methods for operating the maintaining the instrument. This will
also ensure that the user is able to understand and interpret the readings.
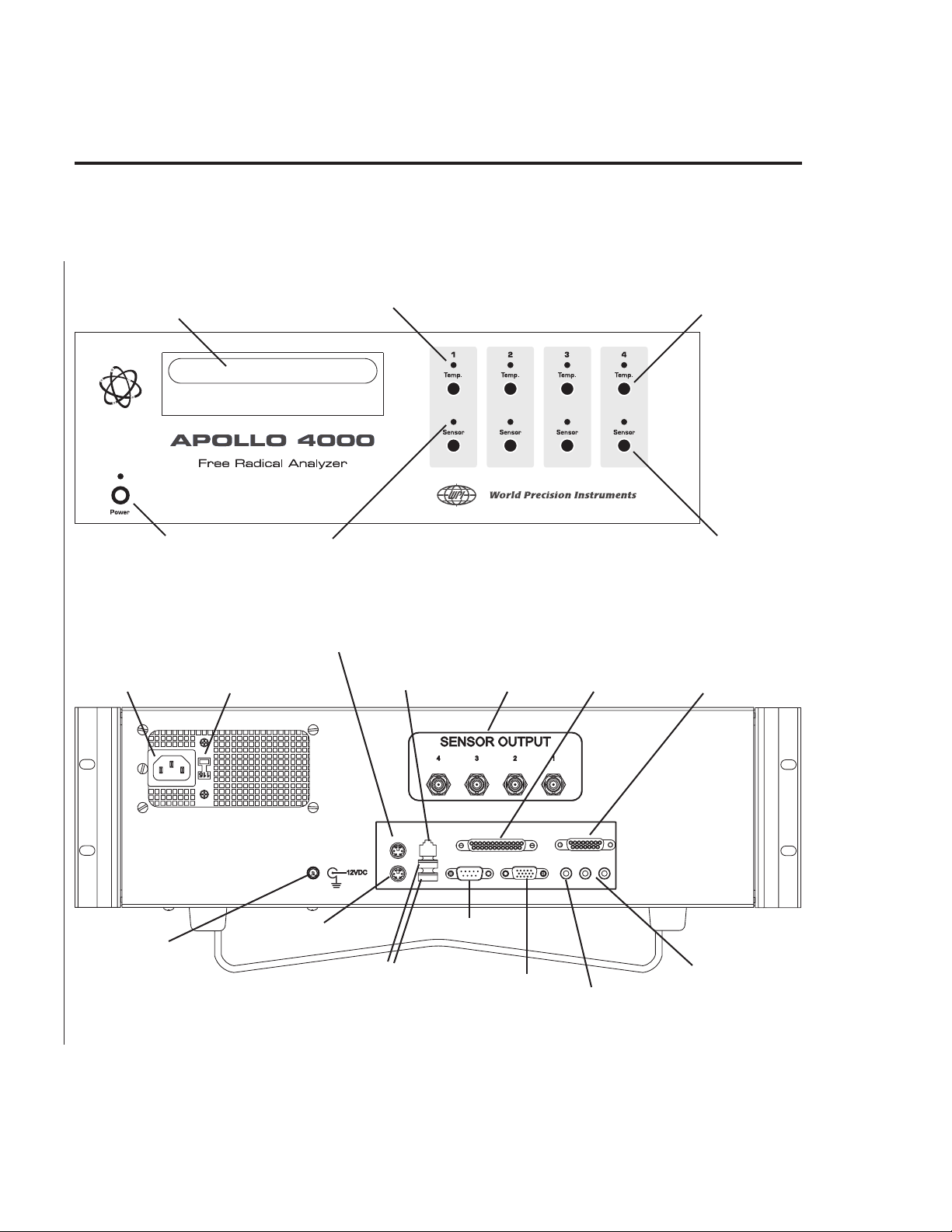
Setting up the APOLLO 4000
1. Place the APOLLO 4000 on a secure, flat surface (
2. Position the LCD display on top of the APOLLO 4000 and connect the
display cable to display output video port located on the rear panel of the
APOLLO 4000 (see A-4).
3. Power the LCD display by connecting one end of the cable from the
provided voltage converter (AC to 12V DC) to the LCD display Power input
receptacle. Connect the other end of the voltage converter to a matching
3-prong grounded wall receptacle and switch on.
4. Connect the power supply cord to the back of the APOLLO 4000 and plug
the other end into a matching 3-prong grounded wall receptacle (see setup diagram below).
NOTE: Ensure that the red voltage selector switch on the rear panel
(next to the power cable receptacle) is set to the correct voltage —
220 or 110.
e.g.
, laboratory bench).
Switching ON the APOLLO 4000
The unit can be turned on by pressing the “Power” pushbutton on the front panel.
After booting up, the system automatically starts the main application software
(APOLLO.exe). If other applications have been used the user can return to
Apollo4000 application by double clicking the “Apollo” icon in the right upper
corner of Windows® desktop.
During the process of booting up no error messages should appear on the screen.
(see Troubleshooting).
Switching OFF the APOLLO 4000
The APOLLO 4000 incorporates a highly advanced single board computer and
WORLD PRECISION INSTRUMENTS B-1
Page 12

APOLLO 4000
OPERATING INSTRUCTIONS
associated electronics. To turn the unit off, it is therefore only necessary to press
the Power button once. It is recommended, however, to close the application
programs
before
computer is not responding it may be necessary to press and hold the Power
button in for 3 seconds before the unit will turn off (see troubleshooting section).
The alternative method for switching the unit off is to position the mouse cusor on
START, click on hold down once, and then choose SHUT DOWN from the pop-up
menu. This method will be familiar to Windows
Precautions for handling sensors
pressing the Power button. In some cases when the embedded
®
users.
The range of free radical sensors offered by WPI vary in their fragility.
However, at
all times the user must exercise caution to avoid damaging the delicate
polymeric membrane covering the end of each sensor.
This membrane
prevents water and dissolved species such as ions and macromolecules from
reaching the electrode surface where they would interfere with normal
measurement and poison the electrode surface. When the sensor membrane
becomes damaged, sample contents are free to react at the electrode surface.
This causes the background current to become very large and/or go off scale
(negative or positive depending on the reacting species). The membrane integrity
of the sensor can always be checked by ascertaining that the current remains low
and stable when the sensor tip is immersed in a 1.0 M saline solution.
Attaching a sensor to the APOLLO 4000
Each channel on the APOLLO 4000 is equipped with two high quality sensor input
receptacles. The channel marked “Temp” is for use only with a compatible WPI
temperature electrode (
only with one of WPI’s free radical sensors.
e.g.
, ISO-TEMP-2). The channel marked “Sensor” is for use
Fig. B1
attaching a
temperature probe
or sensor to the
APOLLO4000,
align the red dot of
the instrument with
the red dot on the
cable connector.
– When
WORLD PRECISION INSTRUMENTSB-2
Page 13

APOLLO 4000
OPERATING INSTRUCTIONS
NOTE:
The Temp and Sensor inputs are not interchangeable but no damage
to the APOLLO 4000 will occur if a sensor/electrode is accidentally inserted
into the wrong input receptacle.
To connect a sensor, simply line up the red dot on the metal connector attached to
the sensor cable with the red dot on the sensor input receptacle and insert the
cable connector (Fig. B1). An LED (located above each sensor input) will light up
immediately indicating the instrument and sensor are connected and working
correctly. The Temp LED is red. The Sensor LED is green. The following LED
indications will inform the user of the status of the system:
LED CONDITION INDICATION
Temp LED (red)
Steady RED light Electrode is performing normally
No RED light Electrode is not connected or is damaged
Sensor LED (green)
Steady GREEN light Sensor is performing normally within the
user-selected current range
Intermittent blinking GREEN light Sensor current is outside the user-
selected linear range.
No GREEN light ERROR sensor current range too low.
Sensor error or sensor not connected.
NOTE: WPI strongly recommends that APOLLO 4000 be powered
through a Back-UPS unit to avoid system failure during power loss.
It is the responsibility of the user as well to install appropriate antivirus protection software.
WPI will not be liable for any loss of data as a result of power loss or virus-attack to the APOLLO 4000 system.
WORLD PRECISION INSTRUMENTS B-3
Page 14

