
BrainPort
®
V100 Device
User Manual
Wicab, Inc.
8313 Greenwa y Blvd.
Middleton, Wisconsin 53562 USA

BrainPort V100 User Manual 2
Table of Contents
CHAPTER 1 ..................................................................... 5
General Information ........................................................ 5
Purpose of Device (Indications for Use) ............................. 5
Description of BrainPort® V100 ......................................... 6
When NOT TO USE the Device (Contraindications) .............. 7
Risks of Use ................................................................... 7
General Warnings ........................................................... 7
Precautions .................................................................. 12
Clinical Experience ........................................................ 13
Product and Package Labeling ........................................ 14
CHAPTER 2 Using the BrainPort® V100 ....................... 17
User Interface Description .............................................. 19
Contrast ................................................................... 19
Status ...................................................................... 19
Image Mode .............................................................. 20
Lock ......................................................................... 20
Test ......................................................................... 20
Volume .................................................................... 20
Power ...................................................................... 20
Intensity and Invert ................................................... 20
Zoom ....................................................................... 21
Camera Tilt ............................................................... 21
Battery Compartment ................................................ 21
Quick Start: Turning on the BrainPort V100 ...................... 24
Starting vRemote .......................................................... 25
Device Announcements.................................................. 26
Batteries ...................................................................... 28
Battery Charger ............................................................ 29
Battery Precautions ....................................................... 30
Care and Maintenance ................................................... 31
CHAPTER 3 Troubleshooting ........................................ 32
Problems with Battery Power .......................................... 33
Controller Troubleshooting ............................................. 35
Headset Troubleshooting ............................................... 37
vRemote Troubleshooting .............................................. 37
CHAPTER 4 Product Specifications and Technical
References ................................................................... 38
General Specifications ................................................... 38

BrainPort V100 User Manual 3
User Profile .................................................................. 40
Electromagnetic Compatibility ........................................ 41
Warranty ..................................................................... 48
Appendix A Cleansers ................................................... 49
Appen dix B Clinical Safety I nformation ........................ 50
BrainPort V100 User Manual 4
Using this Manual
This manual is in tended f or us e af ter you hav e be en
trained o n the o peration o f the B rainPort
®
V100. T his
manual serves as a reference to supplement your training
and to address any questions you may have when you
use the device at home.
The m anual c ontains ge neral i nformation o n s afety,
operation, and troubleshooting. Please read it thoroughly
and become familiar with its contents.
This manual is written in an accessible format.
• Chapter 1 describes the BrainPort V100 and the risks
and benefits of using it.
• Chapter 2 explains how to use the BrainPort V100.
• Chapter 3 includes troubleshooting procedures to use
in the event of problems with the BrainPort V100.
• Chapter 4 lists product specifications and technical
references.
Attention, consult accompanying documents
This label is a reminder for you to consult
this manual or other material you received
with the device for important safety
information.
Rx only
Caution: Federal law restricts this device to sale by or on the
order of a physician.

BrainPort V100 User Manual 5
CHAPTER 1
General Information
The BrainPort V100 is a non-surgical electronic assistive
prescription device for profoundly blind individuals to aid
in orientation, mobility and object recognition. The
BrainPort V100 is indicated as an adjunct to other
assistive devices, such as the white cane and guide dog.
It translates digital information from a video camera to
gentle electrical stimulation patterns on the surface of the
tongue. Users describe the experience as streaming
images drawn on their tongue with small bubbles. With
training, users are able to interpret the shape, size,
location and motion of objects in their environment.
Warning
The BrainPort V100 does not replace the cane or guide
dog. T he BrainPort V 100 is i ntended to aug ment, rathe r
than re place, o ther assistive t echnologies such a s t he
white cane or guide dog.
Training is required before using the
BrainPort V100.
Purpose of Device (Indications for Use)
The BrainPort V100 is intended for use as an electronic
assistive prescription device for profoundly blind
individuals to aid in orientation, mobility and object
recognition. The BrainPort V100 is indicated as an adjunct
to other assistive devices, such as the white cane and
guide dog.

BrainPort V100 User Manual 6
Description of B rainPort® V100
Headset
A digital video camera is mounted on a pair of sunglasses
at the nose bridge. The camera is capable of working
indoors and o utdoors i n t ypical lighting c onditions. T he
camera’s f ield o f v iew is us er-controlled and v aries f rom
narrow to wide angle views. There are two cables
permanently attached to the l eft e ar pi ece: T he I OD
assembly and the headset cable.
Intra Oral Device (IOD)
The IOD (tongue electrode array) contains electrodes that
act as “pixels” for the tongue. The flat side with the
electrodes should be in contact with the front top surface
of the tongue. Close your lips around the thin stem,
maximizing to ngue contact wi th the e lectrodes. T he
stimulus p attern o n the electrode array corresponds t o
the s cene c aptured by the c amera. T here i s o ne c able
exiting the th in s tem o f the IOD that is pe rmanently
attached to the ear piece of the headset.
Controller
The Controller contains the battery as well as the user
control features for the BrainPort V100. The Controller is
generally handheld. A belt clip is provided for hands free
operation.
Battery Charger
A battery charger with factory instructions is included.
Training and Training PC
Training is required before you use the BrainPort V100.
The trainer may use an accessory personal computer
during training. When the personal computer is in use:
• Do not touch the personal computer or your trainer, and
• Do not come into contact with any device plugged into a
wall circuit.
Your BrainPort V100 does not include or require a
personal computer.
BrainPort V100 User Manual 7
When NOT TO USE the Device
(Contraindications)
You should not use the BrainPort V100 if you have any of
the following conditions:
• Numbness or lack of feeling of your tongue
• History of injuries that impair sensation or use of your
tongue
• Any n eurological c ondition t hat c auses im paired
sensitivity to your tongue or loss of consciousness
Risks of Use
Potential risks arising from the use of the BrainPort V100
include:
• Electrical an d e lectromagnetic s afety hazards
associated with battery-operated devices
• Allergic reaction to the materials in the device
• Irritation o f y our t ongue f rom the e lectrodes or
excessive stimulation
You can manage these risks by setting the stimulus level
according to your preferred comfort level, adhering to the
instructions in this manual, and applying the training you
received for the proper and safe use of the device.
General Warnings
• Long-term use . Limited data are available on the long-
term effects of electrical stimulation of the tongue.
Long-term ef fects (b eyond on e y ear) h ave n ot b een
evaluated in clinical trials.
• Device Usage. Limited data is available on use of the
device exceeding an average of between 250 and 400

BrainPort V100 User Manual 8
minutes per month, and a maximum of 1550 minutes
per month. Wicab recommends that you tailor your
use of the device to be within these time limits since
long-term effects (beyond one year) exceeding this
usage have not been evaluated in clinical trials.
• Trainers. Potential trainers o f us ers o f th e B rainPort
V100 s hould h ave r elevant ex perience, s uch a s
experience working with the blind or visually impaired.
Trainers may have professional credentials, such as
certification as a Certified Low Vision S pecialists
(CLVS), C ertified O rientation and Mobility S pecialist
(COMS) or Teachers of the Visually Impaired (TVI).
All potential trainers will be trained by Wicab according
to W icab pro cedures and o nly tho se who hav e
successfully completed the training will be considered
qualified to train users of the BrainPort V100.
• Supervision. T he BrainPort V 100 should o nly be us ed
after you have completed training. Do not give the
device to untrained individuals for use.
• Use only Wicab supplied components and procedures.
Using controls, adjustments, components, or
procedures o ther than tho se s pecified in this m anual
may damage the BrainPort V100, increase risk, or
decrease benefit.
• Oral Health. L imited d ata is a vailable o n s timulation
sensitivity f or individuals w ith oral conditions s uch as
oral u lcerations, he rpes s implex, o ral t hrush, and
geographic to ngue. I f us e o f the de vice c auses
discomfort, discontinue use.
• Oral Health. I ndividuals w ith h igh, nar row p alatal
vaults should discontinue use of the device if use
causes discomfort
• Oral Health. Individuals w ith m axillary o r m andibular
tori that interfere with the IOD placement such that
full contact with the tongue is prevented should seek

BrainPort V100 User Manual 9
additional training to gain the most benefit of the
device.
• Dental Appliances (orthodontic appliances, removable
partial or full dentures, lingual amalgam alloy
restoration, metal crowns, etc.) Electrical stimulation
of t he t ongue w hen m etal a ppliances/surfaces are
present m ay c hange results and/ or c ause unintended
stimulation. If the stimulation causes discomfort,
remove the IOD from your mouth and discontinue use
of the device. If you notice a change in your dental
device or appliance (warmth or looseness),
discontinue the use of the device and contact your
dentist.
• Dental Implants. The B rainPort de vice ha s no t b een
thoroughly evaluated in the presence of dental
implants. T he s afety o f de ntal implants in B rainPort
users i s u nknown. The use o f this d evice p otentially
may cause heating of dental implants; chronic use of
this device potentially may result in loosening and
failure of dental implants.
• Condition of device. Before EACH use, tactilely inspect
the device for damage, for example, anything rough or
loose on the IOD, disconnected cables, worn cables,
cracked or broken glasses, cracked or broken handset,
etc. If you find these or similar issues, contact
Customer Support and DO NOT use the device. Using
a damaged device could expose sharp edges and/or
the dam age c ould pr event no rmal o peration, the reby
increasing the risk of use.
• Proper environment for use. T he BrainPort V 100 is
intended for use as a supplemental assistive device.
o Do not use it in environments that could put you in
danger.
o Do not operate the device in hot or cold conditions
(below 0°C/32°F or above 40°C/104°F). Maintain
conditions between 5% and 95% relative humidity.

