Winncare VAXT6/MA/AUTO, VAXT6/MA/MAX User Manual

User manual
*PA0010122*
MAJ 27/03/2014 UK

ASKLÉSANTÉ
TABLE OF CONTENTS
INTRODUCTION .............................................................................................................................................................................. - 1 -
Necessary information for the patient and those around them ........................................ - 1 -
Advice for use .................................................................................................................................. - 1 -
Indications ......................................................................................................................................... - 2 -
Counterindications ......................................................................................................................... - 2 -
Precautions ....................................................................................................................................... - 2 -
Warning.............................................................................................................................................. - 2 -
Definition of symbols ....................................................................................................................... - 4 -
USE ....................................................................................................................................................................................................... - 4 -
Contents of packaging ................................................................................................................. - 4 -
Operating principle ........................................................................................................................ - 4 -
Installation ......................................................................................................................................... - 5 -
Use ....................................................................................................................................................... - 7 -
Emergency cardiopulmonary resuscitation: CPR .................................................................. - 8 -
Heel Grounding Valves: ................................................................................................................ - 8 -
De-installation .................................................................................................................................. - 9 -
Storage and transport.................................................................................................................... - 9 -
AXENSOR® CONNECT: ............................................................................................................................................................- 10 -
MAINTENANCE - DISINFECTION ............................................................................................................................................- 10 -
Compressor and AXENSOR® system: ....................................................................................... - 10 -
Macro-particle filter: ..................................................................................................................... - 10 -
Mattresses and Covers: ............................................................................................................... - 11 -
Diagram of advised maintenance and disinfection ........................................................... - 12 -
ALARMS and TROUBLESHOOTING ......................................................................................................................................- 13 -
MAINTENANCE - CHECK ..........................................................................................................................................................- 14 -
Frequency of checks: .................................................................................................................. - 14 -
Use of the "info" button ................................................................................................................ - 14 -
DISPOSAL OF THE MEDICAL DEVICE ..................................................................................................................................- 15 -
Protection of the environment .................................................................................................. - 15 -
WARRANTY.....................................................................................................................................................................................- 15 -
TECHNICAL FEATURES ...............................................................................................................................................................- 16 -
ELECTROMAGNETIC COMPATIBILITY..................................................................................................................................- 17 -

ASKLÉSANTÉ
- 1 -
INTRODUCTION
NECESSARY INFOR MATION FOR THE PATIENT AND THOSE AR OUND THEM
This product is a support to help prevent and treat bedsores.
Why have you been prescribed this support?
Your state of health impairs your mobility and puts you at risk of bedsores.
What is a bedsore?
A bedsore is a more or less skin deep lesion caused by putting excessive and
prolonged pressure on tissues between the body and a load bearing surface. This
excessive pressure can obstruct blood flow and lead to bedsores.
A bedsore can take several forms: a simple redness lasting more than one day,
hardening of the skin, or a wound of varying depth, which, in serious cases, can reach
the muscles or the underlying bone.
The appearance of a bedsore may be linked to lack of mobility and/or a chronic
illness.
How does this support work?
This support reduces the pressure supported and allows for better blood flow in the
skin, in order to help prevent bedsores.
ADVICE FOR USE
A support alone cannot prevent bedsores; other preventative measures are also
essential:
– changing position frequently (at least every 2 to 3 hours);
– maintaining skin hygiene and avoiding maceration;
– in cases of incontinence, regularly changing protection;
– monitoring or having the skin condition monitored on a daily basis;
– ensuring sufficient and suitable nutrition;
– regularly drinking a sufficient quantity of liquid.
If one of these measures cannot be taken, it is essential to inform your doctor or nurse
as soon as possible.
Informing your doctor or nurse as soon as possible of any abnormal event such as, for
example, fever, pain or even redness or whitening of support points (head, shoulder,
back, hip, shoulder blade, pelvis, heel, etc.).
It is important to limit the number of layers between the body and the support as much
as possible, with the exception of sheets for a bed support, clothing and any complete
change. Favour loose cotton clothing without seams in the supporting area, if possible.
Do not use folded towels or sheets, an additional pillow, etc.
Ensure the absence of any foreign bodies such as tubing, crumbs, grease, etc.
For hygiene purposes, each support to help prevent bedsores must be used by one
person only.

