Page 1
Instruction Manual
3 CCD HDTV ENDOCAM
GA--A 253 /en/ Index: 08--09--2.0 / ÄM: PDG 09--3778
5550101
Page 2
Important general instructions for use
Ensure that this product is usedonly as intended and described in this instruction manual, by adequately trained and qualified personnel, and that maintenance and repair is only carried out by
authorized specialized technicians.
Use this product only with the combinations and with the accessories and spare parts listed in
this instruction manual. Use other combinations, accessories and replacement parts only if they
are expressly intended for this use and if the performance and safety requirements are met.
Reprocess the products before every application and before returning them for repair as required
by the instruction manual in order to protect the patient, user or third parties.
Subject to technical changes!
Due to continuous development of our products, illustrations and technical data may deviate
slightly from the data in this manual.
CAUTION -- USA only:
Federal law restricts this device to sale by or on the order of a physician.
Safety instructions and levels of danger
Symbol Level of danger
WARNING!
Failure to observe can result in death or serious injury.
CAUTION!
Failure to observe can result in slight injury or damage to the product.
.
.
IMPORTANT!
Failure to observe can result in damage to the product or surrounding.
NOTE!
Tips for optimum use and other useful information.
GERMANY
RICHARD WOLF GmbH
75438 Knittlingen
Pforzheimerstr. 32
Telephone: +49 70 43 35--0
Telefax: +49704335--300
MANUFACTURER
info@richard--wolf.com
www.richard--wolf.com
BELGIUM / NETHERLANDS
N.V. Endoscopie
RICHARD WOLF Belgium S.A.
Industriezone Drongen
Landegemstraat 6
9031 Gent Drongen
Telephone: +32 92 80 81 00
Telefax: +3292829216
endoscopy@richard--wolf.be
www.richard--wolf.be
Marketing Office
U.A.E
RICHARD WOLF Middle East
P.O. Box 500283
AL Thuraya Tower 1
th
Floor,
9
Room 904, Dubai
Telephone: + 9 71 43 68 19 20
Telefax: + 9 71 43 68 61 12
middle.east@richard--wolf.com
www.richard--wolf.com
USA
RICHARD WOLF
Medical Instruments Corp.
353 Corporate Woods Parkway
Vernon Hills, Illinois 60061
Telephone: +1 84 79 13 11 13
Telefax: +1 84 79 13 14 88
sales&marketing@richardwolfusa.com
www.richardwolfusa.com
FRANCE
RICHARD WOLF France S.A.R.L.
Rue Daniel Berger
Z.A.C. La Neuvillette
51100 Reims
Telephone: +33 3 26 87 02 89
Telefax: +33326876033
endoscopes@richardwolf.fr
INDIA
RICHARD WOLF India Private Ltd.
JMD Pacific Square
No. 211 A, Second Floor
Behind 32
Gurgaon -- 122 001
National Capitol Region
Telephone: + 91 12 44 31 57 00
Telefax: +911244315705
india@richard--wolf.com
www.richard--wolf.com
nd
Milestone
UK
RICHARD WOLF UK Ltd.
Waterside Way
Wimbledon
SW17 0HB
Telephone: + 44 20 89 44 74 47
Telefax: +442089441311
admin@richardwolf.uk.com
www.richardwolf.uk.com
AUSTRIA
RICHARD WOLF Austria
Ges.m.b.H.
Wilhelminenstraße 93 a
1160 Vienna
Telephone: +43 14 05 51 51
Telefax: +431405515145
info@richard--wolf.at
www.richard--wolf.at
0
GA--A 253
Page 3

Contents
1 General information 1.......................................................
1.1 Symbols 1..................................................................
1.2 Intended use 2..............................................................
1.2.1 Contraindications 2...........................................................
1.3 Combinations 3..............................................................
1.3.1 General requirements on products/components of a combination 3.................
1.3.2 Specific requirements on the products/components of a combination 4..............
1.4 Electromagnetic compatibility (EMC) 4..........................................
1.4.1 Video mode via DVI / HD--SDI connections 6....................................
2 Illustration 7................................................................
2.1 Front panel 7................................................................
2.1.1 Legend 7...................................................................
2.2 Rear panel 8................................................................
2.2.1 Legend 8...................................................................
3Set--up 9...................................................................
3.1 Preparation 9................................................................
3.1.1 Legend 10...................................................................
3.2 Color bar test image 10........................................................
3.3 Adjustment of LCD monitors 10.................................................
4 Checks 11...................................................................
4.1 Visual check 11...............................................................
4.2 Function check 1 1.............................................................
5Use 12......................................................................
5.1 Operating principle 12.........................................................
5.2 Controls and modes 12........................................................
5.3 Operation of device 13.........................................................
5.3.1 White balance 13.............................................................
5.3.2 Automatic brightness control 13.................................................
5.4 Menu control 14..............................................................
5.4.1 Overview of “OPTIONS MENU” 15..............................................
5.4.2 “PRESETS MENU” 16.........................................................
5.4.3 “BUTTON MENU” (camera button menu) 17......................................
5.4.4 Electronic zoom 17............................................................
5.4.5 Displaying and changing presets 18.............................................
5.4.6 Printer note 18................................................................
5.4.7 Recorder note 18.............................................................
5.4.8 Overview of “SETUP” 19.......................................................
6 Reprocessing and maintenance 20............................................
6.1 Reprocessing of device 20.....................................................
6.2 Maintenance 20...............................................................
6.2.1 Maintenance intervals 20.......................................................
7 Technical description 21.....................................................
7.1 Troubleshooting 21............................................................
7.2 Technical data 23.............................................................
GA--A 253
I
Page 4