BrainPort V100 User Manual 10
The device is intended for operation under normal
atmospheric pressures (700 hPa to 1060 hPa)
o If the controller becomes uncomfortably warm, or
when the ambient temperature exceeds 35°C/95°F,
use the belt clip to carry the device.
o The controller, camera, and other headset
components are not waterproof. Do not use the
device in environments that will allow liquids (such
as rain and snow) to enter these components.
o Electrical shock. To avoid electrical shock, do
not immerse the BrainPort V100 or the
battery charger in liquids.
o Do not use where flammable gases are present.
o Do not come into contact with any device w hich is
plugged into a wall circuit or any person using such
a device.
o Follow the gui dance o utlined i n CHAPTER 4 –
Product Specifications and Technical References
regarding the intended electromagnetic use
environment.
o Contact W icab if y ou hav e que stions o r co ncerns
about a particular use environment.
• Discomfort. Using the BrainPort V100 should not cause
discomfort. I f you ex perience a ny p ain, n umbness or
discomfort including burning or stinging, please stop
using the device. If the symptoms are temporary, you
may re sume us ing the de vice b y re ducing the
stimulation le vel t o a c omfortable le vel. I f t he
condition re curs, s top us ing the de vice and s eek
professional help.
• Risk of Strangulation. Take care in arranging cables to
avoid the r isk o f s trangulation. Small c hildren m ay
become entangled in the cables. Do not allow children
to use the device. Store the device out of reach of
small children.

BrainPort V100 User Manual 11
• Choking. The B rainPort V 100 c ontains s mall par ts.
Check the device for loose or missing parts before
each use. Do not use the device if parts are missing.
Do no t al low c hildren to us e th e de vice. S tore th e
device out of reach of small children.
• Discomfort. Use of the BrainPort V100 may be
contraindicated for young individuals or people with
narrow dental arcades of the upper palate – it may be
difficult to comfortably place the IOD on the tongue for
these individuals.
• Care and Maintenance. Use only the procedures in this
manual to care for your device.
• Intended Purpose. Do not use the BrainPort V100 for
any purpose other than that stated in the Indications
for Use.
• Personal Computer. When us ing a pe rsonal c omputer
with vRemote:
o Do not allow the user to touch the personal
computer;
o Do no t to uch the pe rsonal c omputer and the us er
at the same time;
o Do not allow the user to come into direct or indirect
contact with any device plugged into a wall circuit;
o Do not use a power strip or extension cord to
power the personal computer;
o The personal computer used to run vRemote is an
accessory that i s us ed o nly by trainers or us ers’
sighted c ompanions, and is no t a m edical de vice
when o perated standalone. I t becomes part o f a
medical device system when used for training and
the precautions listed inside the BrainPort V100
Device User Manual must be followed to insure
client safety.

BrainPort V100 User Manual 12
Precautions
• Signal Stimulation. A djust the s timulation to a
comfortable level that allows you to clearly feel and
respond to the signal. Increasing beyond this point
does n ot i mprove ef fectiveness. If the s timulation
causes discomfort, reduce the stimulation setting to a
comfortable level. If discomfort continues, remove the
IOD f rom y our m outh and discontinue u se o f t he
device.
• Sensitivity to stimulation. Although the BrainPort V100
is de signed to m inimize the r isk o f injury due to
stimulation s trength, i f y ou re act ne gatively to the
stimulation from the BrainPort V100, remove the IOD
from your mouth and discontinue use of the device.
• Oral health. If you currently have or develop open
lesions, sores or abrasions in your mouth, discontinue
use of the BrainPort V100 until the situation has
resolved.
• Mouth injuries/Dental Trauma. The IOD is intended to
be held in the mouth during use. Take care so that the
cables do no t be come entangled, p ulling t he I OD out
of your mouth potentially injuring your mouth, teeth
or lips.
• Choking. The IOD is intended to be held in the mouth
during us e. T o m inimize the r isk o f c hoking o n the
IOD, make certain it is securely connected to the
flexible c able and th at the I OD i s positioned p roperly
in the mouth. Do not use the device if the IOD is
damaged.
• Neck Trauma. Take c are s o that y ou do not be come
entangled in the c ables that run f rom the he adset to
the c ontroller. A s udden y ank c ould c ause ne ck
trauma.
• Batteries. Do n ot u se t he d evice with the batte ry
compartment door open. Inspect the battery prior to
use. Do not use the battery if it appears damaged,

BrainPort V100 User Manual 13
corroded, is leaking, or is swollen.
• Connections. Do not attempt to connect the headset
to equipment other than the BrainPort V100 controller.
Doing so may d amage the BrainPort V 100, i ncrease
risk, or decrease benefit.
• Connections. Do not attempt to connect anything
other than the headset that came with your unit to the
BrainPort V 100 c ontroller. D oing s o may damage the
BrainPort V100, increase risk, or decrease benefit.
Clinical Experience
The BrainPort V100 device was evaluated in a single arm,
open label clinical study of 75 enrolled blind/profoundly
blind subjects from 7 s ites i n the U .S. and C anada.
Subjects unde rwent 2 -3 day s ( 10 ho urs) o f tra ining
followed by in-home use over the span of 12 months for
the study.
The p rimary s afety endpoint was no o ccurrence o f a
clinically s ignificant device-related a dverse e vent. A
clinically s ignificant device-related adv erse e vent wa s
defined as any e vent that resulted i n to ngue b urns
attributed to use of the BrainPort V100 device, an allergic
reaction requiring medical care, or negatively altered
changes in taste or numbness that was sustained and/or
resulted in withdrawal from the study. The primary
efficacy e ndpoint w as su ccess i n a t est of ob ject
recognition of at least 50%. The secondary endpoints
measured success rates of at least 50% in tests of word
identification a nd at l east 3 5% i n s ign identification i n a
mobility task. Effectiveness e ndpoints we re m easured at
12 month follow up, and safety events were captured
throughout the study.
Study results demonstrated that the safety objective was
achieved, with no clinically significant d evice-related
adverse e vents thro ughout the s tudy. There w ere n o
serious device-related adverse events. Two (2.67%) of