APOLLO 4000
OPERATING INSTRUCTIONS
APOLLO.EXE Operating Software
The operating software of the APOLLO 4000 is based on a standard Windows
format, hence many of the software control features will already be familiar to the
user.
®
Main Screen
The main working screen is
shown in Fig. B2. The software
automatically recalls the last
settings used, therefore the
appearance of the screen will
depend on how it was last used
immediately before being turned
off.
In the example shown, the set-up
is for a four-channel nitric oxide
application with horizonal scale of
6 sec/division and vertical scales
5000 pA/div for channels 1 to 4.
Data for each channel is
displayed on the left of the screen. Each data channel display shows the following
information:
Fig. B2
1. Zeroed — Relative measurement value (includes any zeroing applied to
signal).
2. Unzeroed — Absolute measurement value (
zeroing) often refered as “background signal”.
3. Temp — Temperature measurement in degrees Celsius.
4. Unit — e.g., pA, nM, etc.
Note: If no temperature sensor is connected there will be no value in the temp
window and no trace in the graph.
i.e.
, true value without
WORLD PRECISION INSTRUMENTSB-4
Page 15
APOLLO 4000
OPERATING INSTRUCTIONS
Menu System
The Apollo 4000 software uses standard Windows® controls. Help notes will
therefore automatically appear when the cursor is positioned on any control
function or word.
The following section contains a brief description of the programs
main menu system. However, in most cases a user familiar with
Windows®-based operating programs will be able to operate the
software efficiently with the minimum of instructions.
Fig. B3
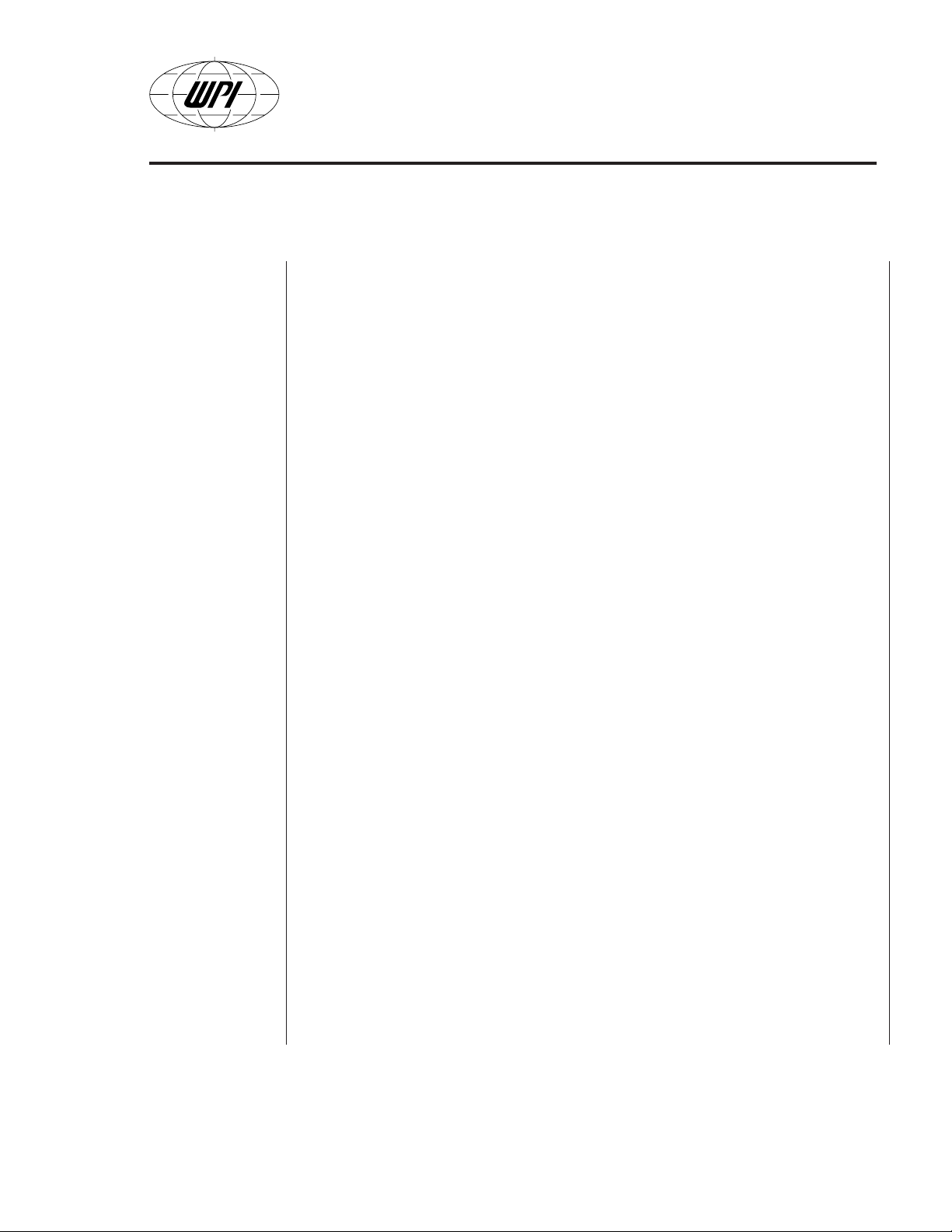
File Menu
Fig. B4
—
File Menu
• New
(Fig. B3) consists of typical Windows® commands:
—
starts a new data file
• Open — opens an existing data file
• Save — saves the current experiment with the default name
• Save as — allows the user to chose the name and the path of the saving
data file
• Print — prints the screen to the system default printer
• Exit — exits the program and returns to the Windows® desktop.
The standard message (Fig. B4) appears when it is needed. For a
detailed explanation of the above commands please refer to a
Windows® textbook.
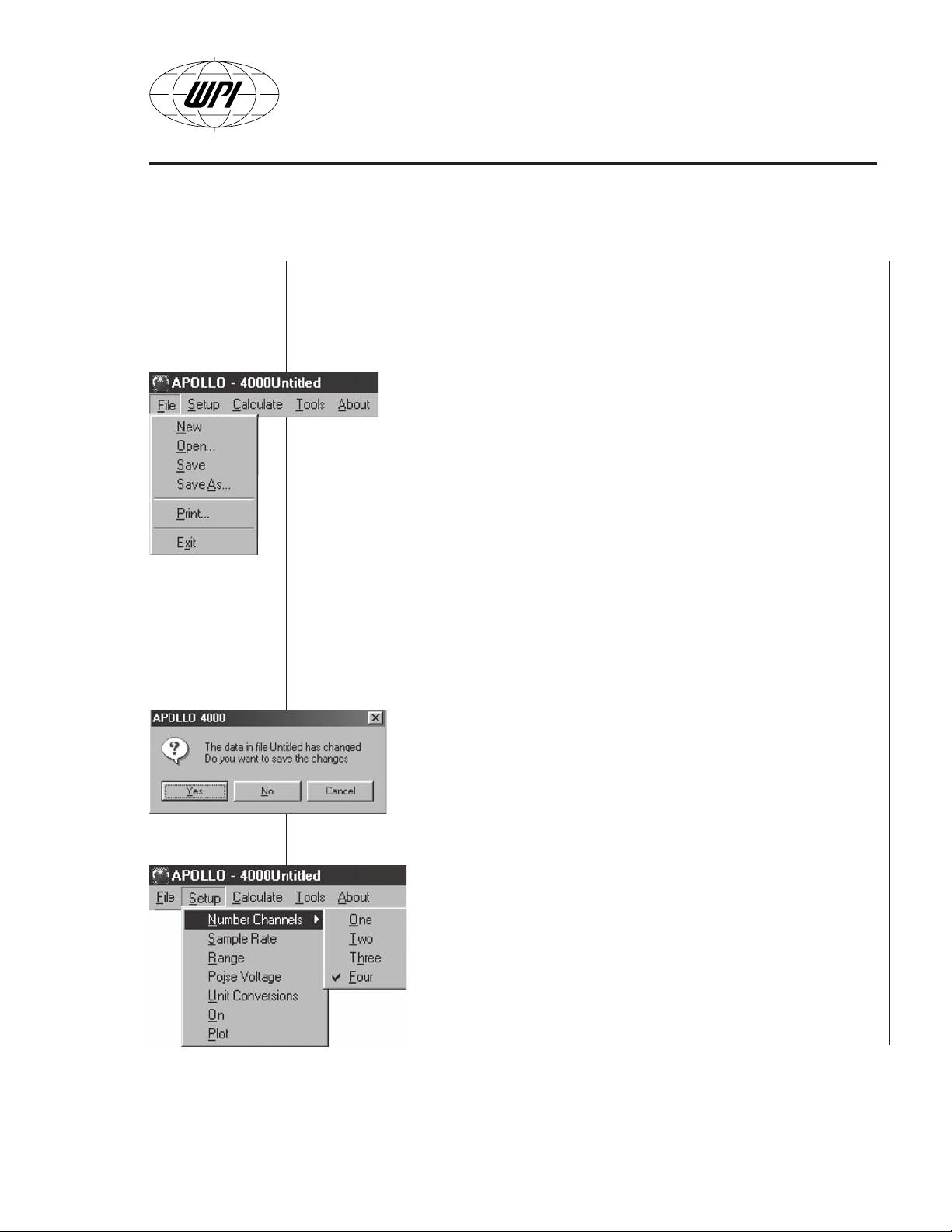
Setup Menu
(Fig. B5) consists of the following menu sub-
commands:
• Number of Channels — Sets the desired number of
displayed channels. When One is selected then only the
Channel 1 is displayed. Select Two Channels and 1 and 2
are displayed. Select Three channels for 1, 2, and 3. Select
Four to display all four channels.
Fig. B5
Setup Menu
WORLD PRECISION INSTRUMENTS B-5
—
Page 16
APOLLO 4000
OPERATING INSTRUCTIONS
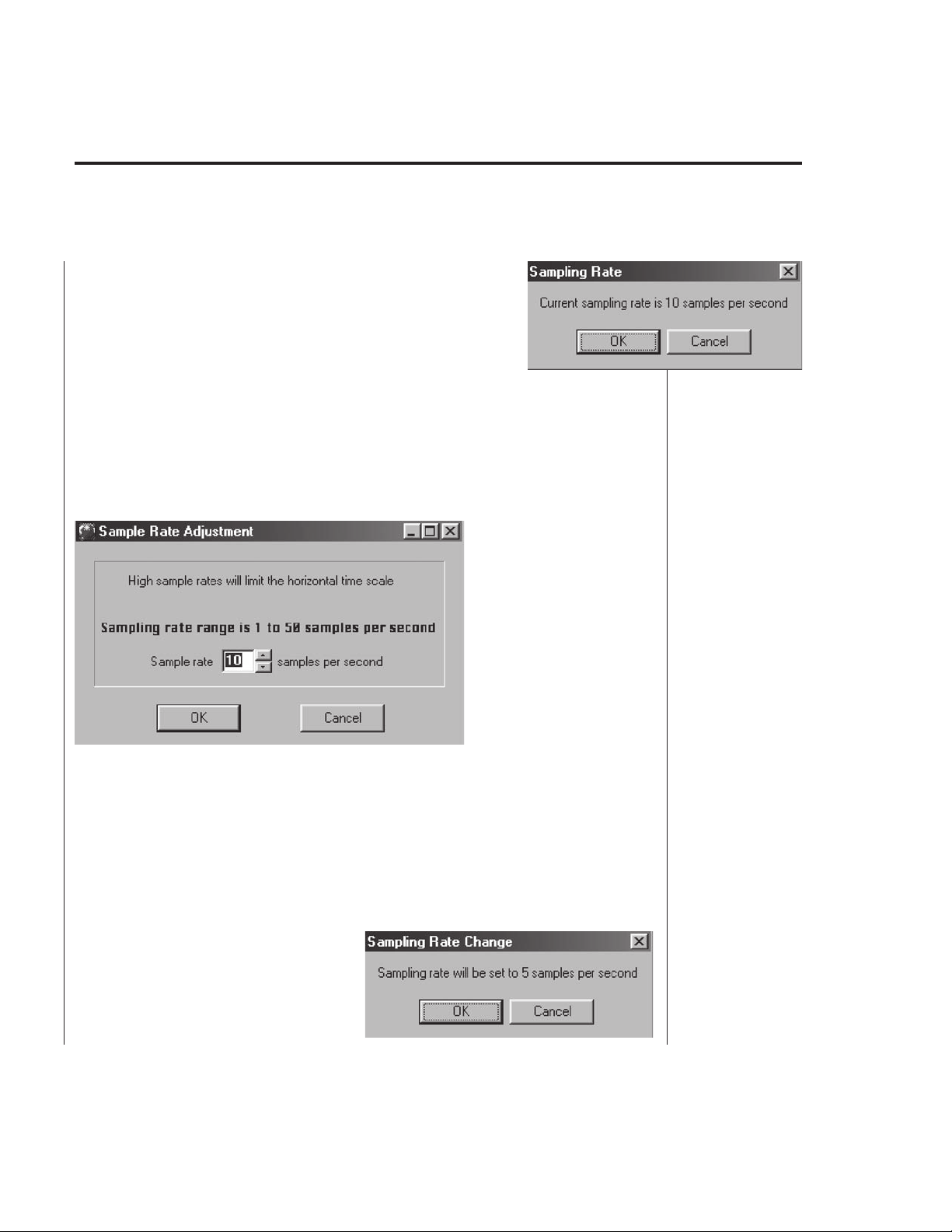
• Sample Rate — Selecting this command triggers the
following sequence of events:
Current sampling rate is indicated (Fig. B6). If this
value is satisfactory, the user can chose Cancel and
continue to work with the current sampling rate.
NOTE: The default sampling rate is 10 samples per second, sufficient
for most applications.
To select a different sampling rate, select OK. The sampling rate change
screen (Fig. B7) will appear following
confirmation screen (Fig. B8).
which may be undesirable. Therefore the user must carefully
sampling rate before the experiment.
50 samples/sec in increments of 5. It is very important for the user to
understand that Windows®-based computers have the limitations of 16,384
pixels per screen and therefore (number of horizontal divisions=10) the
maximum horizontal scale value will be limited to an integer of 1,638.4/
sample rate. After the new sample rate is selected the horizontal scale
factor is set (for clarification) to the absolute maximum value. For instance,
if the sample rate is set to 5
samples/sec the horizontal
scale will be set to 327 sec/div.
The user can reset it to any
lower value (not lower than 1
sec/div).
OK
Sampling rate can be set from 1 to
confirmation by another
The user can cancel the
action and continue to
work with the current
setting. Changing the
sampling rate changes
the data file structure
and it is therefore
necessary to close the
previous file (Fig. B4).
This can interrupt the
current experiment
select the
Fig. B6
Fig. B7
Fig. B8
WORLD PRECISION INSTRUMENTSB-6
Page 17
APOLLO 4000
OPERATING INSTRUCTIONS
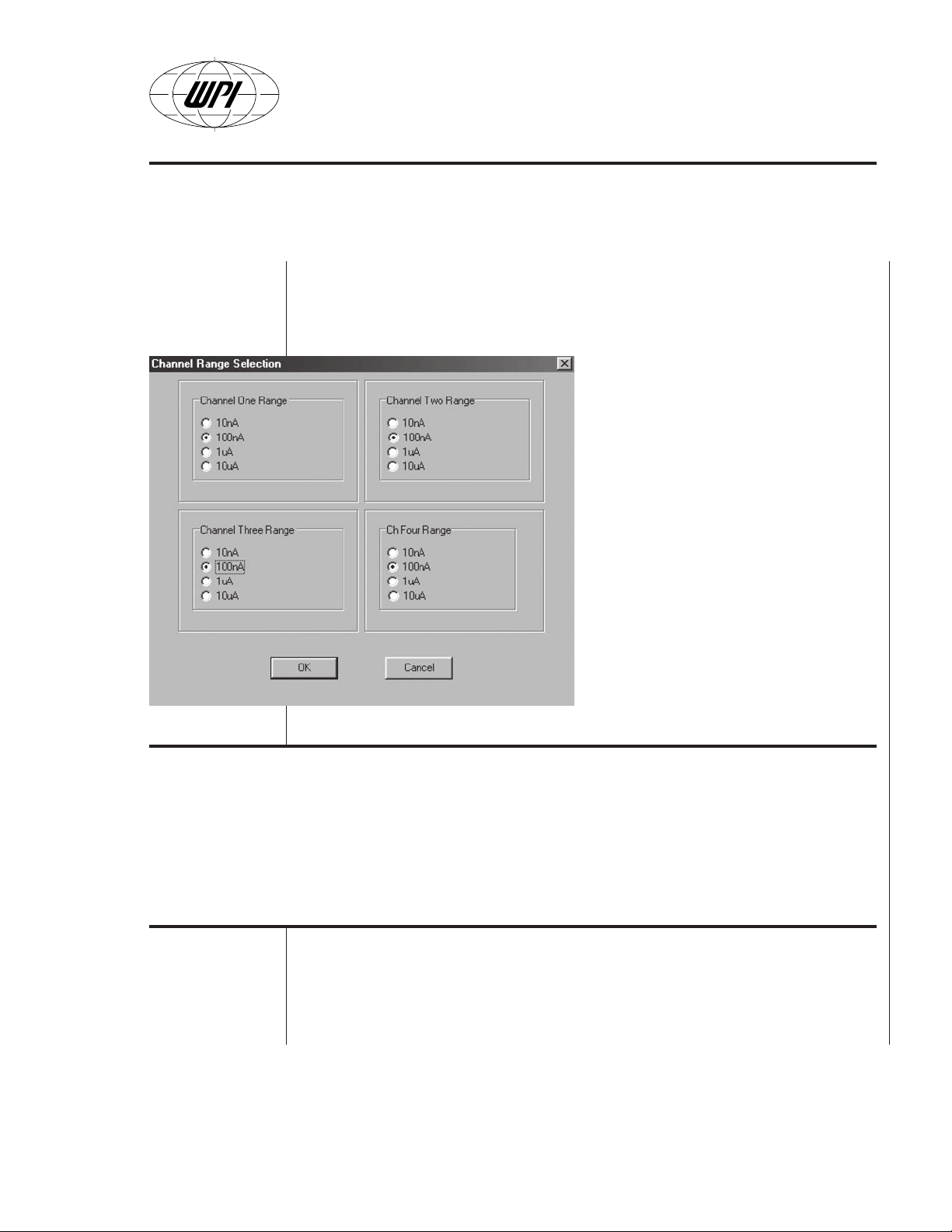
• Range. After selecting this submenu the range control screen appears
(Fig. B9). The user must select a current range for each sensor channel
within the maximum measured/expected value of the experiment. Proper
selection of the measurement range is very important because the
dynamic range of the instrument is
limited to approximately 1,000,000. This
means that the intrinsic background
noise (
i.e.
, “noise floor”) is proportional
to the maximum measured value. For
example:
If 10 nA range is selected, then the
noise floor will be approximately 10 nA
divided by 1,000,000 (
Conversely, if 10 µA range is selected
then the noise floor will be
approximately 10 µA divided by
1,000,000 (
i.e.
, 10 pA).
i.e.
, 10 fA).
If an incorrect range is chosen for any
channel, the Apollo 4000 will indicate
Fig. B9
Green (sensor) LED Indication Remedy
Intermittent blinking Current detected by sensor is too high Select a higher range
for the selected range
No Green Light Current detected by sensor is too low Select a lower range
for the selected range
Steady Green Light Sensor is normal and within the selected range No action required
If the user is satisfied with the current setting he can chose
work as previously.
this as follows:
Cancel
and
WORLD PRECISION INSTRUMENTS B-7
Page 18
APOLLO 4000
OPERATING INSTRUCTIONS
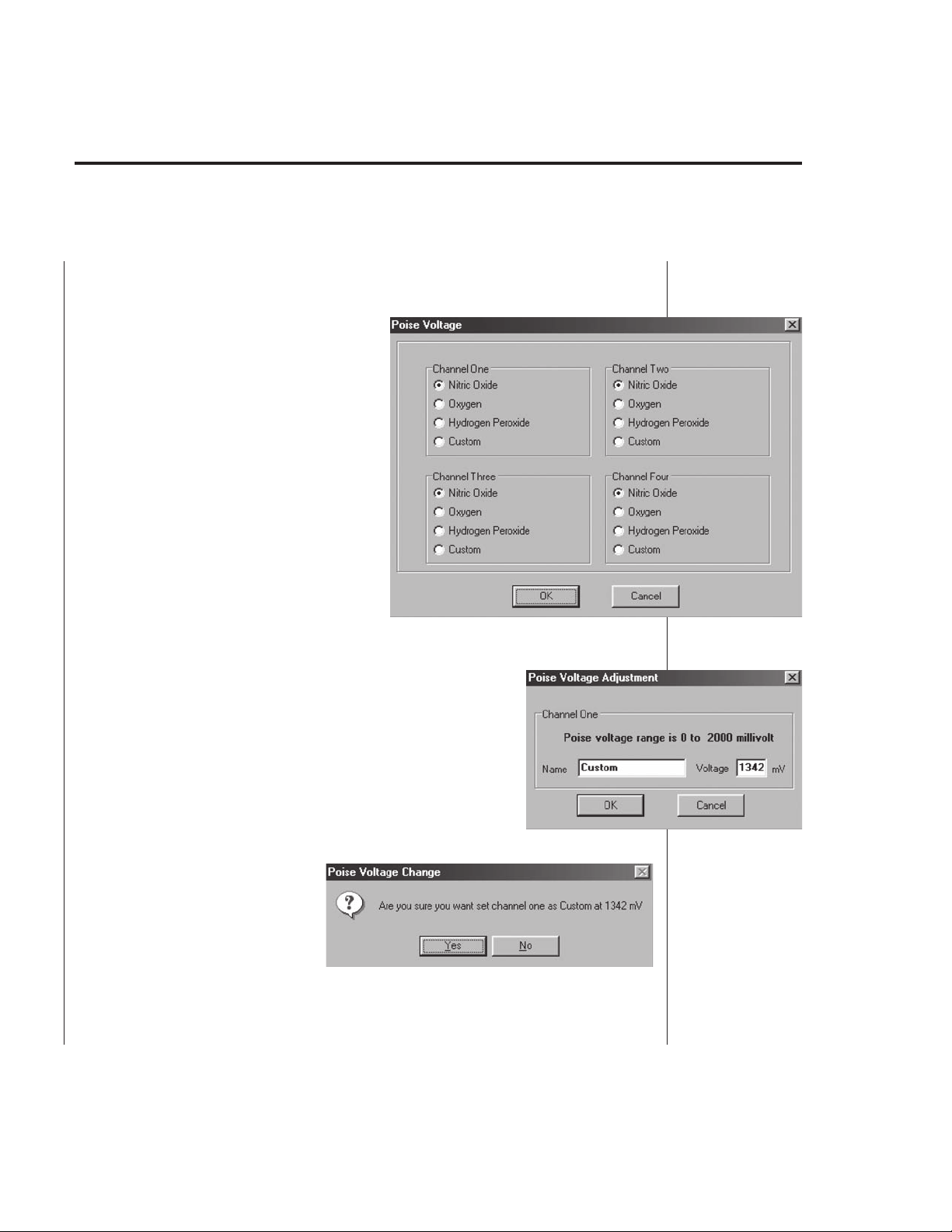
• Poise Voltage. This submenu looks (Fig. B10) and works similarly to the
Range
control. Threre are four choices:
Nitric Oxide —
Automatically configures
the poise voltage (
865 mV) on the selected
channel to measure nitric
oxide.
Oxygen — Automatically
configures the poise
voltage (
the selected channel to
measure oxygen.
Hydrogen Peroxide —
Automatically configures
the poise voltage (
400 mV) on the selected channel to measure
hydrogen peroxide.
i.e.
, 700 mV) on
i.e.
i.e.
,
,
Fig. B10
Custom — Allows the user to manually set
the poise voltage (
(Fig. B11).
Selecting the wrong poise
i.e
., from 0-2000 mV)
voltage will drastically change the results
of an experiment and may render any data
invalid.
confirmation (Fig. B12) prior to changing any
poise voltage.
Hence the program asks for the
Fig. B11
Fig. B12
WORLD PRECISION INSTRUMENTSB-8
Page 19
APOLLO 4000
OPERATING INSTRUCTIONS
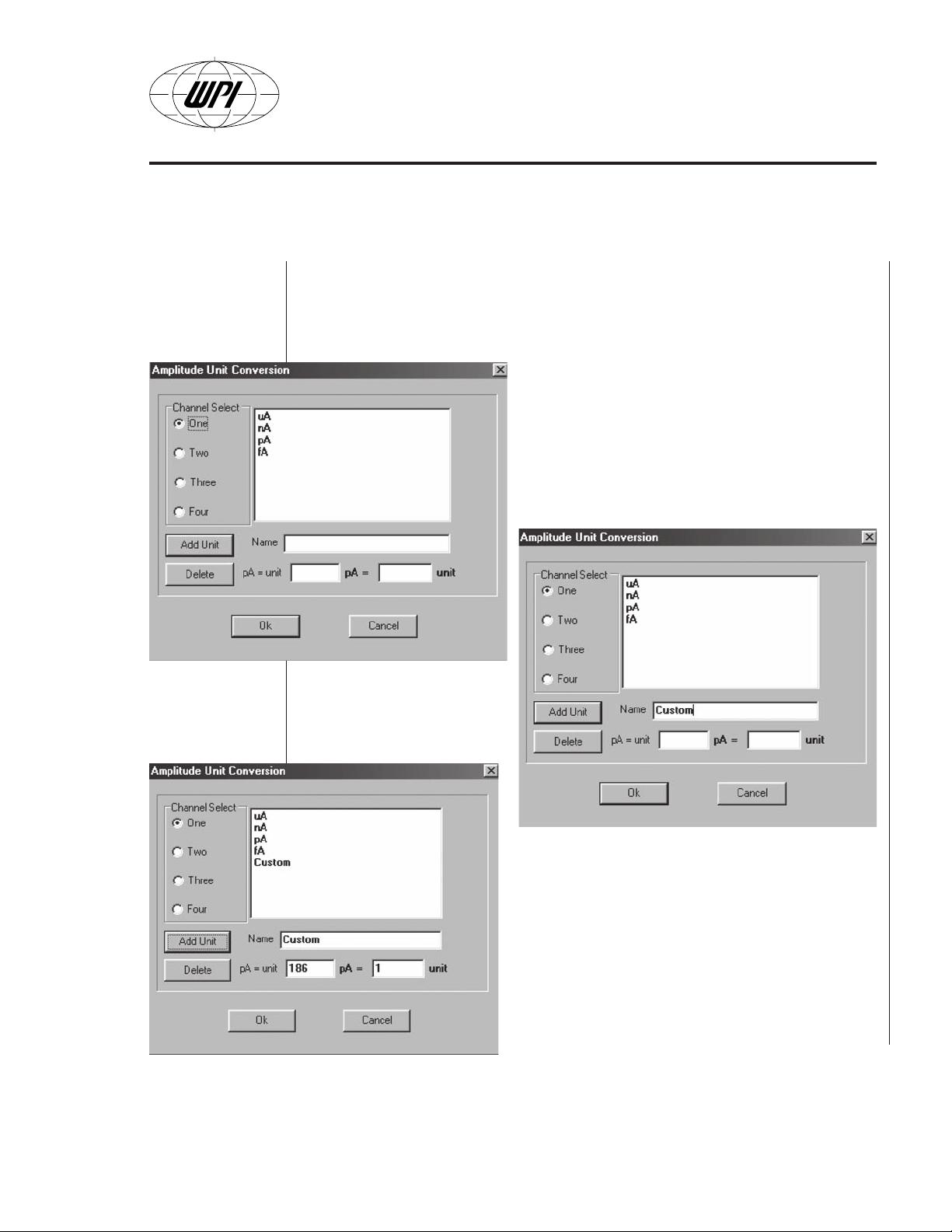
• Unit Conversion. There are four default units of measuring signals: fA,
pA, nA and µA. If a new unit needs to be defined chose the
Conversion
Conversion screen appears (Fig. B13).
submenu in the
Setup
Example: The user defines a new unit called
“Custom” with the conversion ratio 1 unit = 186
pA (Fig.B14). If Add Unit button is selected then
the new unit name appears in the list for the
particular channel (Fig.B15). The user can define
as many custom units as needed as well as
modify the existing custom ones.
control. The Amplitude Unit
Unit
Fig. B13
Fig. B15
Fig. B14
WORLD PRECISION INSTRUMENTS B-9
Page 20