7.2.1 Interfaces 24.................................................................
7.3 Operating, storage, transport and shipping conditions 24...........................
7.4 Spare parts and accessories 24.................................................
7.5 Replacing parts 25............................................................
7.5.1 Device fuses 25...............................................................
7.5.2 Disposal of the product, packaging material and accessories 25.....................
II
GA--A 253
Page 5
1 General information
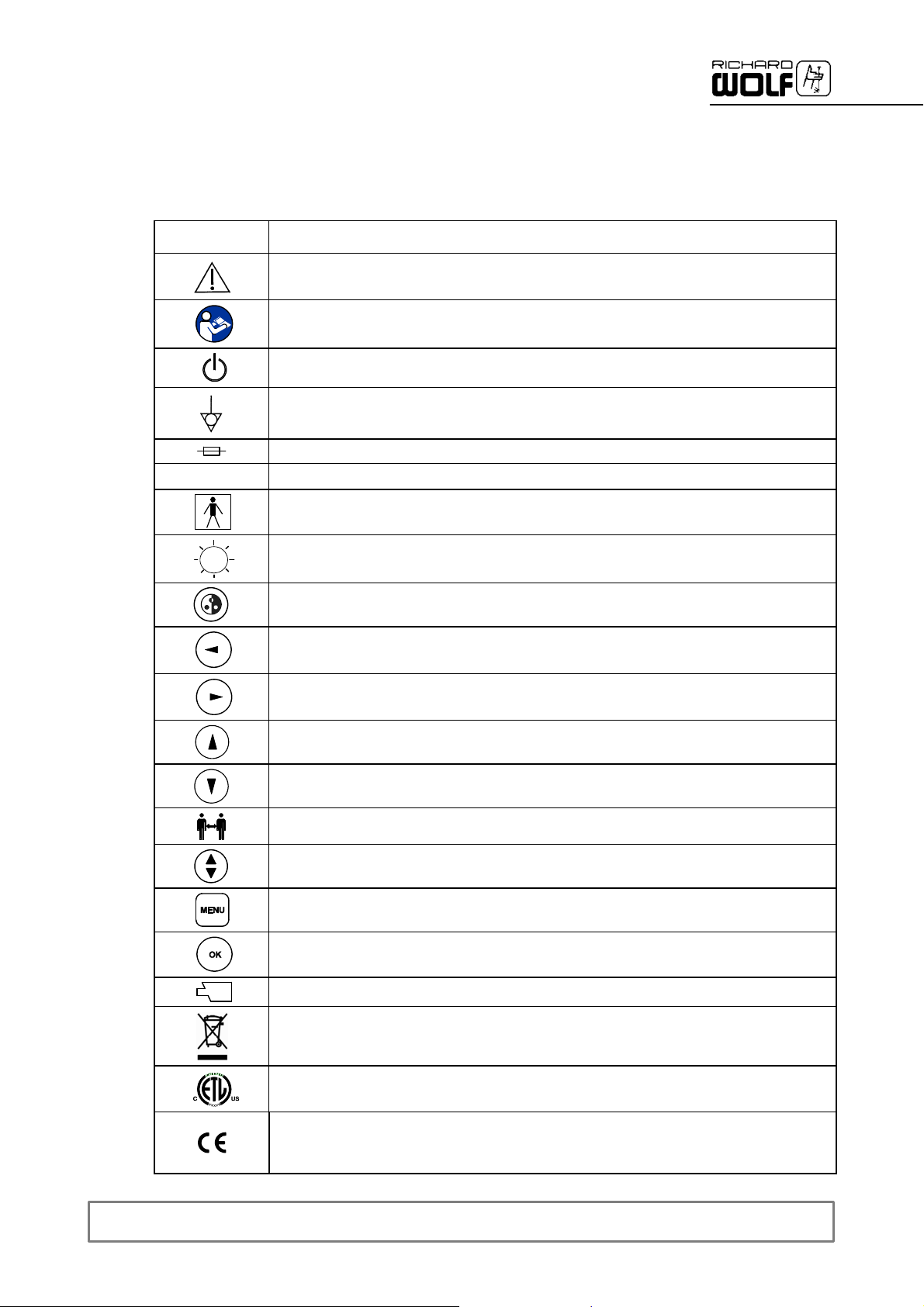
1.1 Symbols
Symbols
μ
Meaning
Caution
Follow instructions for use
Power switch
Potential equalization
Fuse
Alternating current (AC)
TYPE BF APPLIED PART
Brightness
Automatic white balance (AWB)
”LEFT”
”RIGHT”
“UP” or ”PLUS”
“DOWN” or ”MINUS”
Preset
Display or change of preset menu
Display menu
Confirm input
Camera head connection
Do not place product in general waste bin. Recycle separately.
A Registered Trademark of ETL, a Recognized Testing Laboratory, confirm the compliance to the
standard of Medical Electrical Equipment CAN/CSA C 22.2 No. 601.1 (c) and UL 60601--1 (us)
Identification in conformity with Medical Devices Directive 93/42/EEC only valid if the product
and/or packaging is marked with this symbol. Products of category IIa and above, as well as
sterile products or products with measuring function of category I, are additionally marked with the
code number of the notified body (0124)
GA--A 253
1
Page 6