BrainPort V100 User Manual 14
the s tudy s ubjects re ported m ild/moderate e vents
possibly/probably related to the device that led to
decrease i n d evice u se or d iscontinuation of d evice u se.
One subject reported worsening sinus drainage that was
considered a po ssible m etal a llergy and p ossibly re lated
to the device. Device use was discontinued for this
subject. One subject reported tongue soreness that wa s
considered pro bably related to the de vice. D evice us e
decreased for this subject. Both subjects recovered. For
a complete list of device-related adverse e vents that
occurred in the clinical study of the device, see Appendix
B.
In te rms o f e ffectiveness, the pri mary e fficacy o bjective
was achieved with more than 50% of subjects achieving
success o n the object re cognition tas k. Results o f the
secondary efficacy endpoints similarly confirmed device
performance, where approximately 44% of subjects were
able to successfully complete the word identification and
mobility tasks (where subjects with missing data were
counted as failures for a conservative analysis). Based on
all av ailable data at the e nd o f the s tudy, i.e., the “pe r
protocol” subjects who completed 12 month follow up,
57.9% of the study subjects successfully completed the
word identification and mobility tasks.
Overall, evaluation of the safety and efficacy of the
BrainPort V100 device demonstrated a low-risk safety
profile with no serious device-related adverse events, and
strong effectiveness results confirming d evice
performance in the intended user population.
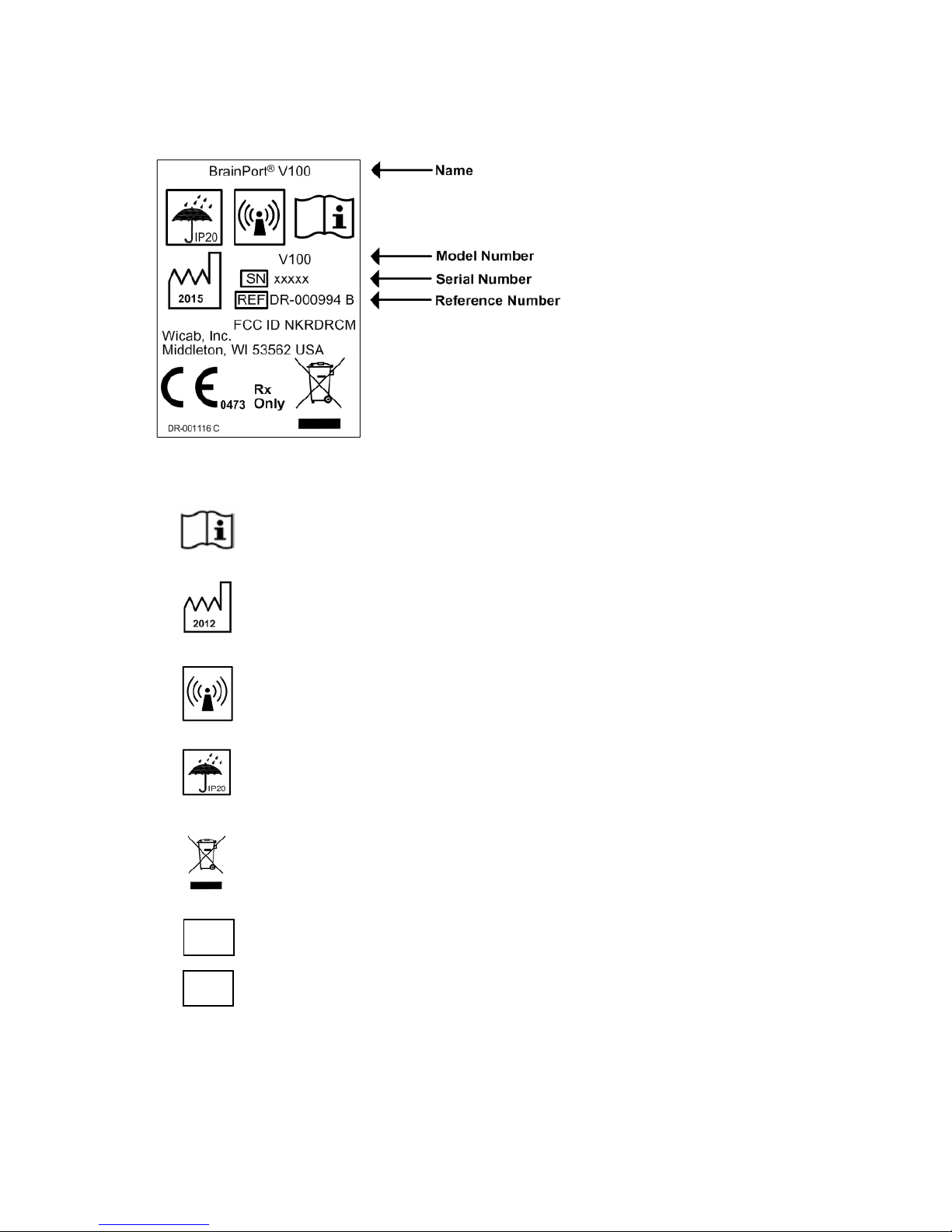
Product and Package Labeling
The labels on the back of the BrainPort V100 controller
and on its packaging provide important information. You
will ne ed the m odel nam e, model num ber, r eference
number, and s erial num ber if y ou call W icab for
assistance. See t he inside bac k c over o f t his m anual for
contact information.
BrainPort V100 User Manual 15
Product Label
Explanation of Symbols
Important safety information is contained in the
documents that accompany the device.
Year of manufacture
The BrainPort V100 includes RF transmitters
Keep the BrainPort V100 dry.
Dispose of in accordance with WEEE
Serial Number
Reference Number
SN
REF
BrainPort V100 User Manual 16
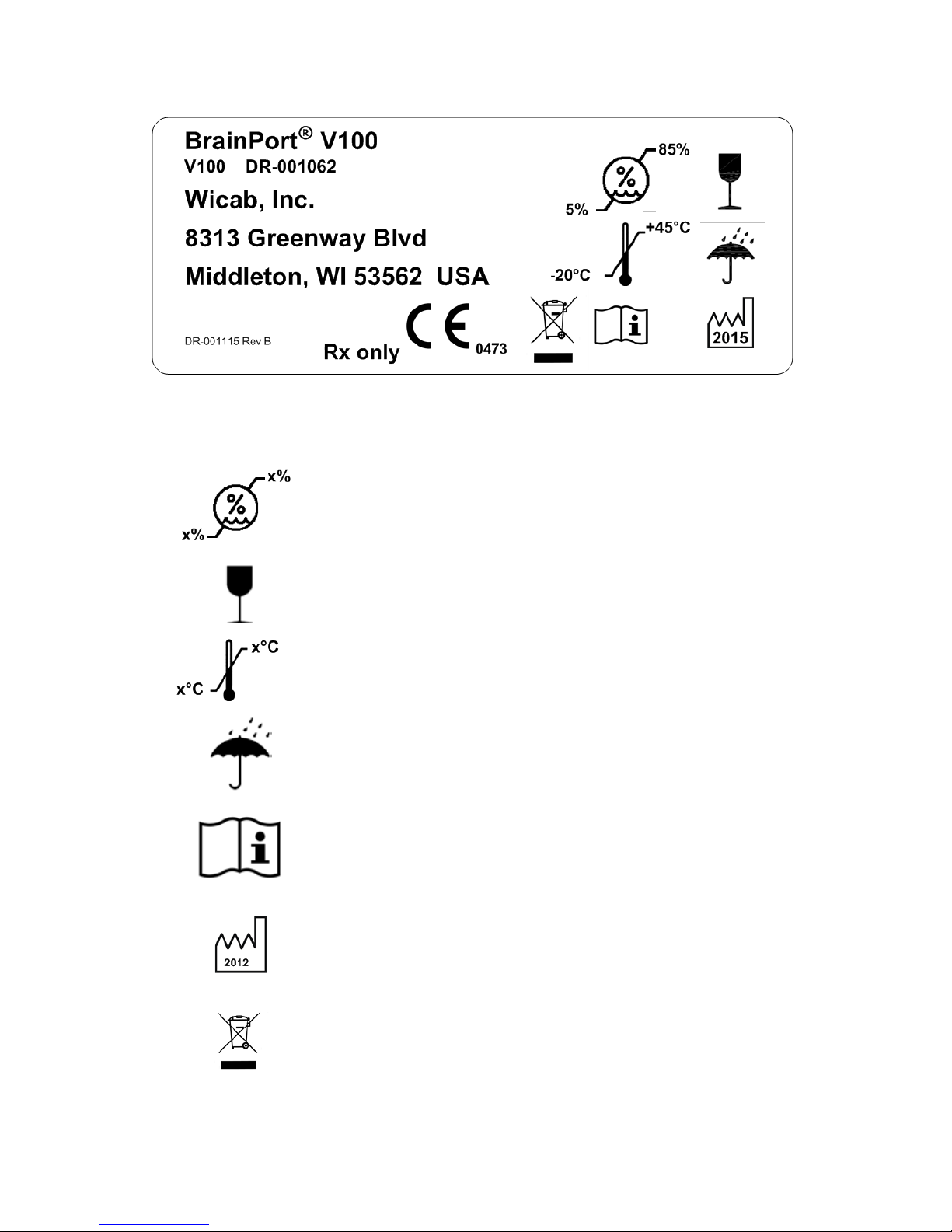
Package Label
Explanation of Symbols
Humidity range for transportation
and storage
Fragile
Temperature range for
transportation and storage
Keep dry
Important safety information is
contained in the documents that
accompany the device.
Year of manufacture
Dispose of in accordance with WEEE
BrainPort V100 User Manual 17
CHAPTER 2
Using the BrainPort® V100
This chapter explains how to set up and use the BrainPort
V100.
(Refer to Figure 1: BrainPort V100 and/or the verbal descriptions below)
Using procedures other than those specified in
this manual may damage the BrainPort V100,
increase risk of injury, and decrease benefits of
use.
Before EACH use, tactilely inspect the device
for damage, for example, anything rough or
loose on the IOD, disconnected cables, etc. If
you find any of these problems, contact
Customer Support and DO NOT use the device.

BrainPort V100 User Manual 18
Figure 1: BrainPo rt V1 0 0
BrainPort® V100 Device Components
1 Headset
2 Camera – Image Sensor
3 Camera Tilt Knob
4 Contrast Button
5 Power Indicator LED
6 Stimulation Indicator LED
7 IOD Receptacle
8 Zoom Dial / Zoom Home
9 Intensity Dial / Invert
10 Status Button
11 Image Gain LOCK
12 IOD (Intra-Oral Device) with
Tongue Array
13 Test Button
14 Image Mode Button
15 Headset Connector
16 Option Button
17 Headset Cable
18 Power ON/OFF Button

BrainPort V100 User Manual 19
User Interface Description
The BrainPort V100 may be held in either hand with the
bottom end of the controller (point of headset connection)
facing downwards and the front panel of the controller
with six raised buttons facing toward the user.
Four of the raised buttons on the front panel of the device
are oriented in a diamond pattern, located at 12 o'clock, 3
o'clock, 6 o'clock, and 9 o'clock. There are two additional
buttons on the front panel near the bottom of the
controller.
On each side of the device there are wheels that both
rotate and act as a button when pressed in. Wicab
recommends holding the device so that your thumb
rotates one wheel and your forefinger rotates the other
wheel.
Finally, on the bottom of the controller, next to the
connector is a button controlling power on and off.
Pressing the buttons or wheels will emit a beep and a
voice will announce the information assigned to each
button. Some actions also trigger a vibration felt in the
hand. Button descriptions and uses are provided below.
Contrast (12 o'clock button- 4): This button toggles
between standard contrast (default) and high contrast
mode. PRESS once to activate high contrast and PRESS
again to return to normal contrast.
Status (3 o'clock button- 10): P
RESS and release of
this button provides a quick status of the battery
percentage and zoom level in degrees field of view.
P
RESS-HOLD the button for one second to hear additional
status information such as battery level, zoom level,
intensity level, lock status, and invert status, tilt, low light
status.