APOLLO 4000
OPERATING INSTRUCTIONS
• On / Off toggle control (also the push button in the
left bottom corner of the screen). This menu starts
and stops the acquisition of data, including writing to
a data file. Before the acquisition starts, the program
notifies the user about the sampling rate and
maximum horizontal scale factor (Fig. B16).
• Plot / Analyze toggle control (also the push button in the left bottom corner
of the screen) commands the program to start and stop plotting of the
incoming data to the screen. In the Analyze mode there is a possibility to
measure different parameters of the acquired data defined by Calculate
menu with the cursors. The cursors appear when the pointer device
(mouse) is moved to a certain position and its left button pushed. There are
a total of two cursors. The second cursor appears after the first one when
the pointer device left button is released
.
Fig. B16
Calculate Menu
program can recalculate from the recorded data stream (Fig.B17):
•Value and Delta — The value as well as time parameter of
plotted data is indicated in the appropriate windows (see
Fig. B2) when the pointer device moves. Clicking of the pointer
device left button fixes the first cursor. While the left button is
depressed the first cursor position data is
data of the current cursor position. When the left button is
released the second cursor is fixed and the difference (delta)
between second and first cursors is measured in the approriate amplitude
and time windows.
• Samples, Average, Min, Max modes enable the appropriate figure
measured
minimum or maximum value accordingly.
includes five different self explanatory parameters that the
between
subtracted
the cursors. It is either number of samples, average,
from the
Fig. B17
WORLD PRECISION INSTRUMENTSB-10
Page 21
APOLLO 4000
OPERATING INSTRUCTIONS
Fig. B18
Fig. B19
Fig. B20
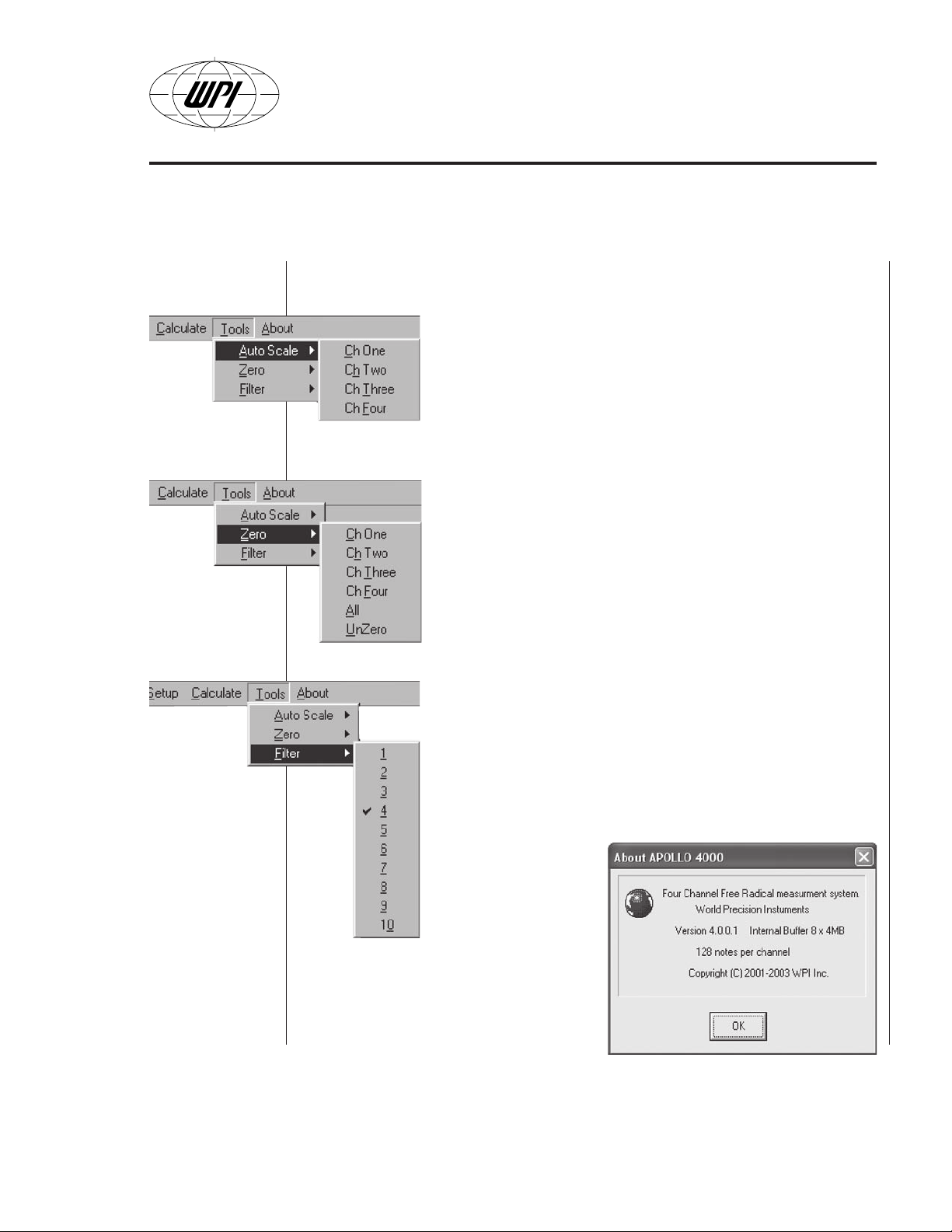
Tools Menu
process.
includes several tools to simplify and improve the acquisition
• Auto Scale. Selecting this option (Fig. B18) allows the user
to quickly find the signal within the plotting windows. This
option moves the vertical zero position and sets vertical
scale to an odd value.
• Zero option subrtacts the current value from the collected
data. The
channel individually, all together or unzero all of previously
“zeroed” channels. The subtracting value is acquired at the
moment of selecting the appropriate action. Zero operation
can be applied as many times as needed. The unzeroed
value is always displayed, too.
• Filter (Fig. B20) applies a moving average digital filter to
the upcoming data. It helps to reduce noise and artifacts if
desirable. Note that the filter does not remove or substitute
data but rather makes the fast transitions of the signal
smoother. There are 10 different orders of filtering from 5
samples averaging (corresponding to filtering level 1) to 50
samples averaging (corresponding to filtering level 10). The
user must select
the most
appropriate filter
for the required
application.
Zero
menu (see Fig. B19) allows to reset each
About Menu
current version of the Apollo 4000 software.
WORLD PRECISION INSTRUMENTS B-11
(Fig. B21) shows the
Fig. B21
Page 22
APOLLO 4000
OPERATING INSTRUCTIONS
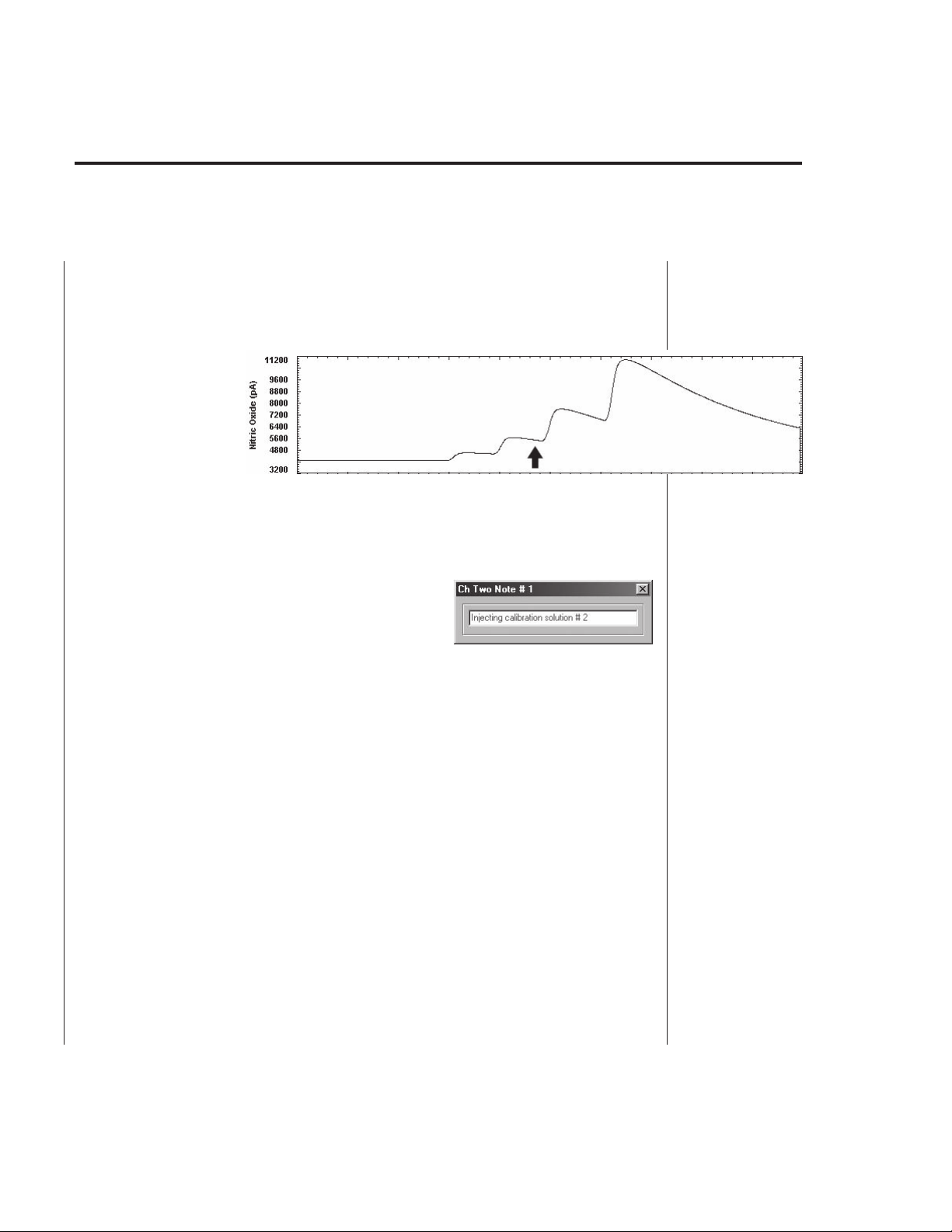
Adding a note: To add a short note to a trace while recording, switch the
operating mode from “PLOT” to “ANALYZE” (see page B-10). The data is still
recorded in the background although the new incoming data is not plotted. Rightclick a point on the trace where you want the Note to be added (see Fig. B22).
This will create a
mark at that place.
Double-click the
created mark. A Note
window will open
where a message of
up to 64 characters can be typed in (see Fig. B23). After the message is entered,
close the window. The mark will remain on the trace and can be double-clicked for
later viewing. Switch to “PLOT” to observe the data as they are being recorded.
To remove a mark from a trace, delete the message from the Note window. When
the Note window is closed, the Note mark will
disappear.
Fig. B22
Fig. B23
WORLD PRECISION INSTRUMENTSB-12
Page 23
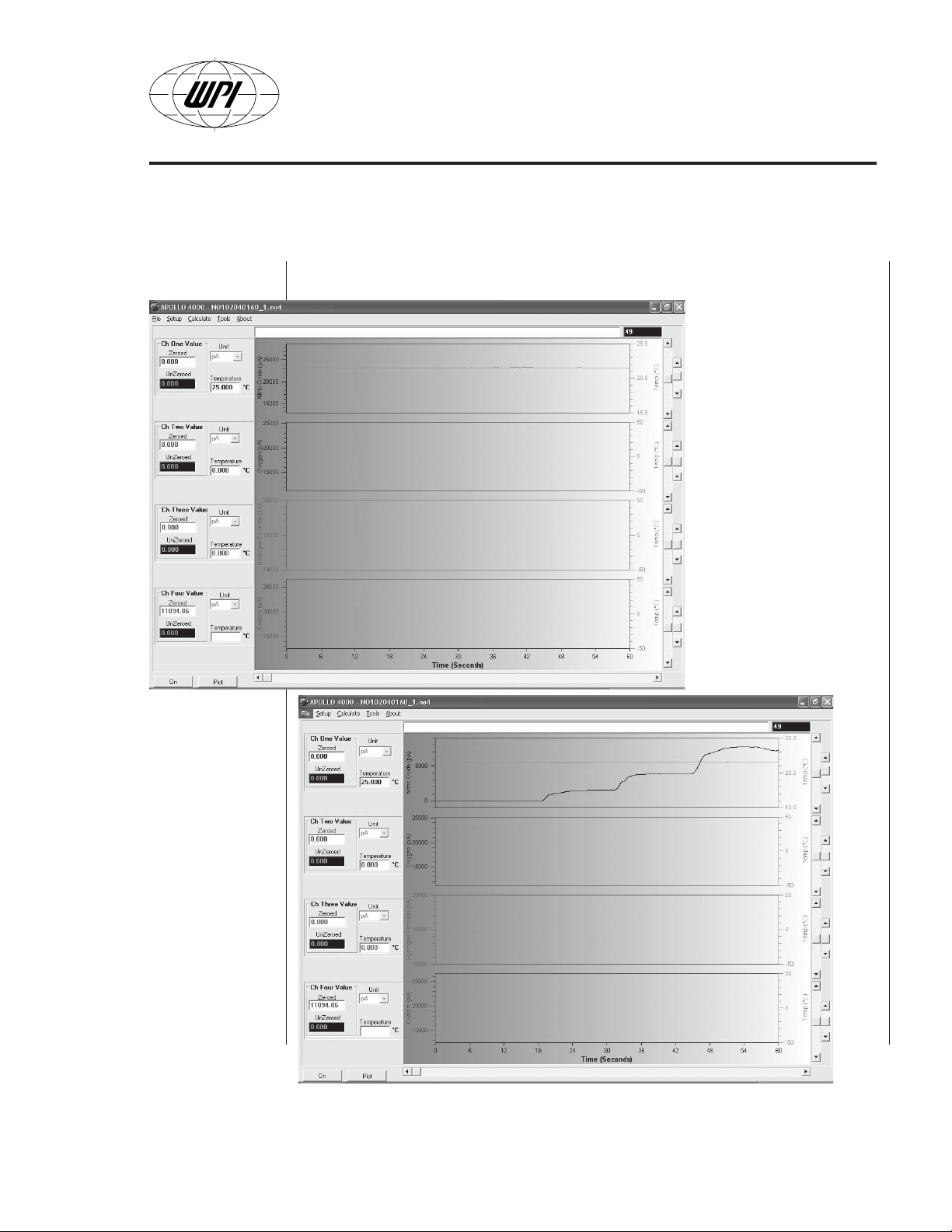
OPERATING INSTRUCTIONS
Example of a real signal application
An example file is
loaded in the Apollo
4000 (Fig. B24). Note
the temperature sensor
is also connected to
channel 1 (
line). However, the
sensor signal is not
seen. If we apply
Scale
appears as follows (see
Fig. B25).
APOLLO 4000
i.e.
, 25°C
Auto
to the channel 1 it
Fig. B24
Fig. B25
WORLD PRECISION INSTRUMENTS B-13
Page 24