1.2 Intended use
1.2.1 Contraindications
The 3 CCD HDTV ENDOCAM 5550 has been designed for the video
endoscopy and can be used for both diagnostic and therapeutic interventions
In conjunction with video storage devices and other video devices, it can be used
for recording and storing video images.
CAUTION!
Possible device failure.
In the case of therapeutic use, a second camera with similar
features should be available.
Contraindications directly related to the product are presently unknown.
On the basis of the patient’s general condition, the doctor in charge must
decide whether the planned application is possible or not. For further notes
and instructions, see the latest medical literature.
2
GA--A 253
Page 7
1.3 Combinations
IMPORTANT!
.
In addition to this instruction manual follow the manuals of the products used in
combination with this product.
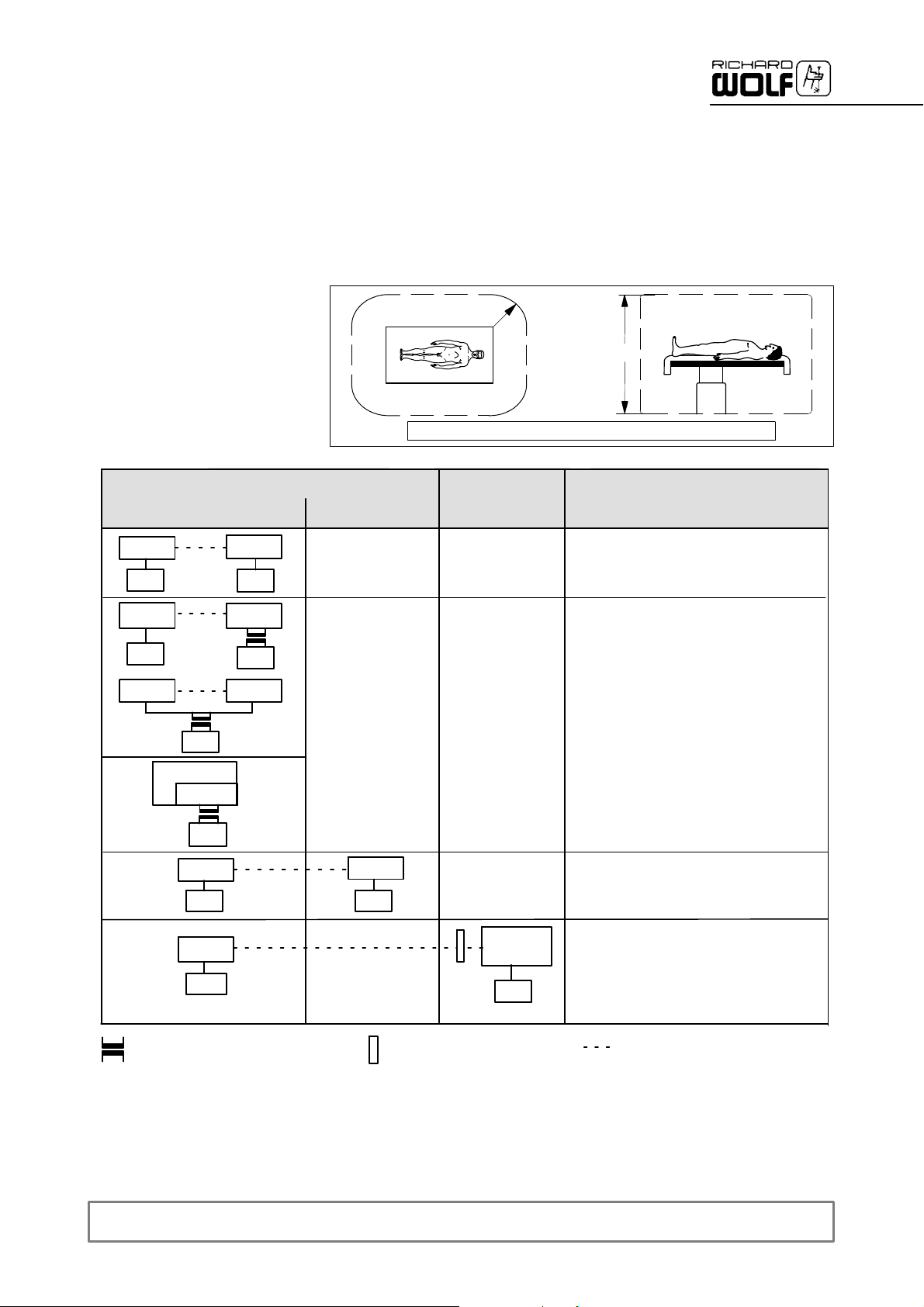
1.3.1 General requirements on products/components of a combination
The general requirements depend on whether the products/components are
inside or outside the patient environment.
Medically used room
Inside the patient environment
MP
μ
MP
μ
MP NMP
μ
MP
NMP
MP
μ
NMP
μ
**
Patient environment
Acc. to UL 60601 -- 1: R = 1.83 m (6 feet) ; h = 2.29 m (71/2feet)
outside the patient
environment
--
--
R=1.5m
Non-- medically
used room
--
--
Patient environment
h=2.5m
Requirements / measures
Leakage currents to clause 19
IEC/EN 60601--1--1
*
--
a) additional protective earth connection
(to be clarified with manufacturer),
or
b) with additional isolating transformer
**
μ
MP
μ
MP
μ
additional isolating transformer
according to IEC/ EN 60601--1--1 **
MP = medical electrical device according to IEC/ EN 60601--1, UL 60601--1, CSA C22.2 No. 601
NMP = non--medical electrical device in accordance with the relevant product--specific IEC/ EN/ UL/ IEC standards
* If connected via a joint mains/power cord under normal conditions the earth leakage current of the system must not exceed 500 μA
(300 μA for systems in acc.with UL 60601--1).
** e.g. Richard Wolf Video Trolley/cart with ”isolating transformer”.
NMP
μ
additional separating device
according to IEC/ EN 60601--1--1
--
MP or
NMP
μ
--
a) common protective earth connection, or
b) additional protective earth connection
(to be clarified with manufacturer), or
c) additional separating device (to avoid
earth/ground loops in the case of a
potential difference)
Functional
connection
μ power supply grid
GA--A 253
3
Page 8
1.3.2 Specific requirements on the products/components of a combination
establishmentsandthosedirectlyconnectedtothepubliclow--voltage
IMPORTANT!
.
Persons combining products to form a system are responsible for not impairing the system’s compliance with
the performance and safety requirements, and that the technical data and the intended use are adequately
fulfilled.
Electromagnetic interference or other types of interference occurring between this product and other products
can cause failures or malfunctions.
When selecting the system components ensure that they meet the requirements for the medical environment
they are used in; in particular IEC/ EN 60601--1 --1. In case of doubt contact the manufacturer(s) of the system
components.
Do not touch connecting devices for electrical connections between the different components (such as signal
input and output connections for video signals, data exchange, control circuits, etc.) and the patient at the
same time.
1.4 Electromagnetic compatibility (EMC)
NOTE: The device or system in the following called product always relates to the 3 CCD HDTV ENDOCAM 5550
Guidance and manufacturer’s declaration -- electromagnetic emissions
The product is intended for use in the environment specified below. The user should assure that the product is used in such an environment.
Emissions measurement/test Compliance Electromagnetic environment -- Guidance
HF emissions to CISPR 11 Group 1
HF emissions to CISPR 11 Class B
Harmonic emissions to IEC 61000--3--2 Class A
In conformity with IEC 61000--3--3 “Voltage fluctuations / flicker
emissions”
The product uses HF energy for its internal function.
The HF emission level is extremely low and it is not likely to cause any
interference in nearby electronic equipment.
The product is suitable for use in all establishments, including domestic
--
power supply network that supplies buildings used for domestic purposes.
Guidance and manufacturer’s declaration -- electromagnetic immunity
The product is intended for use in the environment specified below. The user should assure that the product is used in such an environment.
Immunity tests IEC 60601 test level Compliance Electromagnetic environment -- guidance
Electrostatic Discharge (ESD)
to IEC 61000--4--2
Electrical fast transients, bursts
to IEC 61000--4--4
Surge voltage (surges)
to IEC 61000--4--5
Voltage dips, short interruptions and
voltage variations on power supply
input lines
to IEC 61000--4--11
Power frequency (50/60 Hz) magnetic
field,
to IEC 61000--4--8
* NOTE: UTis the line/mains voltage prior to application of the test level.
± 6 KV contact
± 8KVair
± 2 KV for power supply lines
± 1 KV for input/output lines
± 1 KV differential mode
± 2KVcommonmode
Voltage dip for 0.5 cycle
> 95% U
Voltage dip for 5 cycles
> 60% UT*
Voltage dip for 25 cycles
> 30% UT*
Voltage dip for 5 sec
> 95% UT*
3A/m Yes
*
T
Yes
Yes
Yes
Yes
Floors should be wood, concrete or ceramic tile.
If the floors are covered with synthetic material, the
releative humidity should be at least 30%.
Mains/line power quality should be that of a typical
commercial or hospital environment.
Mains/line power quality should be that of a typical
commercial or hospital environment.
Mains/line power quality should be that of a typical
commercial or hospital environment. If the user of
the product requires continued operation during
power mains/line interruptions it is recommended
that the product be powered from an uninterruptible
power supply or battery.
Power frequency magnetic fields should be at
levels characteristic of a typical location in a
commercial or hospital environment.
4
GA--A 253
Page 9
Guidance and manufacturer’s declaration -- electromagnetic immunity for products that are not life--supporting
The product is intended for use in the environment specified below. The user should assure that the product is used in such an environment.
Immunity test IEC 60601 test levels
Conducted HF interference
to IEC 61000--4--6
Radiated HF interference
to IEC 61000--4--3
REMARKS: At 80 MHz and 800 MHz the higher frequency range applies.
These guidelines may not apply in all situations, as the propagation of electromagnetic waves is affected by absorption and
reflexion from buildings, objects and people.
1 = The field strength of fixed transmitters (e.g. base stations for radio telephones, land mobile radios, amateur radio, radio broadcast and
TV broadcast, ...), cannot be predicted theoretically with accuracy. To assess the EMC environment due to fixed transmitters an
electromagnetic site survey should be conducted. If the measured field strength in the location in which the product is used exceeds
the applicable compliance level above, the product should be observed to verify normal operation.
If abnormal performance is observed, additional measures may be required, such as reorienting or relocating the product.
2 = Over the frequency range between 150 kHz and 80 MHz the field strength should be below 3 V/m.
3V
rms
150kHz to 80 MHz
3V/m
80 MHz to 2.5 GHz
Compliance
level
Yes
Electromagnetic environment -- guidance
Portable and mobile RF communications equipment should be
used no closer to any part of the product, including cables,
than the recommended separation distance calculated from
equation applicable to the frequency of the transmitter.
Recommended separation distance:
d=1.2p P
d=1.2p P for 80 MHz to 800 MHz
d=2.3p P for 800 MHz to 2.5 GHz
P = Nominal power output rating of the transmitter in watts (W)
(according to the transmitter manufacturer)
d = recommended separation distance in meters (m)
Field strengths from fixed RF transmitters, as determined by
an electromagnetic site survey1, should be less than the
compliance level in each frequency range2.
Interference may occur in the vicinity of devices with the
following symbol:
The recommended separation distances between portable and mobile HF telecommunication devices and
devices which are not life--supporting
The product is intended for use in an electromagnetic environment where HF disturbances are controlled.
The user can help prevent electromagnetic interference by maintaining a minimum distance between portable and mobile
HF telecommunications equipment and the product.
Rated nominal output power of the
transmitter (Watts)
0.01 0.12 0.12 0.23
0.1 0.38 0.38 0.73
1 1.2 1.2 2.3
10 3.8 3.8 7.3
100 12 12 23
For transmitters rated at a nominal output power not listed in the table above, the recommended separation distance (d) in meters (m) can
be determined using the applicable equation (observe frequency). P = nominal power of the transmitter in Watts (W).
REMARKS: At 80 MHz and 800 MHz the higher frequency range applies.
These guidelines may not apply in all situations. Electromagnetic propagation is affected by absorption and reflexion
from buildings, objects and people.
150 kHz to 80 MHz
Separation distance as a function of transmitter frequency (m)
d=1.2p P
80 MHz to 800 MHz
d=1.2p P
800 MHz to 2.5 GHz
d=2.3p P
GA--A 253
5
Page 10