BrainPort V100 User Manual 20
Image Mode (6 o'clock button- 14): This button is a
three state toggle. P
RESSing it once makes a bright image
more detailed on the tongue, P
RESSing it twice makes a
darker image more detailed on the tongue, and P
RESSing
it three times returns to default image mode.
Lock (9 o'clock button- 11): This button toggles
between allowing the camera to automatically adjust to
the lighting conditions (default) and locking that feature.
The device will announce whether lock is on or off.
Test (Bottom left button- 13): P
RESSing this button
displays a test pattern on the tongue array to enable
confirmation that the tongue display is active. To stop the
test pattern press the Test button a second time.
Volume (Bottom right button- 16): This button
toggles between three volume levels: high volume, low
volume (default), and mute/vibrate only. Note: You
cannot mute the status button.
Power
(18): Located o n the narrow end o f the
controller, next to the headset connector housing is a
small button controlling power on and off. To turn the
device on or off, P
RESS the button for two seconds.
Intensity and Invert (Left Wheel- 9): On the left side
of the controller is a rotating wheel that also acts as a
button when P
RESSed. This wheel controls stimulation
intensity, while the button controls the invert function.
Turning the wheel up (towards the top of the controller)
increases the intensity of the stimulation on your tongue.
Turning the whe el down ( towards the bo ttom o f the
controller) d ecreases the intensity o n y our t ongue. T he
device will vibrate at the limits of stimulation (highest =
100, l owest = 0 ). At po wer up, s timulation intensity
always re sets to ze ro and m ust be i ncreased to y our
comfortable working level.

BrainPort V100 User Manual 21
A PRESS of t he stimulation wheel activates the Invert
feature that inverts the stimulation intensity values –
strongest becomes weakest and vice-versa. The Invert
Feature is used to toggle between whether bright objects
or dark objects in the field of view stimulate the tongue
array.
Zoom and Home (Right Wheel- 8): On the right side
of the controller is a rotating wheel that also acts as a
button when P
RESSed. The wheel and button control the
zoom level of the camera.
Turning the wheel up (towards the top of the controller)
zooms the camera in (reducing the camera’s effective
field of view). Turning the wheel down (towards the
bottom of the controller) zooms the camera out
(increasing the camera’s field of view). The device will
vibrate at the limits of zoom (widest = 69 degrees,
narrowest = 3 degrees).
P
RESSing the wheel straight in activates the home zoom
position. The default home position is a medium field of
view (24 degrees). To set the home zoom position, use
the right zoom wheel to achieve the desired field of view
and then P
RESS and hold the right wheel for two seconds.
Camera Tilt
(3): On the left side of the headset temple
is a small round wheel with a tactile line in the middle.
Prior to start up, make sure the tactile line is positioned
horizontally. Upon start up, rotating the knob through its
positions (all the way down and all the way up) calibrates
the digital camera tilt. You will hear a tone once tilt has
been initialized. The tilt knob is non-functional until it has
been calibrated. The raised tactile line indicates the
direction of tilt.
Battery Compartment: On the back of the unit is a
sunken well. Insert a finger into the well to grasp the lip

BrainPort V100 User Manual 22
of the battery cover door and pull straight back, away
from the controller, to open the battery compartment.
A fully charged battery should always be used at the start
of each session.
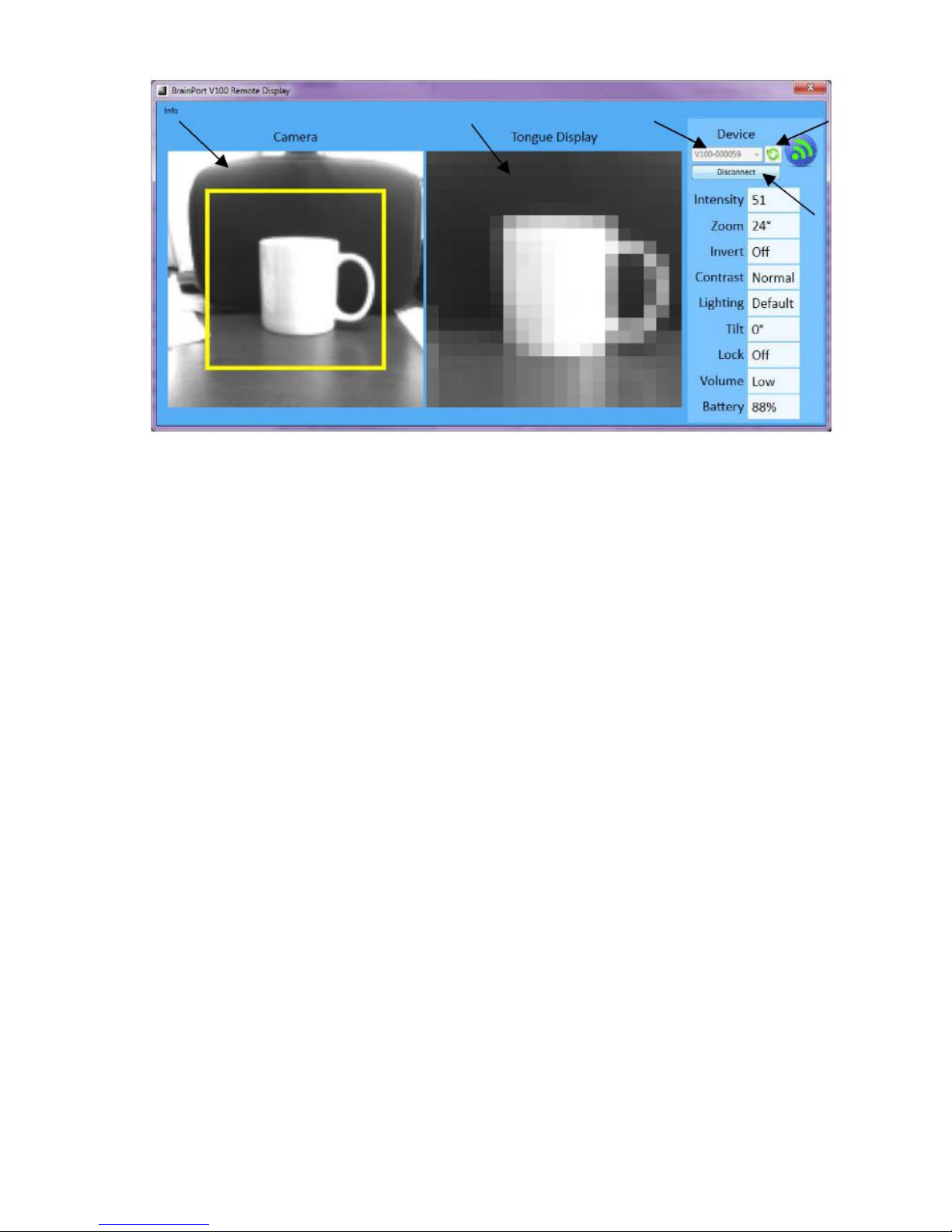
Companion Software (vRemote)
Letters in parentheses refer to Figure 2. vRemote below.
The vRemote software program runs on a personal
computer and can be used by a sighted companion to
view the images collected by the BrainPort V100 camera
and presented to the IOD. The Camera View (A)
represents a generally higher resolution view of the
scene detected by the camera. The yellow box in the
Camera View (A) represents the region of interest
(zoom/field of view) that the user has chosen. The IOD
View (B) is a visual representation of the information
being sent to the IOD (derived from the region of
interest).
vRemote is not to be used by the companion to provide
any instructions to the user, but is simply for watching.
The companion software can be used as an assistive tool
for practice following standard device training with a
Wicab certified trainer.
Note: The personal computer used to run the vRemote
software must have an operating system of Windows XP
or higher (.NET 4.0 or higher), and must be able to
establish an ‘ad-hoc’ Wi-Fi connection (minimum 802.11
a/b) with the BrainPort V100 device.
BrainPort V100 User Manual 23
A B C E
D
Figure 2. vRemote
A. Camer a View
B. IOD View
C. Device Name (Serial No.)
D. Connect/Disconnect Button
E. Refresh Button

BrainPort V100 User Manual 24
Quick Start: Turning on the BrainPort V100
Numbers in parentheses refer to Figure 1: BrainPort V100
1. Fully charge the batteries included with your device
prior to using your BrainPort V100 for the first time.
2. Wicab recommends that you thoroughly clean the
IOD before using it for the first time. Use the alcohol
towelette (included with y our de vice) to clean the
IOD. Allow to air dry. Thoroughly rinse the IOD with
tap water. Your IOD is ready for use after the rinse.
See “Care and Maintenance” for detailed instructions
on how to clean the IOD.
3. The h eadset c able c onnector (15) is attac hed to a
receptacle at the base of the Controller near the
Power On/Off button (18).This will be considered the
bottom of the Controller.
4. Insert a f ully c harged battery into the b ack o f the
Controller.
5. Verify that the Tilt Knob (3) is at its home position by
rotating it up (clockwise) until it reaches its stop
position (do not force past the stop point).
6. There are two way s to u se the B rainPort V 100: i n
standalone mode, or in Wi-Fi mode to connect to the
companion vRemote software.
Standalone Mode
To turn on the BrainPort V100 in standalone mode,
P
RESS the Power On/Off button (18) at the bottom of
the C ontroller. After po wer-up, the re i s a series of
one s econd t icks followed b y ( ~35 se c) the phras e
“active mode” which indicates the BrainPort V100 has
completed its self-test and is ready for use.
WiFi Mode
To turn on the BrainPort V100 in Wi-Fi mode, hold
both wheels (8 and 9) in towards the middle of the
controller t hen P
RESS the P ower O n/Off b utton (18)