APOLLO 4000
OPERATING INSTRUCTIONS
Fig. B26 demonstrates another option that the software offers: a second plotting
window allows the data in any of the channels to be plotted separately for more
detailed viewing. To enable the second window the user must double click inside
the appropriate channel plot. The second window has a distinctive yellow
background color. Only one second window can be opened at the same time. The
size of the second window can be controlled by regular Windows controls
( ).
There are cursors enabled inside the second window and the appropriate value
and delta measurements displayed. Please note that the trace on the expanded
window is a snapshot of the window you double-clicked and is not updated with
time.
Fig. B26
WORLD PRECISION INSTRUMENTSB-14
Page 25

APOLLO 4000
NITRIC OXIDE DETECTION
USING THE APOLLO 4000 TO DETECT NITRIC OXIDE
Initial Set-up
Attach the nitric oxide sensor to the required sensor input channel on the APOLLO
4000. From the main Setup menu of the software, select the correct poise voltage
“Nitric Oxide” and appropriate Range for the selected channel. The electrode must
now be calibrated.
Calibration of the NO Sensor
Accurate measurements of NO require an accurate calibration. Three calibration
methods are described in this section. The first and most convenient method is based
on a simple chemical reaction which generates known amounts of NO (from NO
This method can only be used with the 2.0 mm sensor (ISO-NOP).
-
2
).
The second method is based on the decomposition of the S-nitrosothiol NO-donor,
SNAP using either Cu(I) as described in Method I or Cu(II) as outlined in Method II,
as a catalyst. The NO liberated from SNAP is used to calibrate the sensor.
The third method involves preparing aqueous solutions of NO standards from saturated
NO solutions.
The Calibration Kit
Perform the calibration using the NO calibration kit (WPI catalog #5435) which consists
of the following items:
Plastic stand with two holes; two glass vials; two silicon septums without holes;
two silicon septums with holes and radial slit; one short needle; one long needle.
The chemicals required for the calibration are not provided.
NOTE: The NO chamber (WPI #NOCHM) can be used as an alternative to the use
of the calibration kit. Designed specifically for use with 2.0 mm electrodes, the
chamber can be adapted to other probes. Calibration temperatures from 4 - 40°C
can be controlled using an external circulating bath.
WORLD PRECISION INSTRUMENTS C-1
Page 26

APOLLO 4000
NITRIC OXIDE DETECTION
Calibration by the chemical generation of NO
(Type of NO sensor that can be calibrated with this method: ISONOP)
This method is recommended for use with the 2.0 mm sensor (ISO-NOP). However,
because it requires the use of strong acid it cannot be used with other NO sensors.
The first step is to prepare the following two solutions:
Solution #1: 0.1 M H
+ 0.1 M KI
2SO4
To make 500 mL of solution requires:
4.9 g of H2SO4 (2.7 mL of concentrated H2SO4 {18.4 M})
8.3 g of KI
Slowly
add the sulfuric acid to about 400 mL of distilled water while stirring. Then
add the KI and mix; finally add distilled water to a final volume of 500 mL.
Solution #2: 50 µM KNO
(or NaNO2)
2
The recommended method for preparing this solution is to purchase an ion
chromatography liquid nitrite standard (NaNO2 or KNO2) which may be diluted as
appropriate. Standard Nitrite is available from WPI, catalog #7357.
Alternatively, crystalline reagent KNO
may be used, but the user should note that
2
KNO2 is extremely hygroscopic and degrades once exposed to atmospheric
moisture. It is therefore recommended that if the crystalline reagent is to be used
that the reagent packaged under argon be purchased (available from Eastman
Kodak Chem #105 7462), and that it be stored in a desiccator. While this will
extend the life of the reagent, it will need to be replaced more frequently than will
the liquid standard. The standard nitrite solution prepared from this compound
should be stored in a gas-tight bottle and refrigerated.
This method of calibration is based on the following reaction:
2KNO2 + 2KI + 2H2SO4 → 2NO + I2 + 2H2O + 2K2SO
4
(1)
where a known amount of KNO2 is added to produce a known amount of NO. The
quantity (and so the concentration) of NO generated can be calculated directly
from the stoichiometry if the concentrations of the reactants are known. Since KI
and H2SO4 are present in great excess the limiting reagent is KNO2. Experiments
WORLD PRECISION INSTRUMENTSC-2
Page 27

APOLLO 4000
NITRIC OXIDE DETECTION
have demonstrated that the nitric oxide generated from this reaction will persist
sufficiently long to calibrate the NO sensor easily and accurately.
Since the reaction goes to completion, the equation above states that the ratio
between KNO
tion will be equal to the amount of KNO
be equal to the diluted concentration of KNO
1. Record the value of the sensor current before removing it from the distilled
water in which the tip has been immersed during storage.
2. Immerse the ISO-NOP sensor tip in a strong saline solution (1 M), and after
waiting a few minutes for the current to stabilize record its value. If the current
Figure 4 —
calibration
setup
and NO is 1:1. Therefore the amount of NO generated in the solu-
2
added. The final concentration of NO will
2
in the solution.
2
Calibration Procedure
is offscale or unstable after
several minutes in solution,
it is likely that the membrane
has been damaged and the
sleeve needs to be
changed (refer to the
section on “Changing the
Membrane Sleeve”).
3. Place a magnetic stirring
bar into one of the glass
vials included in the
calibration kit. Add an
appropriate volume (
10 mL) of solution #1.
e.g.
,
4. Note that the calibration
should be carried out at the
temperature at which the
Fig. C1
calibration setup
—
measurements of NO are to be made. This can be accomplished by placing
the vial and stand in a water bath at the appropriate temperature, and allowing
the temperature of the solution in the bottle to equilibrate with the water bath.
WORLD PRECISION INSTRUMENTS C-3
Page 28

APOLLO 4000
NITRIC OXIDE DETECTION
5. Place the stand (and water bath if appropriate) on the magnetic stirrer, and
turn on the stirrer so that the bar is stirring at a moderate rate.
6. Secure the ISO-NO sensor in an electrode holder or micromanipulator (or use
one of the septums included with the start-up kit). Do not push the sensor
tip through the hole — slide the elctrode through the sliced side
of the septum. Carefully lower the sensor into the vial sealing the opening
with the septum. The sensor tip should be immersed about 2-3 mm into the
solution, and should not be in contact with stir bar. Be very careful when
inserting the sensor not to make contact between the cap and/
or bottom of the jar with the tip of the sensor. This could
damage the membrane of the sensor.
7. Wait until the current on the display becomes stable again before continuing.
This may take several minutes if the sensor has undergone a large temperature
change.
8. If you feel it is necessary to degas solution #1 prior to calibration, this can be
done by inserting one of the long stainless steel needles included with the
calibration kit through the septum so that the tip is in the solution. Attach the
needle through appropriate tubing to a source of pure argon gas (nitrogen
may also be used). Insert one of the short needles included with the kit
through the septum such that the needle tip is clearly exposed (not in the
solution) inside the vial. The small needle allows gas to escape, thereby
avoiding a buildup of pressure. Purge the solution at low pressure (5 psi or
less) for 15 minutes.
9. Once purging is complete and the gas source is turned off, remove the purging
and pressure relief needles.
10. One should allow a few minutes for the temperature to equilibrate with the water
bath again since purging with the gas may have changed the temperature.
11. Once the current has achieved a stable value, record this value.
12. Generally, it is not necessarry to pre-purge the calibration solution, since the NO
decays only very slowly in this solution.
WORLD PRECISION INSTRUMENTSC-4
Page 29

APOLLO 4000
NITRIC OXIDE DETECTION
Creating a Calibration Curve
To create a calibration curve, the user measures the current (pA) generated by the
addition of different amounts of KNO
Once the baseline has been set to zero, generate a known concentration of NO in
the solution by adding a known volume of a the NO standard (solution #2). For
example:
Addition 1:
Add 50 µL of solution #2 to 10 mL of solution #1. Then the amount of NO
produced can be calculated by simple dilution factors, as follows:
to the calibration solution.
2
Fig. C2 —
APOLLO4000
Calibration Output
50 µL of 50 µM KNO
(solution #2) into 10 mL solution #1 = 1:201 dilution.
2
Hence, amount of NO produced = 50 (µM) ÷ 201 = 0.2487 µM = 249 nM.
Likewise:
Addition 2:
100 µL of solution #2 added to the above solution
will produce 493 nM NO (
i.e.,
dilution factor =
1:101.5).
The output from the APOLLO4000 will look similar
to the example shown in Figure C2: here three
sequential additions of KNO2 have been made to
solution #1.
From this output a calibration curve can then be
created by plotting the changes in current (pA)
against the changes in concentration (nM). The
slope of this curve indicates the sensitivity of the
probe.
[NO] nM Response (pA)
00
249 332
493 746
966 1486
WORLD PRECISION INSTRUMENTS C-5
Page 30

APOLLO 4000
NITRIC OXIDE DETECTION
Once the sensitivity of the
probe has been ascertained
(in the above example the
sensitivity was 1.557 pA / nM)
the sensor is ready to use
experimentally.
NO2 and NO3 Determination
ISO-NO Calibration Curve Based on Tabulated Data
1600
1200
800
400
0
0 200 400 600 800 1000
Concentration of NO (nM)
Figure C3 —
APOLLO4000
Calibration Curve
We have recently developed a new reagent-less nitrate-reductor, called the
NITRALYZER™. The Nitralyzer is a very useful tool for researchers currently using
enzymatic conversion of NO3 to NO2. Researchers who use the Greiss method for
NO
determination will also be very interested to learn that using the ISO-NOP (and
2
the titration method for calibration described above) a detection limit for NO
as low
2
of 1 nM is routinely possible. This compares to a detection limit using Greiss
reagent of approximately 1 µM. Hence, the ISO-NOP is several orders of
magnitude more sensitive than the Greiss method. Information on the Nitralyzer is
aavailable from WPI.
WORLD PRECISION INSTRUMENTSC-6
Page 31

APOLLO 4000
NITRIC OXIDE DETECTION
Calibration of NO sensor by decomposition of SNAP
This method can be used to calibrated all NO sensors (see Ref. 1: Zhang,
“Novel Calibration Method for Nitric Oxide Microsensors by Stoichiometrical
Generation of Nitric Oxide from SNAP”
S-nitroso-N-acetyl –D,L-penicillamine (SNAP) is a stable NO containing compound
that can be used for quantitative generation of NO in solution. SNAP decomposes
to NO and a disulfide by product when dissolved in water. However, the rate of
decomposition of the SNAP is very slow. The kinetics controlling the decomposition
of SNAP depends on several parameters including, pH, presence of catalyst,
temperature and light.
In the procedure described here, SNAP is used in combination with a catalyst,
cuprous chloride, to generate known amounts NO in solution, which can then be
used to accurately calibrate various NO-sensors. The protocol does not investigate
all parameters involved in SNAP decomposition neither is it intended to propose a
model by which SNAP is decomposed.
Two methods are described here for the calibration of NO sensors based on
decomposition of SNAP. The first method relies on the use of Cu(I) as a catalyst for
the 100% conversion of SNAP into NO. This method is extremely accurate but
technically more demanding than the second method, which relies on the use of
Cu(II) for the partial but quantifiable conversion of SNAP to NO.
Electroanalysis
, 2000, 12: 6).
et al.
,
Method 1: Calibration by decomposition of a Snitrosothiol compound using Cu(I) as a catalyst
This method of calibration results in the 100% conversion of SNAP to NO. The
amount of NO produced, therefore, is based on the final concentration of SNAP.
CAUTION: The described calibration procedure requires the use of cuprous (I)
chloride, CuCl, where Cu (I) is the active catalyst for the conversion of SNAP to
NO. The calibration curve assumes only the presence of Cu (I) and hence a 100%
conversion efficiency of SNAP to NO (see “A novel method to calibrate nitric oxide
microsensors by stoichiometrical generation of nitric oxide from SNAP”, X. Zhang,
L. Cardosa, M. Broderick, H. Fein, I. R. Davis,
WORLD PRECISION INSTRUMENTS C-7
Electroanalysis
, 2000, 12(6),425-
Page 32

APOLLO 4000
NITRIC OXIDE DETECTION
428). However, in the presence of oxygen
Cu (I) is readily oxidized to Cu (II). This will
happen naturally if the compound is
exposed to air and/or there is inadequate
storage of CuCl. The oxidation product Cu
(II) is much less efficient at catalyzing the
conversion of SNAP to NO, and this would
appear during calibration as an apparent
low sensitivity of the electrode to NO.
Since Cu (I) is readily oxidized to Cu (II)
special precautions must be taken to keep
it in its reduced state prior to any
calibration. It is recommended that CuCl be
stored under inert conditions and if used in solution then the solution must be
degassed with inert gas and absent of all oxygen.
NOTE : If your laboratory is not adequately equipped to satisfy the conditions for
storage and use of CuCl please refer to the following section in the manual which
describes a similar calibration procedure based on the use of copper (II) sulfate
(CuSO4) or cupric (II) chloride CuCl
conversion of SNAP to NO.
Getting Started
in which Cu [II] is the active catalyst for the
2,
Figure C4 —
Responses of an
NO electrode to the
successive
additions of
100 nM SNAP into
CuCl saturated
solution (pH=5.5).
Also shown (inset)
is the resulting
calibration plot.
Prepare the following solutions:
#1—Saturated solution of cuprous chloride: This should be prepared by
adding 150 mg CuCl to 500 mL distilled deoxygenated water. The distilled water
can be deoxygenated by purging with pure nitrogen or argon gas for 15 min. The
saturated CuCl solution will have a concentration of approximately 2.4 mM at room
temperature and should be kept in the dark prior to use.
#2—Standard SNAP solution: Dissolve 5 mg EDTA in 250 mL of HPLC pure
water (HPLC grade, Sigma). Deoxygenate the solution using the method described
above. Add 5.6 mg SNAP to the solution. The Molarity of the SNAP solution ( SNAP
f.w.= 220.3) can then be easily calculated. Since the SNAP solution is very
sensitive to light and temperature it should be stored in the dark and in a
refrigerator until required. (Note: The decomposition of SNAP at low temperature,
WORLD PRECISION INSTRUMENTSC-8
Page 33