1.4.1 Video mode via DVI / HD--SDI connections
1
2
DVI / HD --SDI- -IN
S- -VIDEO-- IN
HD- -SDI- -IN
4
S- -VIDEO- -OUT
HD--SDI--OUT
S- -VIDEO- -IN
3
S- -VIDEO- -OUT
HD--SDI--OUT
S- -VIDEO- -IN
Recording device 2
HD--SDI--IN
Recording device 1
HD--SDI--IN
0
DVI / HD --SDI - -OUT
S- -VIDEO- -OUT
HD--SDI--OUT
NOTE!
.
Connect only the cables listed in the table.
Direct connection Connection via recording devices
DVI cable or
BNC cable [1]
6
S--video cable [2], [3] and [4]or
BNC cable (HD--SDI) [2], [3] and [4]
GA--A 253
Page 11

2 Illustration
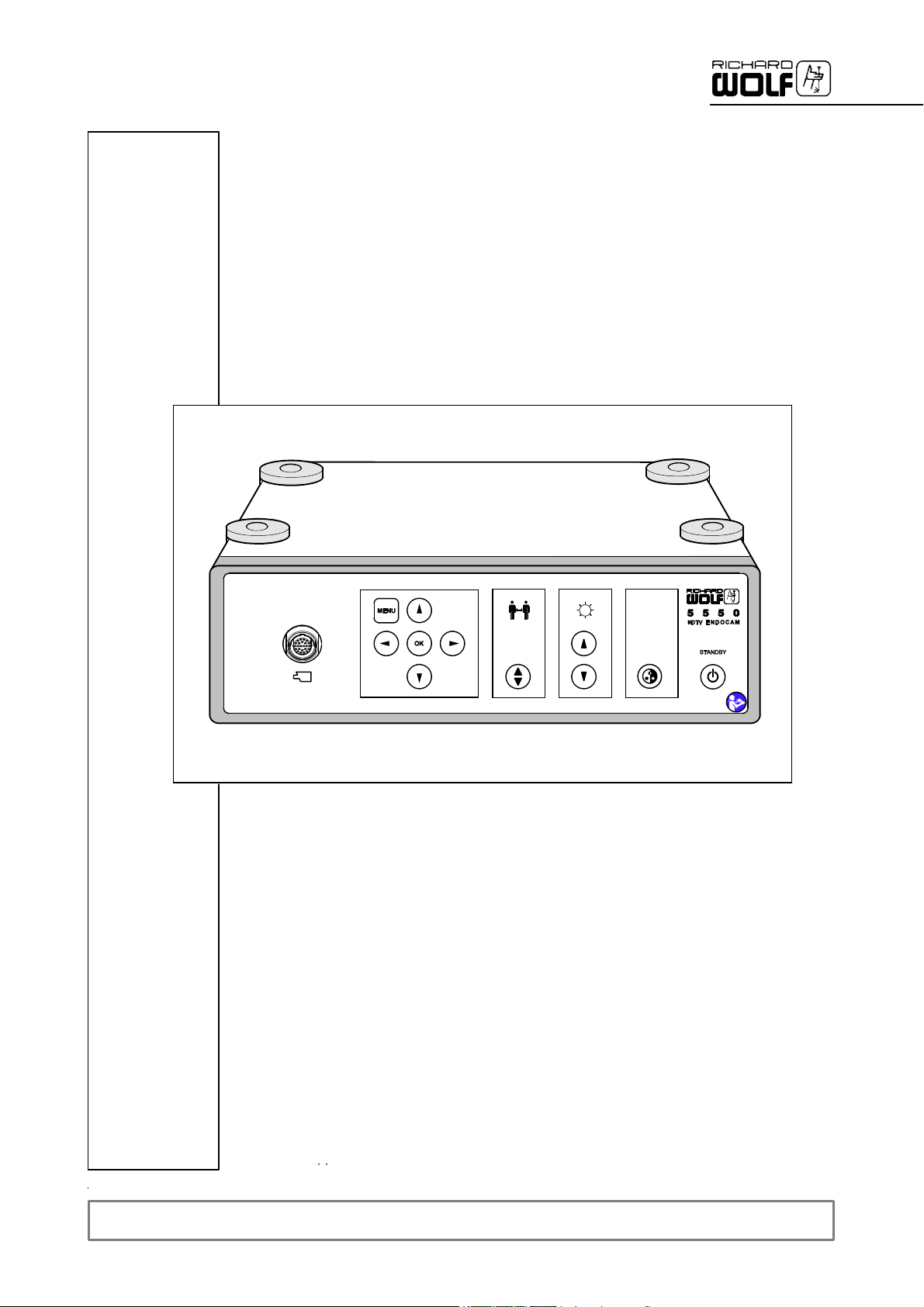
2.1 Front panel
89
1
3
7
2.1.1 Legend
1 Power switch (STANDBY) 6 Control buttons for menu selection
2 ”Automatic white balance” (AWB) button 7 Camera socket
3 ”Brightness PLUS“ button 8 “Display menu” button
4 ”Brightness MINUS“ button 9 “Confirm input” button
5 Display or change the “Preset” menu
6
5
4
2
GA--A 253
7
Page 12

2.2 Rear panel
17 18
16
15
14
13
12
1011
2.2.1 Legend
10 S--Video output 15 Power input receptacle with fuse holder
11 Video output 16 Potential equalization connector
12 DVI output (digital HDTV) 17 System connecton
13 HD--SDI output (digital HDTV)
(not in 1080p mode)
14 Fuse label 19 Identification label
(CAN INTERFACE SERIAL DEVICES,
Software module HD / HDTV ENDOCAM)
18 REMOTE outputs
(e.g. remote control for recorder/printer)
19
8
GA--A 253
Page 13