BrainPort V100 User Manual 25
at the bo ttom o f th e C ontroller. T he C ontroller w ill
double beep, perform the same power up self-testing
as above, and then announce “Wi-Fi enabled” “active
mode”. You may release the wheels after the initial
double beep.
Starting in W iFi m ode i s ne cessary i f y ou wi sh to
connect to vRemote
7. Place the headset onto your head and make any
adjustments for comfort.
8. Initialize camera t ilt b y rotating the t ilt knob (3)
down (c ounter-clockwise) and bac k to its s tart
position, until an initialization double beep is heard.
This ensures that the device tilt range is maximized.
9. Hold the C ontroller in y our r ight han d with the
buttons o f the f ront pane l f acing to ward y ou for
purposes of these instructions.
10. Place the I OD i n y our m outh. U se t he stimulation
intensity d ial o n the left s ide (9) of the Controller to
control stimulation intensity.
11. The c amera f ield o f v iew ( zoom) wi ll d efault to a
medium field of view (24 degrees). This can be
changed by using the Zoom dial (8).
12. To sh ut down the s ystem, P
RESS-HOLD the P ower
On/Off button (18) until all beeps are finished
(approximately 4 seconds).
Starting vRemote
If your sighted companion will be using the personal
computer with vRemote software, follow the instructions
below:
1. Start the V100 in WiFi mode.

BrainPort V100 User Manual 26
2. Power-on the personal computer and wait for the
operating system to boot.
3. Open the vRemote program and click the Refresh
button.
4. Each unit is configured with an Ad Hoc network
corresponding to its serial number. From the dropdown menu in vRemote, choose the wireless network
that matches the numeric portion of the device serial
number of the user’s device. For instance if the device
serial number is SN000079, select wireless network
BPV-000079. Press the Connect button to connect to
the Wi-Fi network. The device will announce “Wi-Fi
connect” and the camera image will appear inside
vRemote.
5. A Camera View (A) and IOD View image (B) should
appear in vRemote on the personal computer. Note:
a. Reminder: Review Personal Computer under
General Warnings.
6. To turn off the system, turn off both the BrainPort
V100 device and the personal computer; order is not
important.
a. Exit the vRemote application and power
down the personal computer.
Device Announcements
During use, the device will produce a variety of
announcements following button presses. These
announcements let the user know which button was
pressed and what c hanges hav e be en m ade to the
camera or IOD. Sometimes the device may detect change
in environmental conditions.
Low Light

BrainPort V100 User Manual 27
In low light conditions, the device will announce “low
light” one time per session. A long P
RESS of the Status
button will reveal whether the conditions remain low light.
This announcement is used to remind users to optimize
their lighting conditions (i.e. turn on a light) in order for
the camera to capture a useful image.
L
OW BATTERY
When the battery l evel decreases to a low level, the
device will repeatedly announce “low battery”. T his
announcement ac ts as a re minder that a re placement
battery should be inserted in order to continue using the
device.
S
TANDBY MODE
The de vice wi ll ann ounce “standby m ode” when t he
headset is not properly connected to the controller. When
the d evice i s in standby m ode, no butto n wi ll f unction
until the device returns to active mode.
A
CTIVE MODE
The device will announce “active mode” upon completion
of start-up testing or when the headset connection has
been restored. The device is ready for use while in active
mode.
W
I-FI MODE
The device will announce “Wi-Fi enabled” upon activation
of the Wi-Fi module and completion of start-up testing.
The device is ready to connect to the vRemote software
while in Wi-Fi mode.
When the device connects with the vRemote software, the
device will announce “Wi-Fi connect”. When the device is
disconnected from vRemote, it will announce “Wi-Fi
disconnect”.

BrainPort V100 User Manual 28
Batteries
The system includes a rechargeable Lithium-Polymer
battery, c harger and c harging instructions. W hen f ully
charged, a batte ry provides approximately three hours of
standard usage. A given battery has an expected life of
more than 5 00 c harge c ycles. A f ully c harged batte ry
should always be used at the beginning of each session.
To replace the battery, open the door on the
back on the Controller by i nserting a f inger
into the re cessed a rea and s winging the
door toward the end of the box where the
cable connects. To remove the battery, grip
the to p of the battery and pull outward. To
replace the battery, align the exposed metal
connectors to the metal connectors in the
Controller and snap the battery into place
and close the door.
To test the battery power while using the BrainPort V100,
use a P
RESS of the Status button. The speaker will report
the appro ximate p ercentage o f c harge remaining f or the
installed b attery. In a low battery c ondition, the d evice
may shutdown automatically to avoid improper operation.
Avoid direct contact with the gold contacts located in the
battery compartment and on the battery.
• If you will not be using the BrainPort V100 for a period
of more than 5 days, remove the battery.
• Storage and Transportation Conditions:
o Temperature: -20°C to +45°C
o Humidity: 5% to 85%

BrainPort V100 User Manual 29
The nearby figure shows the location of the Battery
Manufacturer Part Number.
BrainPort V100 Battery Specifications
Wicab Part Number
DR-000839
Manufacturer Part
Number
56446 702 099
Battery Type
Rechargeable Li-Polymer
Electrical Specification
- 3.7V
- 2260mAh
- 8.4Wh
Storage and
Transportation
Conditions
-
Temperature: -20°C to
+45°C
- Humidity: 5% to 85%
Charge Cycles
> 500
Battery Charger
A c opy o f the batte ry c harger m anual is pro vided w ith
your BrainPort V 100. Follow the manufacturer’s
instructions when charging your battery.
Avoid direct contact with the gold contacts located in the
pocket of the charger. Do not power the charger using a
power strip or extension cord.
Battery Manufacturer
Part Number

BrainPort V100 User Manual 30
Battery Precautions
Avoid short circuits
Do not heat the batteries above 60°C
Do not dispose of batteries in fire
Do not solder directly to the battery
Do not charge with more that 1C and above 4.2V
Do not charge below 0°C or above 45°C
Do not discharge with more than 1C and below 2.7V
Do not discharge below -20°C or above 60°C
Do not disassemble the batteries
Do not insert the batteries in reverse polarity
W
ARNING: FIRE, EXPLOSION, AND SEVERE BURNS HAZARD
Personal injury can occur if the battery is handled
carelessly or improperly. For your safety, follow
these instructions for proper battery handling:
• The battery can ignite or explode if not handled
properly. If you notice any deformities, cracks, or
other abnormalities in the battery, immediately
discontinue use of the battery and contact the
manufacturer.
• Use only authentic, manufacturer-recommend
battery chargers and charge the battery only by
the method described in this user manual.
• Do not place the battery near heating devices or
expose to excessively warm environments, such
as the inside of an enclosed car in the
summertime.
• Do not place the battery in a microwave oven.
• Avoid storing or using the battery in hot, humid
places, such as spas or shower enclosures