APOLLO 4000
NITRIC OXIDE DETECTION
in dark and in absence of trace metal ions proceeds very slowly due to the
presence of chelating reagent, EDTA). The standard SNAP solution can then be
used for many calibrations of NO probes throughout the day. However, since SNAP
will slowly decompose even if stored correctly as described, it is recommended
that a fresh standard stock solution of SNAP is prepared at the beginning of
everyday. This will ensure an accurate calibration of the NO sensor.
The concentration of SNAP
calculated as follows:
Where C is the concentration of SNAP in micromolars, A is the purity of SNAP, W is
weight of SNAP in milligrams and V is the volume of the solution in liters. If SNAP
purity is 98.5%, hence in the above example the concentration of SNAP is:
[C] = [98.5% x 5.6 / (220.3 x 0.25)] x 1000 = 100.1 µM
Note: The purity of SNAP used is extremely important to ensure an accurate
calibration. We recommend the use of high grade SNAP with minimal purity of 98%
or better. SNAP can be purchased from WPI in various amounts.
Calibration Procedure
Within a nitrogen or argon environment, place 10.0 mL of solution #1 (CuCl) in a
20 mL vial (supplied in the calibration kit). Drop a small stirring bar into the
solution, and place the vial on the top of a magnetic stirring plate. Immerse a NO
probe into this solution, and while stirring, allow the background current to decay
and stabilize for 3-5 min. As soon as the background current becomes stable start
recording the data on the APOLLO4000.
Next inject 3 aliquots containing 5 µL, 10 µL and 20 µL sequentially of the SNAP
stock solution (solution #2) into the vial containing cuprous chloride solution.
Depending on the required calibration range (
produced) desired, the volumes of SNAP stock solution could be increased to
produce a greater concentration of NO. It is recommended that calibration range
be kept close to the anticipated experimental concentration of NO.
(and hence NO produced)
[C] = [A•W/(M•V)]1000
i.e.,
the final amount of NO
in the stock solution is
Immediately following the first addition of SNAP into Solution#1 the current (pA) output
from the ISO-NO will be seen to increase rapidly. Within a few seconds the response
WORLD PRECISION INSTRUMENTS C-9
Page 34

APOLLO 4000
NITRIC OXIDE DETECTION
will reach a plateau and the second aliquot of SNAP can then be added. Successive
additions of the remaining aliquots of SNAP can be made in a similar way.
A calibration curve can be constructed by plotting the signal output (pA) vs
concentration (nM) of SNAP. Each addition of SNAP corresponds to equivalent NO
concentration. The response should be very linear from 10 to 1000 nM.
The sensitivity of the NO probe can be established from the gradient of the response
curve. The sensitivity of the ISO-NOP sensor is about 1 pA/nM. Once the slope of the
probe has been determined the value can be entered into the APOLLO4000
software program (see previous section) if the user wishes the observe data in
concentration mode (
Note: Remember that most NO probes are sensitive to temperature changes. It is
therefore recommended that the calibration of a NO sensor is performed at the
experimental temperature.
i.e.
, nM, µM).
Method 2: Calibration by decomposition of SNAP using
Cu(II) as a catalyst
This method of calibration relies on the use of Cu(II) for the partial but quantifiable
conversion of SNAP to NO. This procedure can be used as an alternative to the
previous method in which Cu (I) is the active catalyst for the conversion of SNAP to
NO. In this procedure Cu(II) is substituted as a catalyst for ease-of-handling.
NOTE: Experimentally it has been shown that Cu(II) is less efficient as a catalyst in
the conversion of SNAP to NO (
60%). The accuracy of the calibration may also be reduced (see discussion).
S-Nitriso-N-acetyl-D,L-penicillamine (SNAP) is a stable NO containing compound
that can be used for quantitative generation of NO in solution. SNAP decomposes
to NO and a disulfide byproduct when dissolved in water. However, the rate of
decomposition is very slow. The kinetics of decomposition for this reagent is a
function of several parameters including pH, presence of a catalyst, temperature
and light.
In the procedure described here, SNAP is used in combination with a catalyst,
copper (II) sulfate (CuSO4) or cupric (II) chloride (CuCl2), to generate a known
quantity of NO in solution. Note that this protocol does not investigate the effects of
e.g.,
conversion ratio is reduced to approximately
WORLD PRECISION INSTRUMENTSC-10
Page 35

APOLLO 4000
NITRIC OXIDE DETECTION
all parameters involved in SNAP decomposition nor does it propose a model by
which NO is decomposed. The presented procedure provides an empirical
estimation of the amount of generated NO based on the molarity of a standard
stock solution of SNAP under a controlled set of parameters.
Getting Started
Prepare the following solutions:
Solution #1: Dissolve 5 mg EDTA in 250 mL of water (HPLC grade).
Solution #2: Prepare 250 mL 0.1 M copper (II) sulfate (or cupric (II) chloride) in
distilled water.
Preparing standard SNAP solution:
To prepare the stock solution of SNAP, weigh approximately 5.0 mg +/- 2.0 mg of
SNAP and add it to solution #1. Calculate the Molarity of SNAP solution (SNAP f.w.
= 220.3). Decomposition of SNAP in the stock solution proceeds very slowly due to
the presence of chelating reagent, EDTA. Thus the rate of decomposition is
negligible and the stock solution of SNAP remains relatively stable for at least 5
hours if kept in refrigerator.
Note: The purity of standard reagent, SNAP, is very important for the reported data.
Use high grade SNAP with minimal purity of 95% or better. SNAP can be
purchased from WPI (catalog # SNAP50, SNAP100, SNAP500).
Calibration Procedure
Place 10.0 mL of solution #2 in a 20 mL vial (supplied in the calibration kit). Drop a
small stirring bar into the solution, and place the vial on the top of a magnetic
stirring plate. Immerse a NO probe into this solution, and while stirring, allow the
background current to stabilize for about 3-5 minutes. As soon as the background
current becomes stable start the recording.
Next, sequentially inject three aliquots of SNAP solution, 5 µL, 10 µL, and 20 µL,
into the vial containing copper sulfate solution. The current output will rapidly
increase upon addition of first aliquot and will reach a plateau within a few
seconds. Inject the second aliquot, 10 µL, as soon as the first signal reaches a
plateau. Finally add the third aliquot as the second signal reaches its plateau. If
aliquots are not added promptly when reaching the previous plateau, the signal will
WORLD PRECISION INSTRUMENTS C-11
Page 36

APOLLO 4000
NITRIC OXIDE DETECTION
slowly decline because generated NO is quickly oxidized to nitrite and nitrate
which will not be detected by the probe.
Note: You can change the volume of injected aliquots according to the
concentration of SNAP stock solution. Decrease the volume of aliquot if the
electrode is very sensitive or increase the volume of aliquot if the electrode is less
sensitive.
Because NO sensors can be calibrated in a linear fashion, the magnitude of every
signal should almost double as the volume of SNAP solution added is doubled in
the course of the calibration. Use the recorded data to construct a calibration
curve. The calibration curve can be simply constructed by plotting the signal
output (
every addition of SNAP solution corresponds to a particular NO concentration. This
will be discussed below. After the sensitivity of the NO probe is established, the
APOLLO4000 software can be programmed to display data in either concentration
mode (
e.g.,
in pA) vs. the concentration of SNAP added at that time. Note that
i.e.,
nM, mM) or redox current (
i.e.,
pA, nA).
The standard SNAP solution can be used for the calibration of NO probes
throughout the day. Store the solution in the dark and refrigerate when not in use.
Prepare a fresh stock solution of SNAP in the beginning of every day to ensure
minimal decomposition of SNAP in the stock solution. Concentration of SNAP
decreases to 5-10% of its nominal value after approximately 4-5 hours.
NOTE: Remember that most NO probes are sensitive to changes in temperature.
It is therefore recommended that the calibration of your sensor is performed at
experimental temperature.
WORLD PRECISION INSTRUMENTSC-12
Page 37

APOLLO 4000
NITRIC OXIDE DETECTION
Predicting the level of detectable NO according to the
molar ratio of SNAP in the presence of catalyst
(Method II)
Experiments have shown that SNAP is decomposed instantaneously under the
following set of experimental conditions:
Temperature 25°C
Catalyst solution 0.1M copper sulfate
SNAP WPI, 98% purity. Fresh stock solution with
5 mg/250 mL solution EDTA added.
Copper sulfate is at equilibrium with ambient air (aerobic conditions).
SNAP (RSNO) decomposes to NO and a disulfide byproduct according to the
following equation:
2RSNO → 2NO + RS-SR
Theoretically, the concentration of generated NO should be equal to the final
concentration of SNAP in the copper sulfate solution in the calibration vial if the
decomposition goes to completion and if the generated NO is detected quickly
before it is oxidized to nitrite and nitrate.
However, it is expected that the level of detectable NO will be below the theoretical
value because the copper sulfate solution was at equilibrium with ambient air, and
consequently a portion of the generated NO would have been immediately oxidized
to nitrite and nitrate before it was measured by the NO sensor. In addition, it is
possible that decomposition of SNAP does not go to completion even in the
presence of a catalyst. Results on the kinetics of SNAP decomposition in the
presence of a catalyst in an anaerobic environment are published elsewhere (Zhang
et al.
, “Novel Calibration Method for Nitric Oxide Microsensors by Stoichiometrical
Generation of Nitric Oxide from SNAP”,
Our experimental data indicates a conversion efficiency of SNAP to NO of
approximately 0.6 (
sensor in a solution, which is at equilibrium with ambient air and at the
experimental conditions described above. Hence for each mole of SNAP, 0.6 mole
of NO is liberated under the proposed set of parameters. It is assumed the other
40% of SNAP is either not decomposed or a proportion that is decomposed to NO
i.e.,
60%). This result is only applicable for calibration of a NO
Electroanalysis
, 2000, 12: 6).
WORLD PRECISION INSTRUMENTS C-13
Page 38

APOLLO 4000
NITRIC OXIDE DETECTION
is subsequently oxidized immediately before it is detected by the NO sensor.
Example for creating a calibration curve and related computations
1. SNAP weight = 6.4 mg.
2. SNAP was dissolved in 250 mL solution #1 to obtain the standard stock solution.
3. 20 µL, 40 µL, and 80 µL of SNAP stock were added sequentially into 10 mL of
solution # 2.
4. The current was continuously recorded during the course of the calibration.
5. A standard calibration curve was constructed according to the recorded date.
[SNAP] [NO] = 0.6 X [SNAP] Output Current
232.4 nM 139.4 nM 230 pA
462.0 nM 277.2 nM 488 pA
916.8 nM 550.1 nM 1001 pA
Y = -32.038 + 1.88X
The data from calibration curve indicates that this procedure allows an excellent
linear calibration of NO probes (R2= 1). The accuracy of calibration is
approximately +/- 10% from mean. The source of error arises most probably from
gravimetric measurement of the standard reagent, SNAP. In addition, purity of
SNAP as well as partial oxidation of generated NO in the calibration solution could
contribute to this error. Such a deviation may not be so important when NO is
quantified in biological systems because most often the ability to measure changes
in the basal concentration of NO is more significant than measurement of the
absolute level of NO.
WORLD PRECISION INSTRUMENTSC-14
Page 39

APOLLO 4000
NITRIC OXIDE DETECTION
Calibration of NO sensor using aqueous standards prepared with NO Gas
The following method can be used with all NO sensors.
WARNING: Nitric oxide must be handled only in a well-ventilated
area, usually a laboratory fume hood with forced ventilation
U.S. Occupational Safety and Health Administration has set a time-weighted
average maximum NO value as 25 ppm. That is to say that 25 ppm is cited as the
maximum concentration to which workers may be continually exposed. Brief
inhalation of concentrations as low as 200 ppm could produce delayed pulmonary
edema which may be fatal after an asymptomatic period of up to 48 hours after
the initial exposure.
It is therefore critical that the personnel handling
the gas be thoroughly familiar with the Material Safety Data Sheet
(MSDS) and proper handling procedures.
The precautions recommended
by the gas manufacturer must be followed.
. The
Preparing an NO Standard
This method has the advantage of allowing the user to calibrate NO sensors in the
same environment in which the experimental measurements will be made.
However, it has the disadvantages of added cost, inconvenience, and greater
hazard to the user. All of these factors must be taken into consideration. The setup
for preparing a saturated NO aqueous solution is illustrated in Fig. C5.
1. Be certain the fume hood is functioning. See Fig. C5.
2. Make sure that all fittings and connections are secure. The tubing to be used
should not be permeable to NO. We recommend Tygon® tubing if a polymer
tubing is to be used; this is permeable to NO but has the best performance
compared to other polymer tubing of which we are currently aware. Ideally
glass tubing should be used. If Tygon® tubing is used, note that prolonged
exposure to NO affects its properties; therefore it is recommended that the
tubing be inspected frequently and that it be replaced when it appears to be
WORLD PRECISION INSTRUMENTS C-15
Page 40

APOLLO 4000
NITRIC OXIDE DETECTION
*Lecture bottle
of NO (14.2
liters, 98.5%)
obtained from
Aldrich, catalog
#29.556-6;
telephone 800558-9160
brittle. The pressure regulator and tee purge adaptor should be stainless
steel. This is because nitric oxide is a corrosive gas.
3. Prepare 100 mL of a 10 % (by weight) KOH solution and place it in the
sidearm flask as illustrated above. The flask should be sealed with a stopper
through which the tubing passes by means of a Luer fitting to a syringe needle
which extends almost to the bottom of the flask. Tubing is used to connect the
side arm of the flask to the vial containing the water to be equilibrated with
NO. The KOH solution is used to remove other nitrogen oxides from the NO
gas.
4. Place 20 mL of distilled (preferably deionized) water in a small glass vial. Seal
the vial with a stopper and insert through the stopper a long syringe needle
which extends almost to the base of the vial. Connect this syringe needle to
the tubing from the KOH flask as illustrated. Insert an additional shorter
syringe needle which should not extend into the solution. This acts as
a pressure relief during purging.
Figure C5
Setup for preparing
a saturated NO
aqueous solution
must be in a fume
hood with forced
ventilation. Nitric
oxide is highly toxic
and, as illustrated,
escapes into the
atmosphere during
preparation of the
standards.
—
WORLD PRECISION INSTRUMENTSC-16
Page 41

APOLLO 4000
NITRIC OXIDE DETECTION
5. Place the distilled water vial in an ice-water bath. Reducing the temperature
increases the solubility of NO in solution. Thus when the solution is used at
room temperature you will be assured of a saturated NO solution.
6. Purge the system with argon (or nitrogen) gas for a period of 30 minutes at a
moderate flow rate such that the pressure is maintained at a safe level (1-2
psi). When purging it should be observed that gas is indeed bubbling through
the KOH solution as well as the distilled water. After 30 minutes turn off the
argon source, and switch the tee purge valve to the correct position for
purging with NO from the lecture bottle.
7. Purge the system with NO for 5-10 minutes if using a pure source (longer if the
NO source is not pure). Again make sure that gas is bubbling through both
solutions. Warning: NO is now escaping from the pressure relief
needle in the stopper of the distilled water vial. It is imperative
that the fume hood be running at maximum capacity with the
front panel closed (in the down position).
8. After the time in step 7 has elapsed turn off the NO source.
9. Immediately remove the two needles from the distilled water vial.
10. Set the tee purge valve for purging with argon (or nitrogen) gas, and turn on
the argon source. Purge the system for 5-10 minutes at a moderate flow rate.
Gas should be bubbling through the KOH and then escaping from the flask
into the atmosphere. Again be sure that the fume hood is ventilating well.
11. Turn off the argon (or nitrogen) source, and allow the fume hood to continue to
ventilate for 10-15 minutes so as to ensure that all traces of NO gas are
removed from the atmosphere.
12. The solution of distilled water should now be saturated with NO. The
concentration of NO produced by this saturation is dependent upon the
temperature. At 0° C, the concentration is approximately 3.3 mM, and at 20°C
the concentration is approximately 1.91 mM.
13. Dilutions of known concentration can be prepared from this saturated solution
In preparing a dilution, be careful not to unseal the vial, for this
exposes the solution to atmospheric oxygen.
Once the dilutions are prepared, it is a simple matter to calibrate the instrument.
WORLD PRECISION INSTRUMENTS C-17
Page 42