3Set--up
WARNING!
The device is not protected against explosions.
Explosion hazard.
Do not operate this device in areas where there is a danger of
explosion.
NOTE!
.
Check that the line/mains voltage is the same as the voltage specified on
the identification plate. Connect the device only with the supplied power
cable or a power cable meeting the same specifications.
CAUTION!
Danger of faults and malfunctions.
To guarantee the safety of the user, the patient and others use only
accessories and spare parts specified by the manufacturer of this
product. Other accessories or spare parts can cause the emission
of increased electromagnetic radiation or reduced immunity against
interference.
IMPORTANT!
.
Medical devices are subject to special precautions with regard to electromagnetic compatibility (EMC).
Make sure you observe the notes on EMC for installation and operation.
Medical electrical devices can be influenced by mobile HF communication
devices.
If it is necessary to stack the devices or place them next to each other
and HF interference is observed, make sure you observe the intended
use of the devices.
3.1 Preparation
WARNING!
Power switch ON/OFF.
After switching off using the power switch, the device is in standby
mode.
The device is only complete disconnected from the mains/power
supply if the power plug is disconnected from the socket.
IMPORTANT!
.
Never direct the camera head at the sun or at bright light sources in the
vicinity. High energy radiation in the visible and ultraviolet wave length
can damage the CCD chip surface which may result in incorrect color
rendering and image noise.
If the camera head is not used, make sure that the protection cap is
installed.
Z Connect the auxiliary devices, such as the monitor and the light projec-
tor in accordance with the connection diagrams in Section 1.
Z Switch on the 3 CCD HDTV ENDOCAM.
Z The color bar test pattern appears on the monitor.
Z Prepare the attachable camera head or endoscope for connection (for
this make sure you follow the corresponding menu).
Z Connecting the camera head to the camera controller:
' Hold the camera controller firmly in one hand. Make sure that the
reference point of the camera plug is aligned with the marking of the
camera controller. Then insert the camera plug with your other hand
as far as it will go.
GA--A 253
9
Page 14

' Do not press the buttons on the camera head when connecting the
camera plug because this may cause a functional failure of the buttons (wrong initialization by the camera controller).
Z Separating the camera head from the camera controller:
' Hold the camera controller firmly in one hand, then pull out the cam-
era plug in axial direction with your other hand.
IMPORTANT!
.
Never pull by the camera cable.
Never pinch, grasp or excessively bend the camera cable because this
can damage the wiring resulting in image failure.
Light projector
0
23
14
21
22
Fig. 1
3.1.1 Legend
14 Removable camera head 22 Endoscope
21 Objective lens (integrated) 23 Light cable
3.2 Color bar test image
The color bar test pattern of the camera is used to check color intensity
(chroma) and tint (hue) of the monitor. Via the color bar signal, video and
S--video signals can be adjusted to optimal color rendition.
Z To switch the camera image to color bar signal, pull out the camera
plug.
3.3 Adjustment of LCD monitors
Changing the settings for brightness, contrast, color saturation, chroma,
etc., influences the color rendition of the LCD monitors.
For optimum image results in conjunction with a Richard Wolf
ENDOCAM, we recommend using exclusively LCD monitors offered by
Richard Wolf.
These LCD monitors are preset in the factory, i.e. their color balance has
been properly adjusted.
10
GA--A 253
Page 15

4 Checks
4.1 Visual check
4.2 Function check
IMPORTANT!
.
Run through the checks before and after each use.
Do not use products which are damaged, incomplete or have loose parts.
Return damaged products together with any loose parts for repair.
Do not attempt to do any repairs yourself.
Z Check the device, instruments and accessories for damage, loose or
missing parts, hygienic condition and completeness.
Z Check all connection cables for damage.
Z Any lettering, labeling or identification necessary for the safe intended
use must be legible.
' Missing or illegible lettering, labeling or identification which may lead
to wrong handling and reprocessing must be restored.
IMPORTANT!
.
Before use, check all devices for proper functioning.
Z Connect the camera head to the camera controller and attach the
endoscope to the objective lens.
Z Check that the components are firmly connected.
Z Switch on the camera controller.
' The LED of the power switch changes from yellow “standby” to
green “ON”.
Z Switch on all other video devices.
Z Switch on the light source and connect the light cable to the endo-
scope.
' Direct the endoscope at an object and check the image on the
monitor, ensuring sufficient image brightness.
CAUTION!
Danger of dazzle.
Do not look into the open end of the light cable or scope when it is
connected.
Z Vary the distance between the endoscope and the object. Make sure
you observe the working distances typical for the endoscope used.
' Automatic brightness control will keep the brightness of the monitor
image constant over a wide range.
GA--A 253
11
Page 16

5Use
5.1 Operating principle
.
The 3 CCD HDTV 5550 endocam provides a digital HDTV video signal, in
particular suited for operating a 16:9 LCD monitor for display purposes.
For recording, both the digital and the analog SD signals can be used.
The color properties of the illumination are referred to as color temperatures and are measured in Kelvin (K). Higher color temperatures are bluish, lower color temperatures are reddish.
In order to reproduce a picture in true colors, it is necessary to perform
white balance before the first use and after each change of the light projector or endoscopic equipment. White balance is a procedure where the
color gain for the red and blue portions of the video image is adjusted in
such a way that it suits the color temperature of the light projector and
that the 3 CCD HDTV ENDOCAM provides optimum colors. This includes
that white objects are rendered as completely white.
In the 3 CCD HDTV ENDOCAM, the white balance can be performed for
a temperature range of 2300 K to 6000 K.
IMPORTANT!
Depending on the focal length of the objective lens, image information
may be lost if full format is displayed.
In the case of full format, parts of the endoscopic image do not appear on
the monitor (Fig. 2).
Fig. 2
5.2 Controls and modes
Z The ’Auto White Balance’ (AWB) button serves to start the automatic
white balance procedure.
Z ”Preset” button (user settings) -- initial pressing will display the active
preset.
' If this button is pressed again, the display will change to the next pre-
set.
Z The “brightness selection” buttons serve to increase or decrease the
basic brightness for automatic brightness control (shutter function).
Z “Menu” button -- initial pressing of the menu button will access the
menu. Use the arrow buttons to navigate and the “OK” button to
change the selection.
' Pressing “Menu” button serves to exit the menu.
12
GA--A 253
Page 17

5.3 Operation of device
5.3.1 White balance
.
IMPORTANT!
After each start with the camera head plugged in or plugging in
of the camera head, the user is prompted to perform a white balance.
Z Switch on the light projector and direct the endoscope at a white
surface.
' Make sure that no extraneous light and no colored objects are in the
camera’s field of view.
Z For an initial white balance setting the user can keep depressed either
the “WHITE BALANCE” button of the controller, or any other camera
head button for more than 1 second while “WHITE BALANCE” is displayed.
' During white balance, the “WHITE BALANCE” message appears and
the writing “AWB” blinks.
' If white balance is completed successfully, the white balance symbol
is displayed in green and a green “OK” appears.
' If white balance is completed unsuccessfully, the white balance sym-
bol is displayed in red and a red “FAIL” appears.
The user is prompted to check the setup and repeat the procedure.
If the white balance is not successfully completed, it is not possible to
use the camera head button again (except when the head button is
programmed for white balance function).
Instead, the “WHITE BALANCE” button on the controller front panel
is used.
5.3.2 Automatic brightness control
The automatic brightness control (shutter function or shutter mode)
of the 3 CCD HDTV ENDOCAM allows the use of light projectors without
light control and light projectors with deactivated video control.
The advantage of the shutter function is very fast response to image brightness.
IMPORTANT!
.
To avoid unnecessary heating of the endoscope when using the shutter
function, set the light intensity of the light projector to a middle value.
The shutter function should not be used together with the video control of
the light projector (switch off video control) because this would lead to
undesirable fluctuations in image brightness.
GA--A 253
13
Page 18