ASKLÉSANTÉ
- 2 -
INDICATIONS
According to the CNEDiMTS (the French National Committee for Evaluation of Medical
Devices and Health Technologies) report of 22 December 2009 and in accordance
with expert medical opinions:
Prevention and aid in the treatment of bedsore(s) in stages 1 to 4 (according to
medical opinion) for patients up during the day, in bed for more than 15 hours and
presenting an "average" (AT12) to "high" (AT15) risk of bedsore(s), according to a
validated scale and medical opinion.
COUNTERINDICATIONS
Patients whose weight is over 165kg for the VAXT6/AUTO and 180kg for the
VAXT6/MAX. Use in a hyperbaric chamber. Use on a stretcher.
PRECAUTIONS
Unstabilised bone and/or muscle injury in contact with the support;
In the first few days following bedsore surgery (skin graft or flaps) [use static low
pressure mode] ;
Patient monitored at home with no possibility of medical intervention;
WARNING
In accordance with Annex 1 of Directive 93/42/EEC on essential requirements
applicable to medical devices, only compatibility between the systems assembled
by the manufacturer ASKLESANTE guarantees safe usage of the AXTAIR
AUTOMORPHO AXENSOR® motorised air mattress.
The features and performance of the motorised air support shall only be maintained
by using the pump (ref. VAXT6/PUMP/AUTO) associated with the mattress (ref.
VAXT6/MA/AUTO and VAXT6/MA/MAX), without any modification and, optionally,
the inflation/deflation kit (ref. VKIT/AXT).
The national authority responsible for healthcare safety and health products may, at
any time, take steps to monitor the conditions in which products are put on the
market and take the necessary measures in the event of danger or violation of
regulation. In the event of failing to comply with the instructions for use given above,
the user is likely to be held liable in case of an accident.
Products in the Axtair motorised air range area therapeutic mattresses, as defined
by standard CEI 60601-2-52 on evaluation of protection against the mechanical
dangers of Electro-Mechanical equipment (EM) and EM systems; in this respect, they
are excluded from the scope of application of testing according to figures 201.107,
201.108 and table 201.101 on measuring the Dimension "D".