APOLLO 4000
NITRIC OXIDE DETECTION
Measurement of NO
It is impossible to outline in detail how to use NO sensors to measure NO in every
experimental set up the user may encounter. There are, however, some guiding
principles of which the user should be aware to exploit fully the capabilities of the
technology. These are outlined below.
NO Delivery
For any measurement of NO to be made, the NO must reach the sensor surface so
it can react on the surface of the electrode. This point is of particular concern
because in many experiments the lifetime of NO is quite short. This is especially so
in biological systems where compounds such as hemoglobin can reduce the halflife of NO to less than a second. It is therefore very important that the experimental
set up is designed so as to maximize delivery of NO to the sensor. In particular, the
tip of the sensor should be placed as close as possible to the site of release of NO.
Durability and Handling
The user must excercise caution when handling any NO sensor to avoid actions
which could damage the sensor tip. The sensor membrane and membrane coatings are extremely delicate and improper handling will lead to damage.
Environmental Influences
There are two environmental parameters to which NO sensors are quite sensitive:
temperature and electrical interference, both of which are discussed in greater
detail below.
Temperature
Note that the sensitivity of the NO sensor is temperature-dependent. This is due to
the effects of temperature on the partial pressure of NO in either liquid or gas
samples, on the permeability of the membrane or coatings, and on the
conductivities of various circuit components. It is therefore recommended that any
calibration is performed at the same temperature as the experiment.
Electrical Interference
Although nitric oxide monitoring using the Apollo 4000 involves the measurement
of extremely small currents (
e.g.,
less than 1 pA), the intrinsic noise level of the
WORLD PRECISION INSTRUMENTSC-18
Page 43

APOLLO 4000
NITRIC OXIDE DETECTION
Apollo 4000 and NO sensors is sufficiently low so as to provide accurate
measurements of nitric oxide. However various external electrical sources may
couple to the system and produce large extraneous signals in the output record.
The magnitude of this external noise depends on the environment of the laboratory.
If the interference introduced by the electrical signals in the environment is large,
the first step towards eliminating it is to ground and shield the system properly. See
the section on Grounding and Shielding Procedure for the Apollo 4000.
WORLD PRECISION INSTRUMENTS C-19
Page 44

APOLLO 4000
NITRIC OXIDE DETECTION
Maintenance of NO Sensors
The various NO sensors, if well cared for, will require very little maintenance.
Maintenance of the ISO-NOP
When the ISO-NOP sensor is not being used it should be left connected to the
APOLLO 4000 in
tip suspended in distilled water. The basic structure of the ISO-NOP sensor is quite
simple (see Fig. C6). It consists of an internal NO-sensing working/counter
electrode combination. This electrode fits inside a disposable protective stainless
steel sleeve (WPI #5436)
separated from the external environment by a gas permeable polymeric membrane
covering the end of the stainless sleeve. The other end of the sleeve is flanged.
The locking cap is used to attach the sleeve to the probe handle.
ON
position (or to Pre-Polarizer NSA-1, NSA-2 or NSA-3) with the
which must contain fresh electrolyte
(WPI #7325), and is
When the sensor is fully assembled (
i.e.,
with locking cap and sleeve in place) the
internal electrode should be seen to press gently against the polymeric membrane,
which will then be slightly stretched. This ensures that the electrolyte diffusion layer
will be as thin as possible, which is necessary to minimize sensor response time.
Once a membrane is stretched it is permanently deformed and cannot usually be
reused if the sleeve is removed from the electrode. Four additional membrane
sleeves accompany the ISO-NOP in the start-up kit, together with a MicroFil™
electrolyte filling needle (WPI #MF28G67-5) and 1 mL syringe. Additional sleeves
stainless steel
gas permeable
membrane
combination
working / counter
electrode
sleeve
locking cap
probe
handle
connection
to meter
Figure C6
Basic probe
structure of ISONO sensor
—
WORLD PRECISION INSTRUMENTSC-20
Page 45

APOLLO 4000
NITRIC OXIDE DETECTION
can also be purchased separately (WPI #5436). With proper care and by following
the instructions below a membrane sleeve should last more than one month.
Cleaning the Membrane
The membrane sleeve itself requires very little maintenance. The primary concern
is to avoid damage to the membrane and to keep it as clean as possible. After
each use the membrane should be cleaned by suspending the tip in distilled water
for 20-30 minutes to dissolve salts and remove particles, which may have
accumulated on the membrane. If the probe was used in a protein rich solution the
tip should first be soaked in a protease solution for several minutes to remove
protein build-up, and then in distilled water. Enzymatic detergent (
#7363) can also be used. The membrane sleeves can also be sterilized chemically
using an appropriate disinfectant (
matter can be removed by briefly immersing the tip in an acid or base solution (at
times both may be necessary) for 10 seconds. A good indication of a dirty
membrane sleeve is a sluggish response or an unusually low sensitivity. If these
problems are not rectified by the above cleaning procedures then the membrane
sleeve should be replaced.
e.g.,
Cidex, WPI #7364). Accumulated organic
The probe cannot be used in organic
solvents.
e.g.,
Enzol, WPI
Replacing a Membrane Sleeve
All membrane sleeves will eventually have to be replaced by the user. The
procedure for doing so is simple and straightforward.
1. Unscrew the locking cap from the handle.
2. Hold the stainless steel sleeve and remove it with the locking cap from the
internal electrode assembly, being careful not to bend the electrode assembly
when doing so.
3. Rinse the internal electrode with distilled water (particularly the tip) and let it
soak for at least 15 minutes. Be careful not to let water get into the handle. The
current on the ISO-NO meter should go offscale when the electrode is being
rinsed.
4. Gently dry the electrode with a soft tissue (Kimwipes). Be sure to dry
thoroughly the flat surface at the tip of the electrode. After drying the current
should stabilize fairly quickly to a low value (
the electrode is in good working order.
e.g.,
0 - 20 pA). If this occurs then
WORLD PRECISION INSTRUMENTS C-21
Page 46

APOLLO 4000
NITRIC OXIDE DETECTION
5. If the electrode is not clean, repeat steps 3 and 4. If necessary the ISO-NOP
Rejuvenator (WPI #JUV) can be used restore sensitivity of an old electrode
(contact WPI for assistance).
6. Remove the locking cap from the old used sleeve, and gently slide it onto the
new replacement sleeve.
7. Dip the internal electrode 1-2 cm into the electrolyte provided in the ISO-NO
start-up kit, the current should go offscale during this. Using the MicroFil™
nonmetallic syringe needle and 1 mL plastic syringe, supplied, inject
approximately 100 microliters of electrolyte directly into the new sleeve. The
MicroFil supplied should be less than the length of the sleeve, so that it will not
puncture the delicate membrane at the tip of the sleeve during injection. If the
MicroFil is longer than the sleeve it can be cut to the correct length.
8. Slowly and smoothly insert the electrode into the sleeve, and screw the locking
cap into the handle. The electrode should be observed to press gently against
the membrane.
9. The current displayed on the meter at this time will be high or offscale.
10. Suspend the tip of the new assembled probe in distilled water.
11. After 10-15 minutes the current should no longer be offscale and will gradually
decrease with time. It may take several hours for the sensor current to reach a
low stable value, at which time it will be ready for use.
12. The integrity of the new membrane can be determined by immersing the probe
tip into a strong saline solution (1 M). If the current observed, after a few
minutes in the saline solution, increases dramatically or is offscale then the
membrane integrity is not good and a new membrane will have to be fitted.
13. When the ISO-NOP is not being used it should be stored with the tip
suspended in distilled water.
Additional membrane kits (WPI #5436) may be purchased separately.
Maintenance of Nitric Oxide Microsensors
WPI’s Nitric Oxide microsensors are maintenance-free consumable sensors that
are warranted against defect for 30 days from the date of purchase. The following
information should increase the lifetime of the sensor:
WORLD PRECISION INSTRUMENTSC-22
Page 47

APOLLO 4000
NITRIC OXIDE DETECTION
Tip care
The surface of the sensor tip is very sensitive and is covered with a unique layer of
propriety selective membranes. The tip of the sensor should never be handled as
this will damage the membranes and compromise the electrode’s selectivity for
NO. During use the electrode should be held securely, preferably using a micromanipulator or other similar device that permits accurate positioning.
The electrode should be cleaned periodically in distilled water and dried using soft
tissue paper. Organic contamination can be removed using a mild enzymatic
detergent such as ENZOL (WPI part # 7363)
Sterilization
The following methods can be used to sterilize the sensor:
• Ethylene oxide
• Cidex solution (WPI part # 7364)
Storage
NO microsensors should be stored dry in a cool place away from direct sunlight.
They can also be left attached to an ISO-NO Activator (WPI part # NSA-1, NSA-2,
NSA-3). The Activator maintains the sensor in a polarized state, ready for immediate use when required.
WORLD PRECISION INSTRUMENTS C-23
Page 48

APOLLO 4000
NITRIC OXIDE DETECTION
WORLD PRECISION INSTRUMENTSC-24
Page 49

APOLLO 4000
OXYGEN DETECTION
USING THE APOLLO 4000 TO
DETECT OXYGEN
Initial Set-up
Attach the oxygen sensor to the required sensor input channel on the APOLLO
4000. From the main Setup menu of the software select the correct poise voltage
“Oxygen” and appropriate Range for the selected channel. The electrode must
now be calibrated.
Calibration and Use of Oxygen Sensors
NOTE: Before
using the
ISO‑OXY‑2
dissolved oxygen
sensor for the
first time, connect
the sensor to
the APOLLO
4000 (power ON)
overnight.
The ISO-OXY-2 sensor provides accurate, stable and electrically isolated
oxygen measurements. With a probe tip diameter of just 2 mm and low oxygen
consumption, ISO-OXY-2 is ideal for making measurements in small sample
volumes. Other features include a fast response time and a sturdy stainless steel
probe body.
The ISO-OXY-2 in combination with APOLLO 4000 amperometrically measures
the concentration of oxygen in aqueous solutions and gas mixtures. ISO-OXY-2
is not suited for long-term continuous gas measurements, as it needs to
be calibrated on a daily basis. Measurements can be displayed either as a
percentage of atmospheric pressure, parts per million (ppm), or as a redox
current in nanoamperes (nA). The ISO-OXY-2 houses a platinum working electrode
and a silver counter \ reference electrode inside a stainless steel sleeve. A gas
permeable polymer membrane fits over the end of the sleeve which allows oxygen
to pass while blocking liquids, ions and particulate matter. Oxygen diffuses through
the membrane and is reduced at the platinum cathode which is held at -0.7 V
when the instrument is on. This results in an electrical current being generated, the
magnitude of which is determined by the rate of diffusion to the electrode which
is proportional to the partial pressure of oxygen outside the membrane. Thus the
current serves as a measure of the partial pressure of oxygen.
ISO-OXY-2 comes ready to use. All the user must do is attach the sensor to the
APOLLO 4000, turn the power on and wait for the current to decay to a stable
value. This usually takes several hours. The current can be monitored directly on
the APOLLO 4000. Once the current stabilizes the user may then calibrate the
instrument.
WORLD PRECISION INSTRUMENTS D-1
Page 50

APOLLO 4000
2
3
1
OXYGEN DETECTION
Calibration
For accurate results the sensor probe should be calibrated as closely as is
possible to the temperature at which the measurement is to be made.
Polarization
The user must select the correct poise voltage potential for oxygen applied to the
electrode (Fig. B10). After initially connecting the oxygen sensor to the Apollo 4000
in ON position, the probe current will be high — approximately 10,000 pA. The
current will decrease and settle to a stable value after a period of time — usually
six to eight hours. The ISO-OXY-2 should always remain connected to the APOLLO
4000. When the APOLLO 4000 is turned off it no longer applies a polarizing voltage
to the electrode Hence, it may then take several hours for the background current
of the electrode to become stable once the APOLLO 4000 is switched back on
again.
Zero (Oxygen) Point Calibration
After polarization of the ISO-OXY-2 as described above, a calibration for zero
percent oxygen may be carried out in pure nitrogen gas or in water saturated with
nitrogen. With stirring, the complete saturation of water with nitrogen may take
more than ten minutes. Calibration in pure nitrogen gas is much
faster and generally considered more reliable. A plastic calibration
bottle (Fig. D1) is supplied with the utility kit. Connect a plastic
tube (1) from the side tube to a pure nitrogen gas source at a low
pressure (less than 5 psi) and purge the bottle with nitrogen gas.
Insert the ISO-OXY-2 (2) into the bottle through the top vent hole on
the bottle cap (3). The current should be observed to drop rapidly
(a few seconds) to a stable value. If the current value is not zero it
must be zeroed using the software.
Fig. D1
Scale Factor Calibration
After the instrument has been zeroed (at 0% oxygen) using the
software, it must be calibrated by measuring at least one more
known concentration of oxygen.
WORLD PRECISION INSTRUMENTSD-2
Page 51

Fig. D2 —
Oxygen calibration
trace in nA.
APOLLO 4000
OXYGEN DETECTION
Gas Phase Measurements
Probe calibration for gas phase measurements can be accomplished using the
calibration bottle,as described above for zeroing the instrument with nitrogen,
using a tank of known oxygen composition, for example 100 % O2.
Alternatively, air can be used as the calibration standard but since water vapor
does affect the probe reading it is best to use dry air unless the ambient humidity is
accurately known. Dry air can be obtained by passing room air through a column
containing a solid drying agent such as silica gel or calcium chloride and then into
the calibration bottle for calibration. Ambient humidity may cause a calibration error
of as much as 1% O2. The user may judge if this is acceptable.
WORLD PRECISION INSTRUMENTS D-3
Page 52

APOLLO 4000
OXYGEN DETECTION
The physical interpretation of the
% oxygen is the percentage of
atmospheric pressure that the oxygen
present exerts. For example, in a
100% oxygen environment a reading
of 100 means that the partial pressure
of oxygen is 1 atm (760 mm Hg). A
reading of 21 means that the partial
pressure of oxygen is 0.21 atm (160
mm Hg).
Fig. D2 shows a record of the
calibration procedure described
above. Before point 1 the record
displays the background current
of the oxygen sensor in air. This
current value may vary from sensor
to sensor. At point 1 the sensor is
exposed to 0 % Oxygen. At point 2
the background is zeroed using the software. At point 3 and point 4 the sensor is
exposed to air (21% oxygen) and 100% oxygen, correspondingly. By using the
current at 0% (0 nM) and 100% (236 nA), a two-point calibration curve is built and
the slope is determined at 2.36 nA/%. Alternatively, a three-point calibration can be
implemented by adding the information for the current of the sensor in air and by
using linear regression software.
Fig. D3 —
Creating a new unit
(% oxygen).
To present the data in % oxygen you may use the Setup/Unit conversion option
from the Menu. A new unit for the oxygen-measuring channel must be created by
setting up 2360 pA to correspond to 1 % oxygen (Fig. D3). Switch to % unit as
shown in Fig. D4 and all recorded data as well as incoming data will be displayed
in % oxygen unit.
Note: After the calibration the trace should not be re-zeroed as this will invalidate
the calibration.
WORLD PRECISION INSTRUMENTSD-4
Page 53