5.4 Menu control
To navigate through the various menus:
D press the “MENU” button. This displays the “OPTIONS MENU.
The “OPTIONS MENU” allows access to the “PRESETS MENU” and
the camera head “BUTTON MENU” (menu for camera head buttons).
D If the “PRESETS MENU” or “BUTTON MENU” are selected, their
associated menus will be displayed.
D The “OK” button serves to select and confirm input menu items.
D The “UP”--, “DOWN”--, “LEFT”-- and “RIGHT” buttons are used to
navigate through the menus or menu items..
D In the menu mode, pressing the “LEFT” button takes you back one
step in the menu.
D Pressing the “MENU” button will exit all menus.
14
GA--A 253
Page 19

5.4.1 Overview of “OPTIONS MENU”
Several menus are available to enable the user to customize the camera
to best fit their needs.
PRESETS MENU
OPTIONS MENU
press ”menu” button
PRESET 1
ENHANCEMENT
FIBRE
0...5
BRIGHTNESS
5...120...150
WINDOW SIZE
LARGE
MED
SMALL
GAIN
1...10
ZOOM
MIN...MAX
PRESET 2
ENHANCEMENT
BRIGHTNESS
WINDOW SIZE
GAIN
ZOOM
PRESET 3
GAIN
ENHANCEMENT
BRIGHTNESS
WINDOW SIZE
ZOOM
PRESET 4
ENHANCEMENT
BRIGHTNESS
WINDOW SIZE
GAIN
ZOOM
BUTTON MENU
COLOR
1 ... 6
Fig. 3
GA--A 253
B1 SHORT PRESS
AWB
REMOTE 1
REMOTE 2
BUTTON OFF
PRESETS
B1 LONG PRESS
AWB
REMOTE 1
REMOTE 2
ZOOM
BRIGHTNESS
BUTTON OFF
PRESETS
B2 SHORT PRESS
AWB
REMOTE 1
REMOTE 2
BUTTON OFF
PRESETS
B2 LONG PRESS
AWB
REMOTE 1
REMOTE 2
ZOOM
BRIGHTNESS
BUTTON OFF
PRESETS
15
Page 20

5.4.2 “PRESETS MENU”
The “PRESETS MENU” allows users to customize “PRESETS” (1 to 4)
that can be accessed by the “PRESETS” button on the camera controller
front panel. The setting last used determines what preset will be active at
start--up.Presets 5 to 8 are fixed.
To select the appropriate preset:
Z Press the “MENU” button. This displays the “OPTIONS MENU”.
Z Use the “OK” button to confirm the “PRESETS MENU”.
Z Use the arrow buttons for navigation through the menu “PRESET 1” to
“PRESET 4”.
Z The function presently programmed is highlighted.
Z When a suitable function is highlighted, press the “OK” button to con-
firm.
Z Use the “UP” and “DOWN” buttons to scroll through the functions until
a suitable preset setting is highlighted.
' “ENHANCEMENT” level:
Increasing enhancement improves and accentuates fine details of a
picture. Decreasing enhancement softens the edges of an object.
If instruments with image guides are used there is an anti--Moiré setting called “FIBRE”.
' “BRIGHTNESS” level:
The brightness can be adjusted by the user to obtain the appropriate
brightness level for optimal picture quality.
' “WINDOW SIZE”:
The window size defines what portion of the image is utilized by the
camera controller to determine the brightness level. Small window
determines the brightness based on the inner one third portion of the
image. Large window determines the brightness based on the entire
image.
' “GAIN” factor:
Gain can be utilized to increase light sensitivity at the low--light situations (if there is sufficient light, “Gain” will not be active even if set to
high). There are ten different gain settings. Turning gain from “1” to
“10” may increase the noise level of the picture.
' “ZOOM” factor (magnification factor):
The electronic zoom factor can be customized from “0” to “5”. Zoom
is not working in the “1080i” mode.
' “COLOR” (color setting):
This function allows changing the color intensity.
16
Z Press the “OK” button for selection.
Z Adjustment of the selected function using the “UP” and “DOWN” but-
tons is now possible.
Z Press the “OK” button for confirmation.
GA--A 253
Page 21

5.4.3 “BUTTON MENU” (camera button menu)
The button menu allows the user to customize the functions of the
camera head buttons. There are two camera head buttons and both can
be used for two functions, these can be activated by either pressing and
releasing the button (short press) or pressing and holding the button (long
press).
To program the button functions:
Z Press the “MENU” button to display the “OPTIONS MENU”.
Z Use the “DOWN” button to select the “BUTTON MENU”.
Z Use the “OK” button to confirm the “BUTTON MENU”.
Z Use the arrow buttons for navigation through the menu.
-- “HEAD BUTTON 1 SHORT PRESS” (Press head button 1 briefly)
-- “HEAD BUTTON 1 LONG PRESS” (Press head button 1 for more
than a second)
-- “HEAD BUTTON 2 SHORT PRESS” (Press head button 2 briefly)
-- “HEAD BUTTON 2 LONG PRESS” (Press head button 2 for more
than a second)
Z Press “OK” to activate the highlighted head button to set the
button function.
5.4.4 Electronic zoom
Z Use the “UP” and “DOWN” buttons to scroll through the functions.
' “AWB”; “PRESETS”; “REMOTE 1”; “REMOTE 2”; “BUTTON OFF” --
for short button presses.
' “PRESETS”; “ZOOM”; “AWB”; “BRIGHTNESS”;
“REMOTE 1”; “REMOTE 2” und “BUTTON OFF” -- for long
pressing of the button.
Z When the appropriate function is flashing, press the “OK” button
for confirmation.
In order to operate the electronic zoom feature, the camera head buttons
must be programmed properly in the “Options Menu” (5.4.3).
Adjusting the zoom level:
Z Press and hold the camera head button that is programmed for zoom
to display the “Zoom Level” adjustment screen.
Z For “zooming in”, press camera head button 1.
' The cursor will move to the right with each pressing until the
maximum zoom is obtained.
Z For “zooming out”, press camera head button 2.
' The cursor will move to the left with each pressing until the minimum
zoom (neutral) is obtained.
' The “Zoom level adjustment” screen disappears after a few seconds
when no further action is taken.
NOTE!
.
Zoom default at the product is the neutral zoom level.
Electronic zoom is not available in the “1080i” mode!
GA--A 253
17
Page 22

5.4.5 Displaying and changing presets
NOTE: Users can customize the parameters in the presets 1 to 4 (see
diagram Section 5.4.1).
The parameters of presets 5 to 8 are fixed.
Presets 1 to 4:
The camera controller has different preset configurations designed to give
the user the optimal image quality for different types of procedures and
different types of scopes.
Z Initial pressing will display the active preset.
Z Press the “PRESET” button again and the camera will change to the
nextpreset configuration.
Presets 5 to 8:
The parameters of the presets 5 to 8 are set user--specifically and not be
modified:
D Preset 5: LAPAROSCOPY 1
D Preset 6: LAPAROSCOPY 2
D Preset 7: SMALL SCOPES
D Preset 8:FIBRE
5.4.6 Printer note
5.4.7 Recorder note
Z If printer is programmed for either the camera head button 1 or head
button 2, the remote cable must be connected between the camera
controller “Remote” (18) connector and the printer.
Z If recorder is programmed for either the camera head button 1 or head
button 2, the remote cable must be connected between the camera
controller “Remote” (18) connector and the recorder.
18
GA--A 253
Page 23