BrainPort V100 User Manual 31
Care and Maintenance
• If you will not be using the BrainPort V100 for a period
of more than 5 days, remove the battery.
• Thoroughly rinse the IOD with tap water as needed to
remove c ontaminants. Y ou m ay us e i t i mmediately
following such a rinse.
• Wicab recommends that you clean the IOD once a
week. Use the cleaning solutions listed in Appendix A
on a c otton bal l o r cotton swab to g ently w ipe the
surfaces of the IOD. DO NOT SCRUB! Thoroughly rinse
the IOD with tap water to remove any residual
cleaning solution and allow it to air-dry.
• As n eeded, u se a cloth slightly dam p with wate r to
wipe the ex terior of the c ontroller, he adset, and
battery charger. DO NOT rinse or immerse the
controller, he adset, or the batte ry c harger. A fter
cleaning, a llow the controller, he adset, and b attery
charger to dry completely before use.
• DO NOT store or transport the device in extreme hot
or c old c onditions ( except f or batte ries – see a bove
section).
Storage and Transportation Conditions:
o Temperature: -25°C to +70°C
o Humidity: 5% to 95%
•
DO NOT bend, fold, or crush the IOD cable as this may
permanently damage the IOD.
• Always protect the IOD. T o avoid damaging the IOD,
follow these instructions:
o Do not drag the IOD electrode surface across an object;
o Do not strike the IOD against a hard object;
o Do n ot a llow t he I OD t o c ome i n c ontact w ith o ther
electrical e quipment s uch a s c ell p hones, c ell p hone
chargers, portable music players, etc.
BrainPort V100 User Manual 32
CHAPTER 3
Troubleshooting
This chapter describes steps you can take if the BrainPort
V100 does n ot s eem to be wo rking pro perly. Y ou m ay
need the assistance of a sighted individual to help with
troubleshooting. If the troubleshooting procedures do not
help, if you need further assistance, or if you think the
device may need service, use the information on the
inside back cover of this manual to contact Wicab.
The BrainPort V 100 has n o u ser-serviceable parts and
does no t re quire ro utine adj ustment o r c alibration. Y ou
will void your warranty if you attempt to service the
BrainPort V100.
DO NOT attempt to repair the BrainPort V100
yourself.
To insure that y ou receive accurate real time information
representative of the scene captured by the camera, the
device self-monitors and transitions from active mode to
standby m ode ( stimulation d isabled) w henever an
irregularity o ccurs. T his is the no rmal and i ntended
behavior o f the de vice as i t insures that you d o no t ac t
upon potentially compromised or significantly time
delayed visual information. You must take action to
return to ac tive m ode. The d evice pre vents a re turn to
active m ode i f the i rregularity pe rsists. If the de vice
emits 3 warning beeps and stops functioning, simply turn
the power off, then restart the device as usual.
BrainPort V100 User Manual 33
Problems with Battery Power
The B rainPort V 100 batte ry s tatus re ports the estimated
percentage of power remaining, assuming a fully charged
battery was installed at the beginning of t he session. In
cases wh ere a p artially c harged batte ry i s us ed, the
battery report may be misleading and put the device into
low battery states more quickly than expected.
When the battery in the BrainPort V100 nears the e nd of
its useful life it may take longer to recharge and the
duration of use following a recharge may be shorter. If
you notice these changes, or suspect that you have other
battery related problems, execute the steps below before
contacting Wicab.
The BrainPort V100 uses a rechargeable battery
that contains electronic components that
address safety requirements and that increase
its useful life. These electronic components are
not present in over-the-counter batteries. Using
any other battery may damage the device and
increase risk of use.
The battery charger supplied with the BrainPort
V100 is specifically designed for use with the
battery of the BrainPort V100. Using any other
battery charger may damage the device and
increase risk of use.
Do not use the device with the battery
compartment door open.
Perform t he f ollowing s teps t o i dentify t he s ource of a
battery power problem.
1. Follow the instructions in the battery c harger m anual
to insure the charger is working properly. If the
behavior o f y our batte ry c harger d oes no t m atch the
operation described in the manual, the problem is
most l ikely to be wi th the batte ry c harger. U se the
information on the inside back cover of this manual to

BrainPort V100 User Manual 34
contact Wicab to report the charger problem.
2. If the charger functions correctly, fully charge a
battery. O nce c harged, p lace the batte ry into the
BrainPort V100 controller and attempt to power up the
controller. I f the controller d oes no t illuminate the
green power-on light or begin the startup ticks, repeat
the pri or te st w ith y our s econd batte ry. I f the
controller s till do es n ot i lluminate the green power-on
light o r e mit the s tartup t icks, the pro blem i s most
likely to be with the controller. Use the information on
the inside back cover of this manual to contact Wicab
to report the controller problem.
If, ho wever, the gre en po wer-on l ight illuminates o r
startup ticks begin with one, but not the other, of your
batteries, the pro blem i s most likely w ith the battery
that fails to power up the controller. Use the
information on the inside back cover of this manual to
contact Wicab to report the battery problem.
3. With the f ully c harged batte ry inserted, turn y our
system o n and no te the ti me. I f af ter t wo ho urs o f
continuous operation, if the device is functioning, your
battery i s f unctioning as e xpected. Repeat the tw o
hour te st on y our second batte ry. Should e ither
battery fail to operate the device for two hours or
more, the batte ry is l ikely ne aring the e nd o f i ts
natural life. Use the information o n the i nside bac k
cover of this manual to contact Wicab.
4. If you reach this point, your batteries are mostly likely
functioning properly. If you continue to have
problems with your device, proceed to the controller
troubleshooting section below.

BrainPort V100 User Manual 35
Controller Troubleshooting
Perform the following steps if you suspect a malfunction
with the controller. If you identify a problem with the
controller and c ontact W icab, t he s upport s taff m ay as k
for the serial number of the controller. This is found on
the system label on the rear of the unit (see Product
Label in Chapter 1).
1. Place a f ully c harged b attery i nto the c ontroller and
attempt to po wer up the c ontroller. I f th e c ontroller
does not illuminate the green power-on light or begin
the s tartup t icks, pe rform the bat tery po wer
troubleshooting procedure described above before
continuing with troubleshooting of the controller.
2. If the c ontroller illuminates the gre en po wer-on l ight
and begins the startup ticks, but does not produce the
ready for operation announcement of “active mode”,
the problem is most likely to be with the controller.
Use the information on the inside back cover of this
manual to c ontact Wicab to re port th e controller
problem.
3. If t he c ontroller s uccessfully c ompletes t he s elf-test,
depress each of the buttons and dials in turn. The
controller s hould b eep wi th e ach d epression. I f th e
controller d oes n ot beep f or ev ery d epression of a
button o r d ial, the p roblem is m ost likely t o b e w ith
the controller. Use the information on the inside back
cover of this manual to contact Wicab to report the
controller problem.
4. If the controller successfully recognizes button and dial
depressions, rotate the stimulation dial and verify the
stimulation light illuminates yellow and that you feel
stimulation on your tongue from the IOD. If the
yellow stimulation light fails to illuminate or you do not
feel any stimulation, the problem is most likely to be
with the controller. Use the information on the inside
back cover of thi s m anual to c ontact Wicab to re port
the controller problem.

BrainPort V100 User Manual 36
5. If the yellow stimulation light illuminates or you can
feel stimulation, remove the IOD from your mouth.
Rotate b oth the s timulation and zo om d ials through
their f ully up and f ully do wn po sitions. V erify the
controller v ibrates whe n the m aximum a nd m inimum
stimulation and zoom levels are reached. If the
controller does not vibrate when a limit is reached, the
yellow s timulation light s tays i lluminated, or y ou f eel
stimulation when the stimulation dial is rotated to
minimum, the problem is most likely to be with the
controller. U se the i nformation o n the i nside bac k
cover of this manual to contact Wicab to report the
controller problem.
6. If you reach this point, your controller is mostly likely
functioning properly. If you continue to have
problems with your device, proceed to the headset
troubleshooting section.

BrainPort V100 User Manual 37
Headset Troubleshooting
1. Perform the following step if you suspect a malfunction
with the headset. If you identify a problem with the
headset and contact Wicab, the support staff may ask
for the serial number of the headset. This is found on
the l abel located o n the i nside o f the gl asses on the
left hand s ide. P erform the c ontroller tro ubleshooting
section; including a test of your batteries as noted in
step o ne. I f bo th y our b atteries and c ontroller are
functioning properly, then the problem is most likely
with the he adset. U se the i nformation on the in side
back cover of thi s m anual to c ontact Wicab to re port
the headset problem.
vRemote Troubleshooting
1. Delayed camera image. Due to Wi-Fi limitations an
occasional delay in the camera image may occur. To
minimize delay the distance between the personal
computer and Controller should be less than 6 meters
(20 feet).
2. Intermittent connection problems. Replace the battery
with a fully charged battery in the Controller. Follow
instructions in “Starting vRemote”.
3. Device serial number not listed in “Wireless
Connection” dialog on the personal computer. Replace
the battery with a fully charged battery in the
Controller. Follow instructions in “Starting vRemote”.
If you continue to experience difficulties with the device
or the vRemote software, contact Wicab for assistance.

BrainPort V100 User Manual 38
CHAPTER 4
Product Specifications and
Technical References
This chapter provides technical reference material that
may be useful to you, your doctor, your trainer, or local,
state, and national regulatory agencies.
General Specifications
Type BF Equipment
Physical
Length 13.3 cm
width 5.6 cm
height 3.5 cm (excluding belt clip)
weight <175 g (including battery)
Cable length to headset 106 cm
Power
Internally powered by a lithium polymer
rechargeable battery (3.7V, 2260mAh,
8.4Wh)
Output Wavef orms
Monophasic
Capacitive coupling
Pulse Frequency
200 Hz
Pulse Width
25 µs
Surface Area
0.46 mm² (per electrode)
Voltage
0 to 1.414 V rms (per electrode)
0 to 14.14 V rms (device)
Current
0 to 0.518 mA rms (per electrode)
0 to 20.7 mA rms (device)
Energy/pulse
10.35µJ (see note below)
Materials
The BrainPort V100 is not made with
natural rubber latex
Disposal
Many localities have recycling requirements
for batteries, electronic equipment, and
packaging. Recycle or dispose of the device
and its packaging in accordance with local
ordinances.
Environmental
Conditions
Operating Conditions:
- Temperature Range: 0°C to +40°C
- Humidity Range: 5% to 95%