APOLLO 4000
OXYGEN DETECTION
Fig. D4 —
Oxygen calibration
trace in % oxygen.
WORLD PRECISION INSTRUMENTS D-5
Aqueous measurements
For aqueous calibration, fill the calibration bottle with distilled water to
approximately two thirds of its full volume. Immerse the probe tip into the water via
the top hole. Aerate, for a few minutes, by bubbling air through the side arm of the
bottle at a low pressure using a simple aquarium aeration pump. The scale reading
should be allowed to settle to a stable reading. Dissolved oxygen calibration is
corrected for the effect of water vapor by the following equations :
(1) pO2 = 21% × (1 - pH2O)
or
(2) pO2 = 21% × (1 -p'H2O/760)
Page 54

APOLLO 4000
OXYGEN DETECTION
where pH2O and p'H2O are the partial pressure of water vapor at standard
atmospheric pressure in atmospheres and in mm Hg, respectively (see Table 3).
For example, the pH2O in water- saturated air at 24 deg is 22 mm Hg (see Table 3).
Therefore the pO2 = 21% x (1 - 22/760) = 20.4 %. Note that for purposes of oxygen
measurements liquid water is considered to be “water-saturated air”. The values of
water vapor pressures at different temperatures can be found in Table 3.
You may obtain zero % oxygen concentration by adding to your solution of several
mg Na2S2O3 per 20 mL of solution.
To present dissolved oxygen concentration in parts per million (ppm), refer to Table
1a. This table gives the solubility of oxygen in water at different temperatures at
an ambient pressure of 1 atm. If the solution temperature is 25°C, for example, the
oxygen concentration when the probe is in water is 8.4 ppm. You do not need to
correct for the water-vapor effect for a ppm calibration since the values in Table 1a
are obtained in “water-saturated air” at an atmospheric pressure of 760 mm Hg.
The unit ppm is equivalent to mg/L. This is illustrated as follows. The solubility of
oxygen in water at 0 deg according to the Merck index is 4.889 mL per 100 mL.
Using the ideal gas law we can calculate the number of moles of oxygen present in
100 mL :
PV = nRT
n = P*V/R*T
n = (0.21)*(4.889x10-3) / (0.08206)*(273)
n = 45.8 x 10-6 moles
Where P is the partial pressure of oxygen, V is the volume of oxygen, n is the
number of moles of oxygen, R is the universal gas constant, and T is the absolute
temperature. From the number of moles of oxygen we can calculate the number of
grams of oxygen :
45.8 x 10-6 mol * 32 g / mol
1.46 x 10-3 g
Therefore there will be (1.46 x 10-3 g / 0.1 L) 14.6 mg of oxygen per liter. Since
1 L of water has a mass of 1000 g, and there are 1 million mg in 1000 g, the
concentration in ppm shall be:
(14.6 x 10-3 g/L) / (1000 g/L) = 14.6 ppm
WORLD PRECISION INSTRUMENTSD-6
Page 55

APOLLO 4000
OXYGEN DETECTION
Note that this value corresponds to that given in Table 1a.
For accurate results the temperature of the water sample and the fluid being tested
should be identical. They should be continuously stirred using a magnetic stirrer.
When measuring fluid samples for dissolved oxygen, periodically rinse the
exterior of the probe with distilled water, blot the membrane dry and recheck the
electrode’s calibration as described above.
Creating a Calibration Curve
To create a calibration curve, the user measures the current (nA) of the sensor
at various concentrations of oxygen. The calibration could be either a two-point
or three-point calibration, including measurements at 0% oxygen, 100% oxygen
or air (21% oxygen) with the corresponding correction for water vapor in case of
aqueous measurements (see Table 3).
Note: For absolute measurements in % and ppm units, zeroing function should
not be used after the initial zeroing.
After calibration the user can select to show the oxygen concentration in % or
ppm units. The conversion factor %-to-nA or ppm-to-nA (sensitivity of the probe)
is determined from the slope of the calibration curve and must be typed into the
corresponding field (see Fig. B15). Only after selecting ppm or % unit will oxygen
concentration be displayed.
Calibration Method for O2 Measurements in Living Tissue
or Blood
The APOLLO 4000 and ISO-OXY-2 probe may be used extensively in applications
involving O2 measurements in vitro or in vivo in living tissue or fluids such as
blood. You may still use the calibration procedure in this manual for these
measurements since a membrane-covered amperometric oxygen electrode will
always measure oxygen activity, not concentration. Although it is normal to think in
terms of dissolved oxygen concentration, it is actually more appropriate to define
oxygen in solution in terms of activity, since this is the “effective concentration”.
For example, in distilled water the activity coefficient, γc, is close to unity; but
in solutions with high salt concentration the activity coefficient is different from
unity and concentration and activity of dissolved oxygen are no longer equal: the
oxygen concentration falling with salt concentration increase, while activity remains
constant. For a membrane-covered oxygen electrode this is an important effect
WORLD PRECISION INSTRUMENTS D-7
Page 56

APOLLO 4000
OXYGEN DETECTION
since an oxygen detector only responds to the difference in activity across the
membrane rather than the concentration difference. So in samples containing an
electrolyte, while the oxygen concentration falls with increasing salt concentration
the probe current remains constant.
Thus, if it is necessary to have a measure of dissolved oxygen in terms of
concentration, then the calibration is somewhat more complicated since the
relationship between activity and concentration may change with the change of
salt concentration in the samples. The activity coefficient, a ratio of the activity to
the concentration, generally cannot be predicted and one must rely on empirical
determinations since the compositions of living fluids such as blood are extremly
complicated. One may directly use the fluid to be tested as a “solvent” to prepare a
calibration stantard. Alternatively, one may use the Bunsen absorption coefficient,
α, to calculate oxygen concentration in blood in terms of the results with the
oxygen electrode. The equation is:
where K is a conversion factor depending on the unit of pressure chosen (1 for
atm), pt and pH2O are the total pressure of gas and the partial pressures of water,
respectively. pO2 is the partial pressure of oxygen in blood obtained from the
measurements with the oxygen electrode. Bunsen Coefficients for solubility of
oxygen in plasma and blood can be found in Table 4. However, it is very important
to calibrate at the same temperature as that of the measurement site.
Probe Structure and Assembly
Fig. D5 shows the principal components of an ISO-OXY-2. A gas permeable
membrane (3) is cemented to the tip of the outer stainless steel sleeve (1). The
interior of the probe is a slender wand containing the platinum cathode and silver
counter / reference electrode inside the sleeve. The wand is permanently mounted
in the probe’s plastic handle (2). After electrolyte has been deposited inside the
sleeve (see below), the wand is slowly inserted into the sleeve and secured by
screwing the sleeve cap (4) gently into the probe handle. If the membrane on the
stainless steel sleeve becomes damaged, the entire sleeve must be replaced. Two
spare sleeves are provided with the ISO2 kit, and the user may purchase more.
Should the user wish to sterilize the probe this should be done with alchohol or
some other chemical for an assembled probe. The membrane sleeves can be
WORLD PRECISION INSTRUMENTSD-8
Page 57

Fig. D5
1
2
3
4
— Principal
components
of ISO-OXY-2
electrode.
APOLLO 4000
OXYGEN DETECTION
autoclaved seperately
from the probe should the
user desire to do this and
then place the sterilized
membrane sleeve onto
the probe.
Changing the
membrane
sleeve
All membrane sleeves
replacing will eventually
have to be done by the
user. The procedure for
doing is simple and straightforward.
1. Unscrew the locking cap from the handle.
2. Hold the stainless steel sleeve and remove it with the locking cap from the
internal electrode, being careful not to bend the electrode assembly when
doing so.
3. Rinse the internal electrode with distilled water (particularly the tip) and
let it soak for at least 15 minutes. Be careful not to let water get into the
handle. The current on the APOLLO 4000 should go offscale when the
electrode is being rinsed.
4. Gently dry the electrode with a soft tissue (Kimwipes). Be sure to dry
thoroughly the flat surface at the tip of the electrode. After drying the
current should stabilize fairly quickly to a low value (e.g., 0 - 20 pA). If this
occurs, then the electrode is in good working order.
5. If the electrode is not clean, repeat steps 3 and 4.
6. Remove the locking cap from the old used sleeve, and gently slide it onto
the new replacement sleeve.
7. Dip the internal electrode 1-2 cm into the electrolyte , the current should
go offscale during this. Using the MicroFil™ non-metallic syringe needle
and 1 ml plastic syringe, supplied, inject approximately 100 uL of
electrolyte directly into the new sleeve. The MicroFil supplied should be
WORLD PRECISION INSTRUMENTS D-9
Page 58

APOLLO 4000
OXYGEN DETECTION
less than the length of the sleeve, so that it will not puncture the delicate
membrane at the tip of the sleeve during injection. If the MicroFil is longer
than the sleeve it can be cut to the correct length.
8. Slowly and smoothly insert the electrode into the sleeve, and screw the
locking cap into the handle. The electrode should be observed to press
gently against the membrane.
9. The current at this time will be high or offscale.
10. Suspend the tip of the new assembled probe in PBS buffer solution.
11. After 10-15 minutes the current should no longer be offscale and will
gradually decrease with time. It may take several hours for the sensor
current to reach a low stable value, at which time it will be ready for use.
12. If the current observed, after a few minutes in the saline solution,
increases dramatically or is offscale, then the membrane integrity is not
good and a new membrane will have to be fitted.
13. When the ISO-OXY-2 is not being used it should be stored with the tip
suspended in distilled water.
Clean the Membrane
The membrane sleeve itself requires very little maintenance. The primary concern
is to avoid to damage the membrane and to keep it as clean as possible. After
each use, the membrane should be cleaned by suspending the tip in distilled
water for 20-30 minutes to dissolve salts and remove particles ,which may
have accumulated on the membrane. If the probe was used in a protein rich
solution the tip should first be soaked in a protease solution for several minutes
to remove protein build-up, and then in distilled water. Enzymatic detergent
(e.g., Enzol, WPI #7363) can also be used. The membrane sleeves can also be
sterilized chemically using an appropriate disinfectant (e.g., Cidex, WPI #7364).
Accumulated organic matter can be removed by briefly immersing the tip in an
acid or base solution (at times both may be necessary) for 10 seconds. A good
indication of a dirty membrane sleeve is a sluggish response or an unusually low
sensitivity. If these problems are not rectified by the above cleaning procedures
then the membrane sleeve should be replaced. The probe cannot be used in
organic solvents.
WORLD PRECISION INSTRUMENTSD-10
Page 59

APOLLO 4000
OXYGEN DETECTION
Care of the Electrode
The reduction of oxygen and other trace impurities causes a decrease in the
surface activity of the working electrode. This phenomenon is referred to as
“poisoning”, and over time has the effect of gradually reducing the electrode’s
capability to generate a sufficient redox current. As such, it is recommended to use
the following guidelines to maximize the life of the electrode:
If the oxygen electrode is being used on a daily basis, it’s recommended that the
instrument be left ON with the electrode connected to maintain polarization (see
“Polarization” on page D-2). However; if the electrode is not to be used for a period
of more than 2-3 days, it’s recommended that the electrode be disconnected from
the instrument, and stored with the tip immersed in distilled water. This practice
will reduce the possibility of a gradual reduction of electrode surface activity (as
discussed above) under long term polarization. If the electrode will not be used for
a long period of time (several months), refer to the section titled “Storage” below.
Handling Precaution
When passing the O2 probe through small holes, gaskets or O-rings, allow
sufficient clearance so that the probe tip is not damaged by abrasion. The probe
tip should slide through openings easily before sealing the probe shaft to assure
an air-tight fit.
Storage
For long term storage (several months) unscrew and remove the sleeve from the
probe handle, rinse the electrode tip and the sleeve with distilled water. When
both are dry, replace sleeve to protect probe wand but do not screw the sleeve
completely onto the handle.
WORLD PRECISION INSTRUMENTS D-11
Page 60

APOLLO 4000
OXYGEN DETECTION
Table 1a
°F °C ppm °F °C ppm
32 0 14.6 66 19 9.4
34 1 14.2 68 20 9.2
35 2 13.8 70 21 9.0
37 3 13.5 72 22 8.8
39 4 13.1 73 23 8.7
41 5 12.8 75 24 8.5
43 6 12.5 77 25 8.4
45 7 12.2 79 26 8.2
46 8 11.9 81 27 8.1
48 9 11.6 82 28 7.9
50 10 11.3 84 29 7.8
52 11 11.1 86 30 7.6
54 12 10.8 88 31 7.5
55 13 10.6 90 32 7.4
57 14 10.4 91 33 7.3
59 15 10.2 93 34 7.2
61 16 10.0 95 35 7.1
63 17 9.7 97 36 7.0
64 18 9.5 99 37 6.9
Table 1b
Chloride Concentration of Seawater
°C 5 g/l 10 g/l 15 g/l 20 g/l
0 13.8 13.0 12.1 11.3
1 13.4 12.6 11.8 11.0
2 13.1 12.3 11.5 10.8
3 12.7 12.0 11.2 10.5
4 12.4 11.7 11.0 10.3
5 12.1 11.4 10.7 10.0
6 11.8 11.1 10.5 9.8
7 11.5 10.9 10.2 9.6
8 11.2 10.6 10.0 9.4
9 11.0 10.4 9.8 9.2
10 10.7 10.1 9.6 9.0
11 10.5 9.9 9.4 8.8
12 10.3 9.7 9.2 8.6
13 10.1 9.5 9.0 8.5
14 9.9 9.3 8.8 8.3
15 9.7 9.1 8.6 8.1
16 9.5 9.0 8.5 8.0
17 9.3 8.8 8.3 7.8
18 9.1 8.6 8.2 7.7
20 8.7 8.3 7.9 7.4
21 8.6 8.1 7.7 7.3
22 8.4 8.0 7.6 7.1
23 8.3 7.9 7.4 7.0
24 8.1 7.7 7.3 6.9
25 8.0 7.6 7.2 6.7
26 7.8 7.4 7.2 6.7
27 7.7 7.3 6.9 6.5
28 7.5 7.1 6.8 6.4
30 7.3 6.9 6.5 6.1
Table 1a:
Solubility of oxygen
in parts per million
(ppm) in fresh
water at different
temperatures, in
equilibrium with
air at barometric
pressure of
760 mm Hg
(101.3 kPa)
and oxygen
partial pressure
of 159 mm Hg
(21.1 kPa).
Table 1b:
Solubility of oxygen
(milligrams/liter)
in seawater of
different salinities,
in equilibrium with
air at barometric
pressure of
760 mm Hg
(101.3 kPa)
and oxygen
partial pressure
of 159 mm Hg
(21.2 kPa).
WORLD PRECISION INSTRUMENTSD-12
Page 61

APOLLO 4000
OXYGEN DETECTION
Table 2:
Oxygen solubility
obtained from
Table 1a or
Table 1b should
be corrected
if barometric
pressure is different
than 760 mm Hg or
at altitudes other
than sea level.
Table 2
Altitude Pressure Solubility
(feet) (mm Hg) Correction
Factor
-540 775 1.02
Sea Level 760 1.00
500 746 0.98
1000 732 0.96
1500 720 0.95
2000 707 0.93
2500 694 0.91
3000 681 0.90
3500 668 0.88
4000 656 0.86
4500 644 0.85
5000 632 0.83
5500 621 0.82
6000 609 0.80
Table 3
Water-vapor partial pressure in mm Hg:
Temp. Pv Temp. Pv
°C mm Hg °C mm Hg
0 5 20 18
2 5 22 20
4 6 24 22
6 7 26 25
8 8 28 28
10 9 30 32
12 11 32 36
14 12 34 40
16 14 36 45
18 16 38 50
40 55
WORLD PRECISION INSTRUMENTS D-13
Page 62

APOLLO 4000
OXYGEN DETECTION
Table 4
Bunsen Coefficients (α) for Solubility of Oxygen
in Plasma and Blood
Temp –––––––––––– Blood Hb g/100 mL ––––––––––––
°C Plasma 5 g 10 g 15 g 20 g
15 0.0302 0.0310 0.0312 0.0316 0.0323
20 0.0277 0.0282 0.0284 0.0287 0.0293
25 0.0257 0.0261 0.0263 0.0265 0.0271
28 0.0246 0.0249 0.0251 0.0253 0.0259
30 0.0238 0.0241 0.0243 0.0245 0.0251
35 0.0220 0.0226 0.0227 0.0229 0.0234
37 0.0214 0.0220 0.0221 0.0223 0.0228
40 0.0208 0.0221 0.0212 0.0214 0.0219
WORLD PRECISION INSTRUMENTSD-14
Page 63