5.4.8 Overview of “SETUP”
Z To activate the “ “Setup” menu, first press and hold “OK” and then
press the “MENU” button.
”SETUP”
MENU
LOCK
ON
OFF
CAMERA
SETTINGS
RESET
PRESET 1
PRESET 2
PRESET 3
PRESET 4
HEAD COUNT
YES
NO
YES
NO
YES
NO
YES
NO
YES
NO
RED GAIN
determined after white balance
FRAME RATE
50 Hz
59,94 Hz
BLUE GAIN
determined after white balance
HD FORMAT
720P
1080P
1080I
SD FORMAT
CROP
LETTERBOX
WAKEUP
PRESET 1
PRESET 2
PRESET 3
PRESET 4
LAPAROSCOPY 1
LAPAROSCOPY 2
SMALL SCOPES
FIBRE
LASTLAST
NEXT PAGE
page 2
SHUTTER
OFF
ON
NEXT PAGE
page 3
Fig. 4
GA--A 253
FACI LITY
NAME
FACI LIT Y
BACK
PRESET 1 PRESET 2 PRESET 3
NAME NAME NAME
PRESET 1
BACK
PRESET 2
BACK
PRESET 3 PRESET 4
BACK
SPEAKER
OFF
LOW
MEDIUM
HIGH
PRESET 4
NAME
BACK
POWERONMODE
NEXT PAGE
page 4
STANDBY
LAST
AUTO
SCROLL SPEED
0...4
19
Page 24

6 Reprocessing and maintenance
6.1 Reprocessing of device
WARNING!
Make sure that no humidity enters the device.
Danger of electric shock.
Before reprocessing switch off the device and disconnect it from
the power supply.
Clean the device with a soft cloth moistened with surface disinfectant,
alcohol or spirit.
Follow the disinfectant manufacturer’s instructions.
IMPORTANT!
.
Make sure that no humidity enters the device. Do not use any cleaning
agents, scouring agents or solvents on this device.
6.2 Maintenance
IMPORTANT!
.
In the case of inquiries or in your correspondence please always indicate
the product number and the serial number listed on the identification
plate. Further documentation is available from the manufacturer on
request.
6.2.1 Maintenance intervals
.
IMPORTANT!
To avoid any incidents or damage caused by aging and wear it is necessary to service the product and the accessories at adequate intervals.
Depending on the frequency of use, but at least once a year, have an
expert check the functional and operational safety of the equipment.
20
GA--A 253
Page 25

7 Technical description
7.1 Troubleshooting
IMPORTANT!
.
If you cannot eliminate the problems with the help of this table, please
contact the service department or return the device for repair.
' Do not attempt to do any repairs yourself!
Fault Possible cause Remedy
Device is without function The power switch is not ON
The power cable is not connected
The device fuse is blown
There is no mains / line voltage
Wrong color rendition
Automatic white balance
In general
Image flickers
Shutter function and video--controlled
light projector
Image is overly bright
In general
Automatic white balance incorrect
Wrong color setting on monitor
Light projector hasn’t been allowed to
warm up
The video control on the light projector
is active.
No 75 ohm termination on analog SD
video signal
Monitor incorrectly adjusted
'Activate the mains/power switch
'Connect the power cable
'Replace the fuse
'Check in--house power supply
'Perform automatic white balance
'Readjust color intensity (chroma)
and color phase (tint)
'After switching on the light projector,
wait 3 minutes, then perform white
balance.
'Switch light projector to manual
mode.
'Terminate the last device of the video
chain with 75 ohm.
'Readjust contrast and brightness on
the monitor.
Small image circle diameter
Automatic brightness control (shutter)
is OFF.
Image too dark
In general
Grainy image or image noise. Brightness incorrectly adjusted.
Image out of focus / blurred Objective lens is not focused
Light transmission not optimal
Lamp service life (light source) exceeded
Endoscope, camera or objective window soiled
GA--A 253
'Switch on endoscopic image adaptation for the small image circle
diameter.
'Set the “Shutter” option in the setup
to ON.
'Clean the light entry and exit points
of the endoscope and light cable.
'Replace the lamp
'Increase brightness of light projector
or adjust brightness on the controller.
'Focus objective lens correctly.
'Clean window or endoscope
window, respectively
21
Page 26

Fault RemedyPossible cause
No image No connection between camera out-
put socket and monitor
No power supply
Camera head or camera controller
defective
LCD monitor cannot display the HDTV
signal 720p , 1080i or 1080p.
-- Analog video output
(Video/S--Video)
No image via HD--SDI output socket
Running or offset image
Screen resolution is set to “1080p”.
'Check video cable connection
'Check the connection between the
power cable and the power input
socket. Switch on the monitor and the
components in the video daisy chain.
'Return the device with the camera
head for repair
'Set up the LCD monitor or HDTV signal correctly in the camera controller.
'The frame rate is incorrectly set on
the camera controller when connectingaCRTmonitor.
'For PAL monitors (which cannot be
switched over), set the camera controller to 50 Hz (see Section 5.4).
'For NTSC monitors (which cannot be
switched over), set the camera controller to 60 Hz (59.94 Hz) (see Section 5.4).
'The HD--SDI signals are out of range
-- only DVI and analog video connection is possible.
Switch to 1080i or 720p (Setup)
Zoom function is deactivated
Blurred image or stripes Endoscope, camera or objective win-
Image interference when moving the
camera cable
Stripes on the monitor if HF devices
are used at the same time.
Buttons on camera head do not
function.
White balance is not triggered On--screen display remains on moni-
Screen resolution is set to “1080i”.
dow soiled
Camera cable defective
HF cable too close to camera or video
cable
3 CCD HDTV ENDOCAM and HF
devices are connected to the same
power circuit.
When connecting the camera plug,
the buttons on the camera head were
pressed
(incorrect initialization of camera controller)
tor
'Switch over the image resolution on
the camera controller.
'Clean endoscope and window
'Return camera head with cable for
repair
'Route HF cables at a certain dis-
tance from other cables or avoid parallel cable routing, respectively.
' Connect power cables of HF device
and video devices to different power
circuits. Connect the power cables of
all video devices of the daisy chain to
the same power circuit.
Connect the potential equalization
line.
'Let go of camera head buttons,
disconnect the camera plug, then
connect again.
'Push the camera head buttons for
more than 1 second
22
GA--A 253
Page 27

7.2 Technical data
Camera
controller
5550101 PAL / NTSC
Electromagnetic compatibility (EMC) in acc. with IEC / EN 60601--1--2
Medical Devices Directive 93/42/EEC Class I
Protection against electric shock see identification of camera head
Protection class in acc.with IEC / EN 60601--1;
(UL 60601--1 / CSA C22.2 No.601.1 -- for USA)
Degree of protection against the ingress of liquid IP 20 (Not protected)
Duty cycle Continuous operation
Degree of protection when flammable mixtures are
present
Weight 4.6 kg (12lbs 3oz)
Dimensions WxHxD 330 mm x 100 mm x 360 mm
Frame rate 50 Hz (PAL) , 60 Hz (NTSC)
Automatic brightness control (auto shutter) 1/60 s to 1/10.000 s
Color control Automatic white balance by pressing a button
Brightness control
White balance Temperature range between 2300 K and 6000 K
Maximum gain 10 dB
Maximum resolution 1920x1080 pixel
TV
standard
Voltag e
V μ
100 -- 120
200 -- 240
Frequency
Hz
50/60 50 0.5 T1.0H
(Do not operate this device in areas where explosive
Power
consumption
VA
This device is not protected against explosions
substances are present)
Automatic shutter control
+ automatic gain control
Current
rating
A
I
Fuse
A
NOTE!
.
For the technical data of the camera heads, please refer to the corresponding manuals.
GA--A 253
23
Page 28