BrainPort V100 User Manual 39
-
Atmospheric pressure range of 700 hPa
to 1060 hPa
Storage and Transportation Conditions (except
batteries):
- Temperature Range: -25°C to +70°C
- Humidity Range: 5% to 95%
Storage and Transportation Conditions
(batteries):
- Temperature Range: -20°C to +45°C
- Humidity Range: 5% to 85%
IP Classification
The BrainPort V100 is classified as IP20.
The device is protected against solid foreign
objects over 12 mm.
Regarding 60601-1-11 8.3.1: Other than the IOD
assembly, the V100 device is not designed to
prevent ingress of water or other liquids per test
of IEC 60529:1989 for IPX2. However, in all
cases the device maintains B
ASIC SAFETY and
ESSENTIAL PERFORMANCE (which may include
cessation of operation) after undergoing the test.
The IOD assembly is IPX4 rated (protection
against splashing water)
Intended Con ditions
for Use
- Non-Sterile
- Used in the home environment
- Used to augment white cane or
guide dog. Does not replace
white cane or g uide dog.
- Typical duration of use: multiple
sessions per day, usually less than
an hour per session
Part Numbers
Controller: DR-000994 (V100)
Headset: DR-000649
Battery: DR-000839 (56446 702 099)
Declaration of
Conformity
The CE mark on this product indicates it
complies with the provisions noted in the
93/42/EEC Medical Device Directive.
Note: Stimulation is voltage controlled and limited to 20.0 Volts.
Stimulation current depends on variat ions in us er ph ysiol ogy and with the
pressure of the tongue against the electrode array. Values given for
current and energy per pulse are at maximum voltage into a 2770 Ω
load, for a single electro de. Up to four electrodes m ay be active at an y
given time; therefore the current may exceed 10mA rms.
BrainPort V100 User Manual 40
User Profile
User
For use by individuals who are profoundly
blind.
Education
Completed traditional blindness
rehabilitation: white cane, and/or guide
dog, and rehabilitation in activities of daily
living
Knowledge
Minimum: ability to understand verbal
training instructions
Reading and comprehension at 10th grade
level
Experience
All users must participate in a minimum of
10 hours of supervised training per Wicab’s
training protocol prior to unsupervised use
of the device
Permissible
Impairments
Diagnosis of no light perception or light
perception
Blindness may be acquired or congenital
Absence of oral sensory impairments

BrainPort V100 User Manual 41
Electromagnetic Compatibility
Medical electrical equipment needs special precautions
regarding electromagnetic compatibility. The BrainPort
V100 should be used according to the electromagnetic
compatibility information provided below.
NOTE: The BrainPort V100 ( DR-0001062) uses WiFi
and an antenna. The following information is
included for reference.
Your BrainPort V100 contains a Wi-Fi transmitter and
receiver with the following characteristics:
FCC ID
NKRDRCM
Description
WLAN 802.11B/G CF MODULE
Model No.
DRCM-81
Frequency Range 802.11b/g:
2400~2483.5 MHz
Support channels for 802.11b/g
11 Channels
Antenna Type
Antenna peak Gain
Dipole
2 dBi (11b/g)
Other e quipment m ay i nterfere w ith th is device, even i f
that other equipment complies with CISPR emission
requirements.
This equipment has been designed to comply with the
limits for a Class B digital device, pursuant to Part 15 of
the F CC R ules. These l imits are d esigned to pro vide
reasonable protection against harmful interference in a
residential installation. T his e quipment g enerates, us es,
and c an radi ate ra dio f requency e nergy and , if no t
installed and used in accordance with the instruction, may
cause harmful interference to radio communications.
There is no guarantee that interference will not occur in a
particular installation. If this equipment does cause
harmful interference to rad io o r te levision re ception,
which c an be d etermined by turn ing th e e quipment o ff
and on, the user is encouraged to try the correct the
interference by one of the following measures:

BrainPort V100 User Manual 42
− Reorient or relocate the receiving antenna.
− Increase the s eparation be tween th e e quipment and
receiver
− Consult the d ealer o r an e xperienced rad io/TV
technician for help.
The BrainPort V100 has been tested and found to conform
to IEC 60601-1-2 and is intended for use in the
electromagnetic environment specified in the following
tables. I f y ou m ust us e the BrainPort V 100 in c lose
proximity to other electronic equipment, observe the
device to verify normal operations before starting use.
Note: The f ollowing t ables u se t he Eu ropean c onvention
of representing decimal values with a comma instead of a
period as is done in the United States. For example, the
value “2,5 GHz” in Table 204 is typically written “2.5 GHz”
in the United States.
This device complies with part 15 of the FCC Rules.
Operation is subject to the following two conditions:
(1) This device may not cause harmful interference, and
(2) this device must accept any interference received, including interference
that may cause undesired operation.
Changes or modifications not expressly approved by the
party responsible for compliance could void the user's
authority to operate the equipment.

BrainPort V100 User Manual 43
Table 1: Electromagnetic Emissions
The user of the BrainPort V100 should ensure that it is
used in the intended environment, as specified below.
Guidance and manufacturer’s declaration –
electromagnetic emissions
The BrainPort V100 is intended for use in the electromagnetic environment
specified below. T he cu stomer or t he u ser o f t he BrainPort V 100 should
assure that it is used in such an environment.
Emissions test
Compliance
Electromagnetic
environment – guidance
RF emissions
CISPR 11
Group 1
The BrainPort V100 must emit
electro-magnetic energy in order
to perform its intended function.
Nearby electronic equipment may
be affected. The product is
considered Group 1 - the emitted
energy is below 9kHz.
RF emissions
CISPR 11
Class B
The BrainPort V100 is suitable for
use in all establishments,
including domestic
establishments and those directly
connected to the public lowvoltage power supply network,
which supplies power for
buildings used for domestic
purposes.
Harmonic emissions
IEC 61000-3-2
Not applicable
(internally powered)
Voltage fluctuations/
flicker emissions
IEC 61000-3-3
Not applicable
(internally powered)

BrainPort V100 User Manual 44
Table 2: Electromagnetic Immunity
The user of the BrainPort V100 should ensure that it is
used in the intended environment, as specified below.
Guidance and m a nu facturer’s declaration – electromagnetic
immunity
The B rainPort V 100 is intended for u se in the electromagnetic e nvironment specified
below. The customer or the user of the BrainPort V100 should assure that it is used in
such an environment.
Immunity test
IEC 60601
test level
Complian
ce level
Electromagnetic
environment – guidance
Electrostatic
discharge (ESD)
IEC 61000-4-2
±8 kV contact
±15 kV air
±8 kV
contact
± 15 kV
air
Floors should be wood,
concrete, or ceramic tile. If
floor covering is synthetic
material, the relative
humidity should be at least
30%.
Electrical fast
transient/burst
IEC 61000-4-4
±2 kV for power
supply lines
±1 kV for
input/output
Lines
Not
applicable
(internally
powered)
(No
input/outp
ut lines)
Surge
IEC 61000-4-5
±1 kV line(s) to
line(s)
±2 kV line(s) to
earth
Not
applicable
(internally
powered)
Voltage dips, short
interruptions, and
voltage variations
on power supply
input lines
IEC 61000-4-11
<5% Ut
(>95% dip in Ut)
for 0,5 cycle
40% Ut
(60% dip in Ut)
for 5 cycles
70% Ut
(30% dip in Ut)
for 25 cycles
<5% Ut
(>95% dip in Ut)
for 5 sec
Not
applicable
(internally
powered)
Power frequency
(50/60 Hz)
magnetic field
IEC 61000-4-8
30 A/m
30 A/m
Power frequency magnetic
fields should be at levels
characteristic of a typical
commercial building or
hospital or home.
Note: Ut is the a.c. main voltage prior to application of the test level.

BrainPort V100 User Manual 45
Table 3: Electromagnetic Immunity
Users of the BrainPort V100 should ensure that it is used
in the intended environment, as specified below.
Guidance and m a nu facturer’s declaration – electromagnetic
immunity
The BrainPort V100 is intended for use in the electromagnetic environment
specified below. The customer or the user of the BrainPort V100 should
assure that it is used in such an environment.
Immunity
test
IEC 60601 te s t
level
Complianc
e level
Electromagnetic
environment –
guidance
Portable and mobile RF
communications
equipment should be
used no closer to the
BrainPort V100,
including cables, than
the recommended
separation distance
calculated from the
equation that applies to
the transmitter’s
frequency.
Recommended
separation distanc e
Conducted RF
IEC 61000-4-6
6 Vrms;
150 kHz to 80
MHz
6 V
d =1,2√P
Radiated RF
IEC 61000-4-3
10 V/m;
80 MHz to 2,7
GHz
10 V/m
d =1,2√P
80 MHz to 800 MHz
d =2,3√P
800 MHz to 2,5 GHz
Where P is the maximum
output power rating of
the transmitter in watts
(W) according to the
transmitter
manufacturer, and d is
the recommended
separation distance in
metres (m).
Field strengths from
fixed RF transmitters, as

BrainPort V100 User Manual 46
determined by an
electromagnetic site
survey,
a
should be less
than the compliance
level in each frequency
range.
b
Interference may occur
in the vicinity of
equipment marked with
the following symbol:
Note 1: At 80 MHz and 800 MHz, the higher frequency range applies.
Note2:
These guidelines may not apply in all situations. Electromagnetic
propagation is affected by absorption and reflection from structures, objects,
and people.
a
Field strengths from fixed transmitters, such as base stations for radio
(cellular/cordless) telephones and land mobile radios, amateur radio, AM and
FM radio broadcast, and TV broadcast, cannot be predicted theoretically with
accuracy. To as
sess the e lectromagnetic e nvironment d ue to fixed RF
transmitters, consider doing an electromagnetic site survey. If the measured
field strength in the location in which the BrainPort V100
is used exceeds the
applicable RF compliance level above, observe the BrainPort V 100
to verify
normal operation. If y ou d etect a bnormal performance, additional measures
may be necessary, such as reorienting or relocating the BrainPort V100.
b
Over
the f requency range of 150 kHz to 80 MHz, field s trengths s hould b e
less than 10 V/m.