APOLLO 4000
HYDROGEN PEROXIDE DETECTION
USING THE APOLLO 4000 TO
DETECT HYDROGEN PEROXIDE
Initial Set-up
Attach the HPO sensor to the required input channel of the APOLLO 4000. From
the main Setup menu of the software select the correct poise voltage “Hydrogen
Peroxide” and appropriate Range for the selected channel. The electrode must
now be calibrated.
The structure of the HPO sensor
The basic structure of the ISO-HPO-2 sensor is identical to that of the ISO-NOP
nitric oxide sensor (see Fig. C6), although there are significant differences in the
actual type of materials used.
It consists of an internal working/counter electrode combination. This electrode fits
inside a disposable stainless steel sleeve (WPI#600012) which must contain fresh
refillable electrolyte (WPI#100042), and is separated from the external environment
by membrane covering the end of the stainless sleeve. The other end of the sleeve
is flanged. The locking cap is used to attach the sleeve to the probe handle.
When the sensor is fully assembled ,the internal electrode should be press gently
against the membrane, which will then be slightly stretched. This ensures that the
electrolyte diffusion layer will be as thin as possible, which is necessary to minimize
sensor response time.
Once a membrane is stretched, it is permanently deformed and cannot usually
be reused if the sleeve is removed from the electrode. Four additional membrane
sleeves (WPI#600012), a MicroFil™ electrolyte filling needle (WPI#MF28G67-5)
and 1 mL syringe are provided in the ISO-HPO startup-kit (WPI#600011).
With proper care and by following the instructions below, a membrane sleeve
should last more than one month.
WORLD PRECISION INSTRUMENTS E-1
Page 64

APOLLO 4000
HYDROGEN PEROXIDE DETECTION
Calibration of the HPO Sensor
Hydrogen Peroxide (H2O2) is a very important product in the biological system. The
determination of H2O2 requires an accurate method of calibration. Amperometeric
(electrochemical) determination using the Apollo is a very reliable method to
measure H2O2. The Apollo measures the amount of H2O2 oxidized on the surface
of the sensor using a poise voltage of +400 mV. The oxidation of H2O2 at the
sensor surface produces a small current (pA), which is detected by the Apollo.
The amount of current produced is linearly proportional to amount of H2O2 in the
experiment.
Items Required
• Plastic stand with two holes
• One glass vial
• 1.0mM H2O2 standard solution (available from WPI)
• 0.01M PBS buffer solution (PBS buffer tablets available from WPI)
NOTE: The multi-port measurement chambers (WPI#NOCHM or #NOCHM-4) can
be used as an alternative calibration kit, specifically for use at different temperature
condition. Calibration temperatures from 4 - 40°C can be controlled using an
external circulating bath (contact WPI for information).
Calibration Procedure
1. Turn on the instrument Apollo 4000, set all the parameters that are needed
in experiment. Select the poise voltage for hydrogen peroxide in the
software.
2. Add an appropriate volume (e.g.,10 mL) of PBS buffer solution (0.01M) in
glass vial. Turn on the stirrer so that the bar is stirring in moderate rate.
3. Remove the sensor from the electrolyte solution in which the tip has been
immersed during storage. Immerse the ISO-HPO-2 sensor tip in PBS
buffer solution (0.01M), The sensor tip should be immersed about 0.3-
0.5mm into the solution, and should not be touched by stir bar.
4. Record the current value. If the current is offscale or unstable after a half
hour in solution, it is likely that the membrane has been damaged and
the sleeve needs to be changed (refer to the section on“Changing the
Sleeve”).
WORLD PRECISION INSTRUMENTSE-2
Page 65

APOLLO 4000
-100
100
300
500
700
900
1100
1300
-30 20 70 120 170
time (sec)
current (pA)
0.5 µM
1 µM
2 µM
4 µM
8 µM
y = 73.296x + 6.4857
R2 = 0.9988
0
100
200
300
400
500
600
700
0 2 4 6 8 10
Concentration of H2O2 (µM)
Current (pA)
HYDROGEN PEROXIDE DETECTION
NOTE: The calibration should be carried out at the temperature at which the
samples of H2O2 are to be measured. This can be accomplished by placing the
vial and stand in a water bath at the appropriate temperature, and allowing the
temperature of the solution in the bottle to equilibrate with the water bath.
5. Once the current has achieved a stable value. It is ready for calibration.
Creating a calibration curve
To create a calibration curve, the user measures
the current (pA) generated by the addition of
different amounts of H2O2 to the calibration
solution.
1. After the current is stable, zero the baseline.
2. Add a known volume of the H2O2 standard
solution to the PBS buffer solution.
Figure E1
— H2O2 calibration
output.
Figure E2 —
ISO-HPO-2
calibration curve
based on Figure 1
WORLD PRECISION INSTRUMENTS E-3
For example, add 0, 5, 10, 20, 40, 80mL H2O2
standard solution (1.0mM) into 10mL PBS buffer solution.
The output from the H2O2 will like similar to the example shown in Fig. E1.
From this output, a calibration curve (Fig. E2) can
be created by plotting the changes in current (pA)
against the changes in concentration (mM). The
slope of this curve indicates sensitivity of the probe.
Page 66

APOLLO 4000
HYDROGEN PEROXIDE DETECTION
Interference
Temperature
The background current of the sensor will usually increase with increasing
temperature of the experiment. Although, the sensitivity of the sensor does not
change significantly within the range 20-37°C. It is therefore recommended that
any calibration should be performed at the same temperature as the experiment.
pH
Between pH 3-10, changing the pH of the solution does not affect the sensitivity
of the HPO sensor. However if the pH is below 3.0, the noise of the sensor
will increase. At pH 10.0 and higher, the response of the sensor will diminish
significantly.
Maintenance of HPO Sensors
When the ISO-HPO-2 sensor is not being used (and for short-term storage)
it should be connected to the APOLLO 4000 with power ON and with the tip
suspended in distilled water. TThis will keep the sensor polarized and ready for
immediate use.
Clean the Membrane
The membrane sleeve itself requires very little maintenance. The primary concern
is to avoid to damage the membrane and to keep it as clean as possible. After
each use, the membrane should be cleaned by suspending the tip in distilled
water for 20-30 minutes to dissolve salts and remove particles ,which may
have accumulated on the membrane. If the probe was used in a protein rich
solution the tip should first be soaked in a protease solution for several minutes
to remove protein build-up, and then in distilled water. Enzymatic detergent
(e.g., Enzol, WPI#7363) can also be used. The membrane sleeves can also be
sterilized chemically using an appropriate disinfectant (e.g., Cidex, WPI#7364).
Accumulated organic matter can be removed by briefly immersing the tip in an
acid or base solution (at times both may be necessary) for 10 seconds. A good
indication of a dirty membrane sleeve is a sluggish response or an unusually low
sensitivity. If these problems are not rectified by the above cleaning procedures
then the membrane sleeve should be replaced. The probe cannot be used in
organic solvents.
WORLD PRECISION INSTRUMENTSE-4
Page 67

APOLLO 4000
HYDROGEN PEROXIDE DETECTION
Changing the Membrane Sleeve
All membrane sleeves replacing will eventually have to be done by the user. The
procedure for doing is simple and straightforward.
1. Unscrew the locking cap from the handle.
2. Hold the stainless steel sleeve and remove it with the locking cap from the
internal electrode, being careful not to bend the electrode assembly when
doing so.
3. Rinse the internal electrode with distilled water (particularly the tip) and
let it soak for at least 15 minutes. Be careful not to let water get into the
handle. The current on the Apollo – 4000 should go offscale when the
electrode is being rinsed.
4. Gently dry the electrode with a soft tissue (Kimwipes). Be sure to dry
thoroughly the flat surface at the tip of the electrode. After drying the
current should stabilize fairly quickly to a low value ( e.g., 0 - 20 pA). If this
occurs, then the electrode is in good working order.
5. If the electrode is not clean, repeat steps 3 and 4. If necessary the ISONOP Rejuvenator (WPI #JUV) can be used to restore sensitivity of an old
electrode (contact WPI for assistance).
6. Remove the locking cap from the old used sleeve, and gently slide it onto
the new replacement sleeve.
7. Dip the internal electrode 1-2 cm into the electrolyte , the current should
go offscale during this. Using the MicroFil™ non-metallic syringe needle
and 1 ml plastic syringe, supplied, inject approximately 100 uL of
electrolyte directly into the new sleeve. The MicroFil supplied should be
less than the length of the sleeve, so that it will not puncture the delicate
membrane at the tip of the sleeve during injection. If the MicroFil is longer
than the sleeve it can be cut to the correct length.
8. Slowly and smoothly insert the electrode into the sleeve, and screw the
locking cap into the handle. The electrode should be observed to press
gently against the membrane.
9. The current at this time will be high or offscale.
WORLD PRECISION INSTRUMENTS E-5
Page 68

APOLLO 4000
HYDROGEN PEROXIDE DETECTION
10. Suspend the tip of the new assembled probe in PBS buffer solution.
11. After 10-15 minutes the current should no longer be offscale and will
gradually decrease with time. It may take several hours for the sensor
current to reach a low stable value, at which time it will be ready for use.
12. If the current observed, after a few minutes in the saline solution,
increases dramatically or is offscale, then the membrane integrity is not
good and a new membrane will have to be fitted.
Storage
Store the electrode with its tip immersed in electrolyte solution in the sealed vial
provided with the electrode.
.
WORLD PRECISION INSTRUMENTSE-6
Page 69

APOLLO 4000
TROUBLESHOOTING
Troubleshooting for APOLLO 4000
Problem Solution
1) The current reads too high or offscale on the
display.
2) The sensor is unresponsive; current is close
to zero and/or there is no response to NO/
HPO/O2 during calibration.
a) If the membrane sleeve has just been
changed, this is normal. Refer to the section
“Replacing a Membrane Sleeve.”
b) The membrane may be damaged. Replace
the membrane sleeve.
c) The electrode has just been attached to the
Apollo 4000. This is normal.
a) Make certain that the contents of the
solutions used in the calibration procedure
are correct.
b) The membrane may be heavily
contaminated. Clean the membrane as
described in the section, “Cleaning the
Membrane.”
c) The electrolyte inside the sleeve may no
longer be functioning. Refer to the procedure
“Replacing a Membrane Sleeve”.
d) Open circuit in cable. Check cable
connections.
3) The baseline is noisy and/or drifts.
WORLD PRECISION INSTRUMENTS F-1
a) Make sure that the experimental set up is well
grounded and shielded as outlined in the
appendix on “Grounding and Shielding”.
b) Check that the electrode has not been
damaged. If it has, replace the sleeve or fit a
new sensor.
Page 70

APOLLO 4000
TROUBLESHOOTING
WORLD PRECISION INSTRUMENTSF-2
Page 71

APOLLO 4000
BASIC GROUNDING & SHIELDING PRINCIPLES
APPENDIX: BASIC GROUNDING &
SHIELDING PRINCIPLES
While the current model of the Apollo 4000 is protected against EMI it may still be
necessary under very noisy conditions to provide additional shielding. Enclosing
the system in a iron shield (Faraday cage) is the best way to shield against stray
electric fields. The best Faraday cage is constructed of solid sheet metal, but in
NO measurements it is usually necessary to see the measurement system. The
alternative is to use copper screening but it must be soldered completely along
any joining seams. Place all the instruments and the sample into a
Faraday cage
It may not always be possible to put the whole measurement system in a Faraday
cage for shielding, as for example with a flow-through system when the probe is
immersed into the effluent of a perfusion system or placed directly into the vein of
the heart of an animal. In this case grounding the external bathing fluid, vein, or
tissue with a stainless steel needle or Ag/AgCl reference electrode will often help
significantly. If pumps or other electrical instruments are to be used in a flowthrough system, the associated equipment or instruments should be grounded as
well. Use a common ground for all equipment in the experiment.
.
grounded
After careful grounding and shielding of the electronic equipment and the probe
system, sometimes it is found that movement of people in the immediate vicinity
causes current fluctuations. These are due to variations in the resulting stray
capacitance. There are several ways to minimize these effects. When the
measurements are made
addition, the operator may need to be grounded because large static charges can
be generated by the operator’s body. Wrist straps connected to ground the
operator may be helpful.
.
WORLD PRECISION INSTRUMENTS G-1
in vivo
, it is good practice to ground the animals. In
Page 72

Page 73

* Electrodes, batteries
and other
consumable parts are
warranted for 30 days
only from the date on
which the customer
receives these items.
APOLLO 4000
Warranty
WPI (World Precision Instruments, Inc.) warrants to the original purchaser that this equipment, including its
components and parts, shall be free from defects in material and workmanship for a period of one year* from
the date of receipt. WPI’s obligation under this warranty shall be limited to repair or replacement, at WPI’s
option, of the equipment or defective components or parts upon receipt thereof f.o.b. WPI, Sarasota, Florida
U.S.A. Return of a repaired instrument shall be f.o.b. Sarasota.
The above warranty is contingent upon normal usage and does not cover products which have been modified
without WPI’s approval or which have been subjected to unusual physical or electrical stress or on which the
original identification marks have been removed or altered. The above warranty will not apply if adjustment,
repair or parts replacement is required because of accident, neglect, misuse, failure of electric power, air
conditioning, humidity control, or causes other than normal and ordinary usage.
To the extent that any of its equipment is furnished by a manufacturer other than WPI, the foregoing warranty
shall be applicable only to the extent of the warranty furnished by such other manufacturer. This warranty will
not apply to appearance terms, such as knobs, handles, dials or the like.
WPI makes no warranty of any kind, express or implied or statutory, including without limitation any warranties
of merchantability and/or fitness for a particular purpose. WPI shall not be liable for any damages, whether
direct, indirect, special or consequential arising from a failure of this product to operate in the manner desired
by the user. WPI shall not be liable for any damage to data or property that may be caused directly or
indirectly by use of this product.
Claims and Returns
• Inspect all shipments upon receipt. Missing cartons or obvious damage to cartons should be noted on the
delivery receipt before signing. Concealed loss or damage should be reported at once to the carrier and an
inspection requested. All claims for shortage or damage must be made within 10 days after receipt of
shipment. Claims for lost shipments must be made within 30 days of invoice or other notification of
shipment. Please save damaged or pilfered cartons until claim settles. In some instances, photographic
documentation may be required. Some items are time sensitive; WPI assumes no extended warranty or any
liability for use beyond the date specified on the container.
• WPI cannot be held responsible for items damaged in shipment en route to us. Please enclose
merchandise in its original shipping container to avoid damage from handling. We recommend that you
insure merchandise when shipping. The customer is responsible for paying shipping expenses including
adequate insurance on all items returned.
• Do not return any goods to WPI without obtaining prior approval and instructions (RMA#) from our returns
department. Goods returned unauthorized or by collect freight may be refused. The RMA# must be clearly
displayed on the outside of the box, or the package will not be accepted. Please contact the RMA
department for a request form.
• Goods returned for repair must be reasonably clean and free of hazardous materials.
• A handling fee is charged for goods returned for exchange or credit. This fee may add up to 25% of the
sale price depending on the condition of the item. Goods ordered in error are also subject to the handling
fee.
• Equipment which was built as a special order cannot be returned.
• Always refer to the RMA# when contacting WPI to obtain a status of your returned item.
• For any other issues regarding a claim or return, please contact the RMA department.
Warning: This equipment is not designed or intended for use on humans.
World Precision Instruments, Inc.
International Trade Center, 175 Sarasota Center Blvd., Sarasota FL 34240-9258
UK: Astonbury Farm Business Centre • Aston, Stevenage, Hertfordshire SG2 7EG • Tel: 01438-880025 • Fax: 01438-880026 • E-mail: wpiuk@wpi-europe.com
WORLD PRECISION INSTRUMENTS 5
Germany: Liegnitzer Str. 15, D-10999 Berlin • Tel: 030-6188845 • Fax: 030-6188670 • E-mail: wpide@wpi-europe.com
Tel: 941-371-1003 • Fax: 941-377-5428 • E-mail: sales@wpiinc.com
Page 74

 Loading...
Loading...