7.2.1 Interfaces
Digitalvide
o
Video outputs PAL output levels NTSC output levels
All specified levels with color bar signals (75% color bar)
Video (BNC) composite 1.0 V
S--Video (Mini--DIN 4--pin)
p--p
Y: 1.0 V
C: 0.30 V
/75Ohm 1.0 V
/75Ohm
p--p
Burst/75Ohm
p--p
Y: 1.0 V
C: 0.286 V
/75Ohm
p--p
p--p
/75Ohm
Burst/75Ohm
p--p
Analog video
Normally open contact (NOC): PIN 1 (max. 15V DC pull--up) and
PIN 3 (GND)
2x3.5mmministereoplug
Remote output
PIN 2 must not be assigned!
PIN 1
PIN 2
PIN 3
HDTV video outputs Output format
Digital video
DVI
HD SDI
1280 x 720 (50 Hz / 60 Hz)
1920 x 1080 (50 Hz / 60 Hz)
1920 x 1080 (50 Hz / 60 Hz)
1280 x 720 (50 Hz / 60 Hz)
1920 x 1080 (50 Hz / 60 Hz)
Progressive
Progressive
Interlaced
Progressive
Interlaced
7.3 Operating, storage, transport and shipping conditions
Operating conditions
Storage, transport and shipping conditions
NOTE!
.
To prevent damage during the transport or shipment of the products we recommend using the original
packaging material.
+10°Cto+40°C , 30% to 75% rel. humidity,
atmospheric pressure 700 hPa to 1060 hPa
-- 2 0 °Cto+60°C , 10% to 90% rel. humidity,
atmospheric pressure 700 hPa to 1060 hPa
7.4 Spare parts and accessories
Quantity Product number Designation
1 64268002 Device fuse T 1.0 H (10/pkg.)
1 244003 Power cable (Europe), 3.0 m
1 103115 Video cable BNC, 1.5 m
1 10313 Video cable BNC, 3.0 m
1 103501 Video cable, S--VHS, 2.5 m
1 5502991 Remote control cable, 1.5 m
1 103830 DVI--D cable, 3.0 m
' Further accessories on request
24
GA--A 253
Page 29
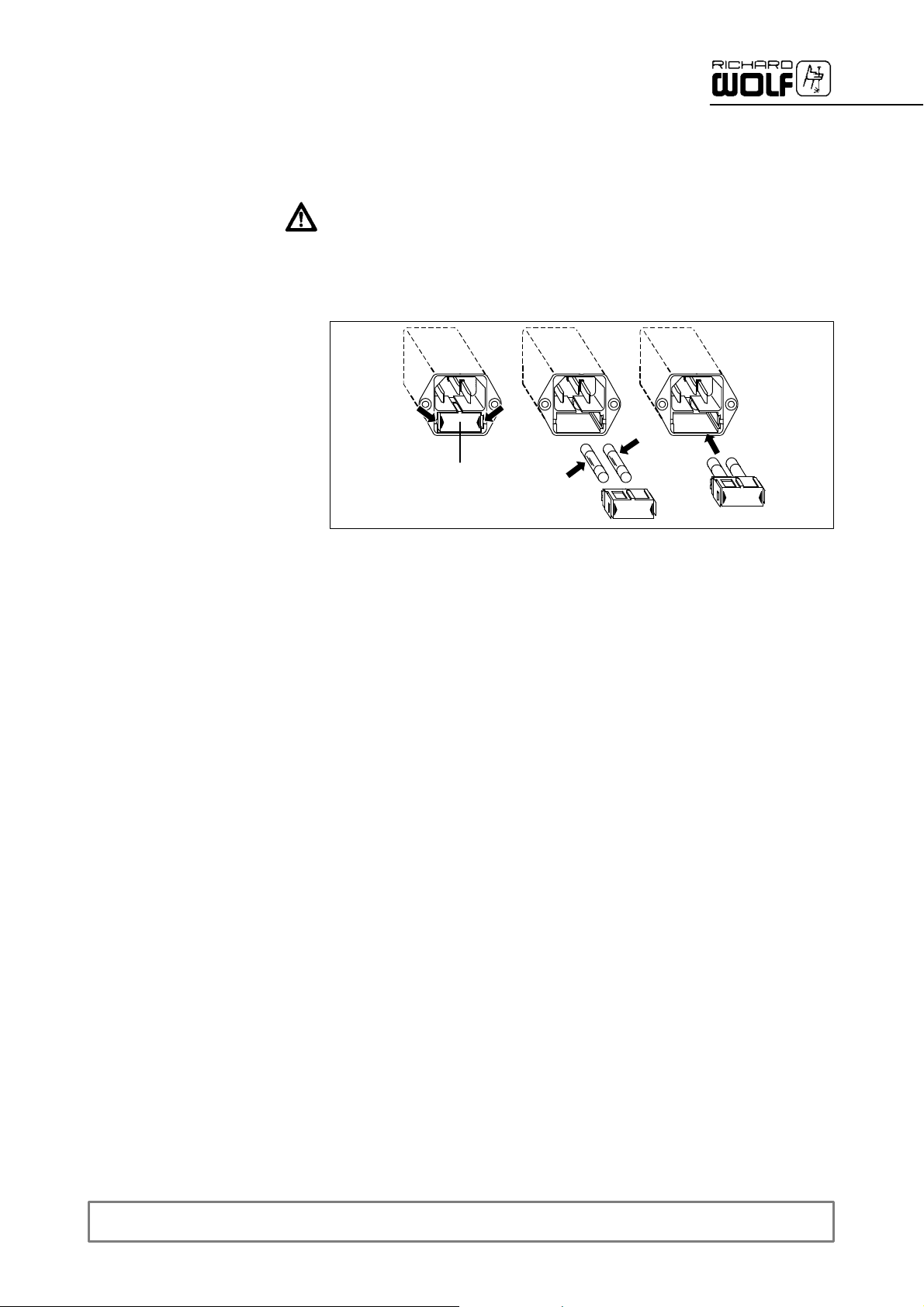
7.5 Replacing parts
7.5.1 Device fuses
CAUTION!
The specification of the fuses in the device must correspond with
the fuse ratings on the identification plate.
Use only the fuses specified in the spare parts list.
L Power input connector with fuse holder
2
1
Z Switch off the device and disconnect the power cable from the wall
socket and from the power input connector of the device.
Z Push together the clamps [2] of the fuse holder [1] and pull out the fuse
holder.
Z Pull out and replace fuses [3].
Z Reinsert fuse holder [4] and push it in until it snaps into place.
7.5.2 Disposal of the product, packaging material and accessories
For the disposal observe the relevant regulations and laws valid in your
country.
' For further information please contact the manufacturer.
2
3
3
4
GA--A 253
25
 Loading...
Loading...