ASKLÉSANTÉ
- 3 -
In order to comply with risk assessment under ISO standard 14971, an assessment was
carried out on all "hospital bed - therapeutic mattresses (Axtair) - accessories". The
risk assessment shows that there may be a risk of bodily trapping if the bedridden
person presents mental confusion and/or agitation. Use of the device has been
approved in order to improve the service rendered in terms of therapeutic aid
and/or prevention of bedsores.
We have approved this product in order to improve the service offered in the
prevention and aid in treatment of bedsores versus the risk of trapping of the
bedridden person.
Keep packaging, the transporting bag as well as the mattress out of reach of
children in order to avoid any risk of suffocation.
In order to avoid any risk, any modification of this product or unspecified use of its
accessories is prohibited.
ASKLÉSANTÉ
- 4 -
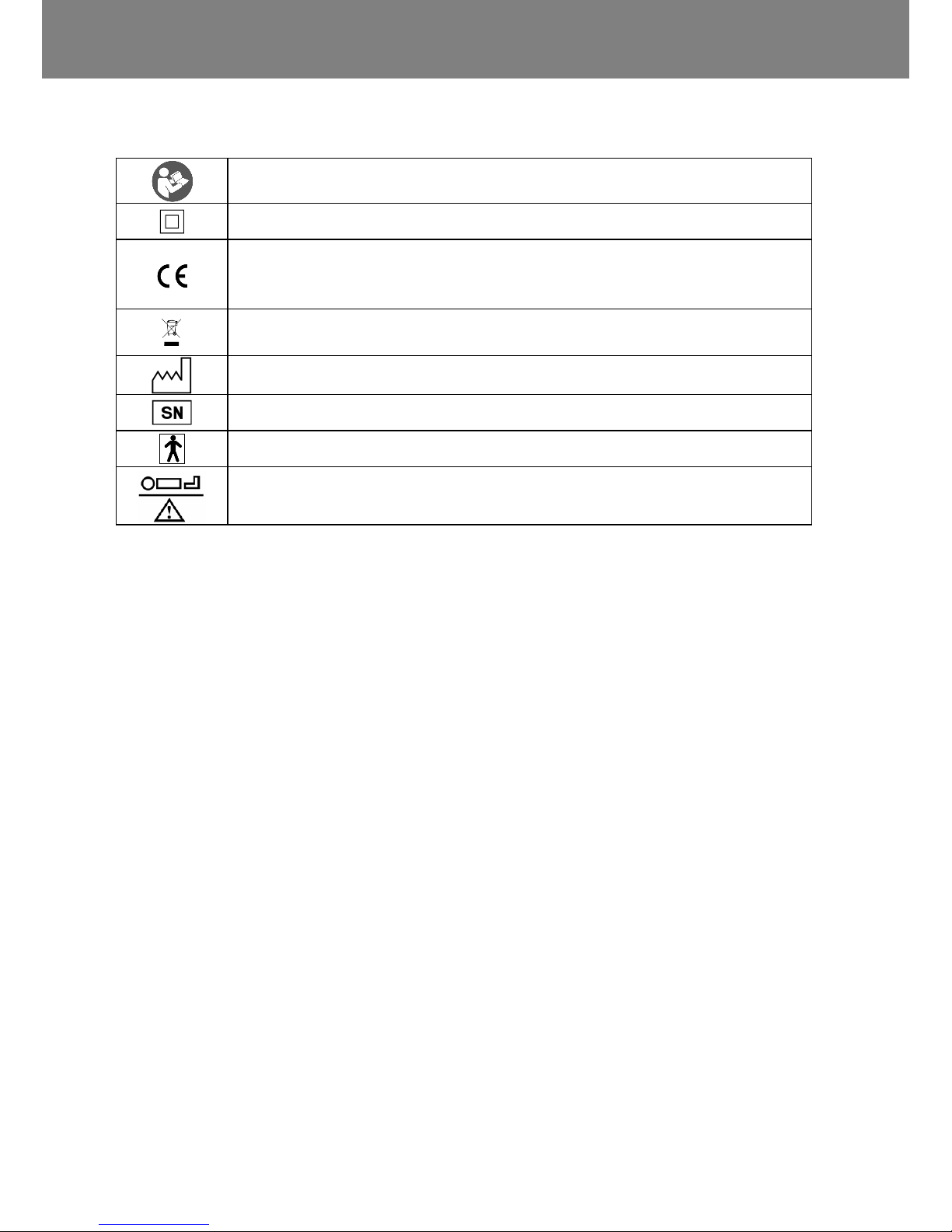
DEFINITION OF SYMBOLS
Warning - see user manual and (or) instructions for use
Class II device (Double insulation)
In accordance with the essential requirements of European
Directive 93/42/EEC, applicable to medical devices (modified
2007/47/EEC)
Warning - electrical and electronic equipment subject to
separate collection of waste
Manufacturer
Series number
BF electrical device (applied to blankets)
Patient weight range
USE
CONTENTS OF PACKAGING
1 mattress, rolled up in a transporting bag
1 dirty/clean label
1 identification label
1 AXENSOR® device, located in the blanket cover
1 compressor integrated into a rolled-up mattress
1 electrical power cable with 2 hooks systems for connecting to the sides of a
hospital bed
1 user manual
OPERATING PRINCIPLE
Alternating pressure helps to avoid prolonged vascular compression which is likely to
lead to tissue hypoxia.
The static low pressure mode allows for the care of people requiring immobilisation
(fractures, neurological injury, etc.), reducing secondary pain to a minimum,
promoting the patient's rest and carrying out a weaning process before putting in
place a static mattress, etc..
The "care" mode (fixed) allows for the maintenance of certain acts of care and
transfers.
Adjustment of the level of inflation is automatic based on the patient's shape and
size and the angle of the backrest, thanks to AXENSOR® technology. No external
intervention is necessary.
 Loading...
Loading...