BrainPort V100 User Manual 47
Table 4: Recommended Separation
Distances
Users of the BrainPort V100 should ensure that it is used
in the intended environment, as specified below.
Recommende d se paration distanc es between porta ble and
mobile RF commu nications equipment and the BrainPort V100.
The BrainPort V100 is intended for use in an electromagnetic environment in
which radiated RF disturbances are controlled. The customer or user of the
BrainPort V100 can help prevent electromagnetic interference by maintaining
a minimum distance between portable and mobile RF communications
equipment (transmitters) and the BrainPort V100 as recommended below,
according to the maximum output power of the communications equipment.
Rated maximum
output power of
transmitter
W
Separation di st ance according to
frequency of t ra nsmitter
M
150 kHz to
80 MHz
d = 1,2√P
80 MHz to
800 MHz
d = 1,2√P
800 MHz to
2,5 GHz
d = 2,3√P
0,01 0,12 0,12 0,23
0,1 0,38 0,38 0,73
1 1,2 1,2 2,3
10 3,8 3,8 7,3
100 12 12 23
For transmitters rated at a maximum output power not listed above, the
recommended separation distance d in metres (m)can be estimated using the
equation applicable to the frequency of the transmitter, where P is the
maximum output power of the transmitter in watts (W) according to the
transmitter manufacturer.
Note 1: At 80 MHz and 800 MHz, the separation distance for the higher
frequency range applies.
Note 2: These guidelines may not apply in all situations. Electromagnetic
propagation is affected by absorption and reflection from structures, objects,
and people.

BrainPort V100 User Manual 48
Warranty
Limited Product Warranty. Wicab, Inc. (the “Company”) warrants to the
purchaser (“Purchaser”) (limited to the original Purchaser and to no other
person) that the Product and the component parts thereof manufactured
and distributed by the Company will meet the specifications stated on the
Product specification sheets and shall be free from defects in workmanship
and materials for a p eriod of two years ( Service L ife) measured f rom t he
date the Product is shipped to the Purchaser (“Warranty Period”). If any
component of the Product does not conform to these specifications, the
Company will, at its sole discretion, as its sole and exclusive liability and as
to the Buyer's sole and exclusive remedy, repair or replace the Product
with a new or factory reconditioned Product at no charge or refund the cost
of the Product; provided that notice of nonconformance (setting forth in
reasonable detail the nature of the nonconformance) is given the Company
within the Warranty Period and within 30 calendar days of the discovery of
the defect or nonconformance.
This warranty limits the Company's liability to the repair or replacement of
Product or refund of the cost of Product. No other warranties of any kind,
express o r i mplied, i ncluding w ithout l imitation, a n i mplied w arranty o f
merchantability or fitness f or a particular purpose or noninfringement, are
provided by company. Company shall in no event be liable for personal
injury or property damage or any other loss, damage, cost of repair, or
direct, indirect, incidental, special, consequential, or punitive damages of
any kind, whether based upon warranty, contract, strict liability,
negligence, o r a ny o ther ca use o f a ction, arising o ut o f th e sa le, u se,
results of the use or inability to use product or its components. The
foregoing shall apply, without limitation to losses of profits, business
interruption, d amages t o p urchaser’s r eputation o r a ny o ther s uch
damages.
Limitation of Liability. With respect to any Company l iability not legally
subject to the foregoing terms, the Company's liability shall not exceed the
amount paid by Purchaser to the Company for the Product.
BrainPort V100 User Manual 49
Appendix A Cleansers
Wicab recommends that you clean the IOD once per week
using t he cleansers listed in the table below. I f the
cleanser is i n s olution, we t a c otton ball or c otton s wab
and gently wipe the surfaces of the IOD. DO NOT SCRUB!
Thoroughly rinse the I OD with tap wate r to re move any
residual cleanser and allow it to air-dry.
The cleansers listed below have been tested and shown to
be c ompatible with the materials used in IOD. Follow the
manufacturer’s directions regarding safe use and disposal
for the cleanser.
Please v isit o ur we bsite f or the m ost re cent l ist o f
approved cleansers. Cleansers are add ed to the l ist as
they are tested and shown to be compatible with the IOD.
Cleanser
Source
70% isopropyl alcohol (rubbing
alcohol)
Over the Counter (local
pharmacy)
May be in solution or as
‘wipes’
The IOD may be damaged by bleach or oxidizing
agents. Do not use any product that contains
chlorine, sodium hypochlorite, calcium hypochlorite,
hydrogen peroxide, sodium percarbonate, sodium
perborate, or similar chemical. Household bleach
and over-the-counter whitening agents will damage
the IOD.

BrainPort V100 User Manual 50
Appendix B C linical Safet y
Information
As described in Chapter 1, the BrainPort V100 was
evaluated in a clinical study of 75 enrolled subjects. All
adverse events reported, regardless of whether or not the
events were related to the device, were captured in the
study. The device-related adverse events are presented
in Table A below. Throughout the 12 month study, a
total of 28 device-related adverse events were reported in
18 subjects, but no clinically significant device-related
adverse event occurred.

BrainPort V100 User Manual 51
Table A: Device-Related Adverse Events Reported with
Use of the BrainPort V100 Device (n=75 Subjects)
Type of Event
Number
of
Events
Events
Allergic reaction NOT
requiring medical care
3
Sinus Drainage Worsening
Metallic Taste
Suspected Metal Allergy
Positively altered change
in taste or numbness that
is NOT sustained
2
Tingling sensation in mouth
Hypergeusia (increased taste sensation)
Negatively altered change
in taste or numbness that
is NOT sustained
13
Tongue sensitivity
Taste of metal in mouth
Dysgeusia while using device (metallic
taste)
Tingling on tongue
Metallic taste while using the device
Occasional hyposensitivity to taste after
prolonged use
Intermittent numbness in upper right
dentition- canine and incisor
Intermittent tingling sensation on tongue
Increased sensitivity in teeth
Increased tongue sensitivity
Tongue stinging
Mild sensitivity to teeth
Soreness of tongue
Other event related to
health or condition not
included in above
categories
10
Increased tongue awareness
Headache
Increased salivation
Small erythematous area on hard palate
of mouth
Small 2-3mm irritation in ridge- midline
of mouth
Headaches
Clicking on right side of jaw
Clicking of jaw (more on right than left)
Headaches after prolonged use of device,
intermittent
Clicking of jaw bilaterally
Note: S ome s tudy p articipants r eported m ore tha n 1 e vent; e ach e vent i s
counted individually in the table above.
The IOD component of the BrainPort V100 device has also
been evaluated in patients with balance disorders in
another device (Wicab Balance). The device-related
safety data reported for this device are presented below.
The Balance device is indicated for use in patients with

BrainPort V100 User Manual 52
balance disorders, and thus the reported adverse events
may not be the same as those reported in the profoundly
blind patients that were treated with the BrainPort V100
vision device. However, for a comprehensive evaluation
of the device safety profile, all relevant available devicerelated safety data are presented in Table B (events
reported during the clinical study of the Balance Device)
below.
Table B: Device-Related Adverse Events Reported with the
BrainPort Balance Devic e – Controlled Clinical Trial (n=148
Users)
Type of Event Events
Number of
Events
Oral Conditions
Tingling tongue
4
Dysgeusia
3
Tongue irritation
2
Cheilitis
2
Mouth ulcer
3
Burning tongue
1
Oral pain
1
Ear & Labyrinth Disorders
Vertigo
4
Accident, Injury, And
Procedural Complications
Fall
3
Ankle sprain
1
Nervous System Disorders
Migraine
1
Headache
1
Gastrointestinal Disorders
Nausea
1
Psychiatric Disorders
Concentration deficit 1

BrainPort V100 User Manual 53
User Assistance
If you need assistance setting up, using or maintaining
the BrainPort V100, or to report unexpected operation or
events, please contact Wicab, Inc. by mail, telephone, or
e-mail:
North America
Wicab, Inc.
Attn: Customer Support
8313 Greenway Blvd.
Suite 100
Middleton, WI 53562 USA
Telephone: 1.608.829.4500
Fax: 1.608.829.4501
E-Mail: customersupportus@wicab.com
Internet: www.wicab.com
BrainPort is a trademark or registered trademark of
Wicab, Inc. in the United States and other countries.

BrainPort V100 User Manual 54
0473
Correct Disposal of This Product
(WEEE - Waste Electrical & Electronic Equipment)
This marking on the product, accessories or literature indicates
that the product and its electronic accessories should not be
disposed of with other household waste at the end of their
working life. To prevent possible harm to the environment or
human health from uncontrolled waste disposal, please separate these items
from other types of waste and recycle them responsibly to promote the
sustainable reuse of material resources.
Household users should contact either the distributor where they purchased this
product or their local government office for details of where and how they can
take these items for environmentally safe recycling.
Copyright 2014, Wicab, Inc.
DR-001114 Rev D, January 2015
 Loading...
Loading...