VQ OrthoCare Orthostim 4 Patient Manual

Patient Manual
OrthoStim4 Rehabilitative Electrotherapy
from VQ OrthoCare

VQ OrthoCare is not liable for any misuse or misunderstanding of the
OrthoStim4 product or operating manual. Please call your local
representative if any additional assistance is required regarding this
product and its operating instructions.
This patient manual is proprietary and confi dential. No part of this document
may be reproduced or transmitted in any form or by any means, electronic
or mechanical, including photocopy, recording or information storage and
retrieval system, without permission in writing from VQ OrthoCare.
VQ OrthoCare warrants the OrthoStim4 to be free from electrical circuit
defects in workmanship and materials for a period of three (3) years upon
delivery to the customer. The case, cradle and belt clip are warranted to be
free from defects in workmanship and materials for one (1) year upon
delivery to the customer. VQ OrthoCare will repair or replace, at its
facilities, any OrthoStim4 unit found to have become defective within the
warranty period.
This warranty does not apply to accessories; specifi cally lead wires,
electrodes, batteries, tapes, gel, carrying case and AC Adapter, nor does it
apply to OrthoStim4 units that have been damaged due to misuse, or
repaired or altered by parties other than VQ OrthoCare at its facilities.
This warranty is in lieu of any other warranties expressed or implied.
No person or entity is authorized to bind VQ OrthoCare to any representa-
tion of warranty other than those specifi cally set forth herein.
Warranty

Letter to the Patient ......................................................................2
System Components ...................................................................3
Precautions and Prescription Information ..............................4
Controls and Features .................................................................6
Accessories ....................................................................................8
Starting Your Treatment Sessions ...........................................10
Normal Running Screens ..........................................................13
After Completing Your Treatment Sessions ..........................17
Electrode Use and Care.............................................................18
Cradle/Modem ............................................................................19
Care and Maintenance ..............................................................22
Technical Data and Specifi cations .........................................26
Waveform Diagrams ..................................................................30
Troubleshooting .........................................................................37
Notes .............................................................................................39
Warranty .......................................................................................41
Table of Contents

2
Letter to the Patient
Your physician has selected the OrthoStim4 – a portable multi-modality
device that provides Interferential, Neuromuscular, Pulsed Direct Current
and High-Volt Pulsed Current Stimulation – as the most appropriate treatment for your needs.
The OrthoStim4 electrical stimulator generates small pulses of current that
are delivered through lead wires to stimulating electrodes* placed on your
skin. These pulses pass through the skin and activate underlying nerves.
The OrthoStim4 activates sensory nerves at lower levels of stimulation,
producing a tingling sensation. Motor nerves are activated at higher levels
of stimulation, resulting in muscle contractions.
The OrthoStim4 system includes a cradle equipped with a modem that
enables your doctor or therapist and our technicians to conveniently administer setting changes to the device and keep your prescription information
up to date. As a patient, you may also take advantage of this technology to
make modifi cations to your settings, and to assist all of your healthcare
partners in providing the most personalized service possible.
In order to gain maximum benefi t from your OrthoStim4 treatment, it is
important that you follow the therapy regimen prescribed by your physician or therapist. He or she is familiar with your specifi c requirements and
the technical specifi cations of the OrthoStim4, and will instruct you on the
proper mode of operation and correct degree of stimulation for your
individual treatment.
Please read the following sections carefully before using the OrthoStim4.
• Precautions and Prescription Information
• Controls and Features
• Starting Your Treatment Sessions
• Care and Maintenance
NOTE: The OrthoStim4 is not a substitute for full medical evaluation and
treatment. Always consult your physician or therapist if you have specifi c
questions or problems regarding the use of your OrthoStim4. The OrthoStim4 should not be given to or used by any individual other than the
person for whom it is prescribed.
* For use only with stimulating electrodes, commonly referred to as “electrodes” throughout this manual
3
System Components
The OrthoStim4 multi-modality stimulation system is operated with:
(1) OrthoStim4 electrotherapy device
(1) package of non-sterile, reusable electrodes
(1) lead wire
(2) Power Packs
The OrthoStim4 system also includes:
(1) carrying case with removable shoulder pouch
(1) Patient Manual (this booklet)
(2) additional packages of non-sterile, reusable electrodes
(depending on your doctor’s protocol)
(8) adhesive remover towelettes
(4) additional Power Packs
(1) AC Adapter
(1) cradle with modem
(1) telephone cord with splitter
A
A
B
B
C
C
D
D
E
E
F
F
G
G
H
H
I
4
Precautions and Prescription Information
CAUTION: Federal law restricts this device to sale by, or on the order of,
a practitioner licensed by the law of the State in which he/she practices to
use or order the use of this device.
NOTE: Please read the following prescription information carefully before
using the OrthoStim4. If you have any questions regarding this information,
consult with your physician or therapist before proceeding.
Indications
The High Volt Pulsed Current Stimulation and Neuromuscular Electrical Stimulation
can be used in the following applications:
• Relaxation of muscle spasms
• Prevention or retardation of disuse atrophy
• Increases local blood circulation
• Muscle re-education
• Maintaining and increasing range of motion
• Immediate post-surgical stimulation of calf muscles to prevent venous thrombosis
Interferential Stimulation can be used in the following applications:
• Relieves acute pain
• Relieves chronic pain
• Relaxation of muscle spasms
• Maintaining and increasing range of motion
• Increases local blood circulation
Pulsed Direct Current stimulation can be used in the following applications:
• Reduction of edema (under negative electrode)
• Relaxation of muscle spasm
• Increasing local blood circulation
• Retardation or prevention of disuse atrophy
• Muscle re-education
• Maintaining or increasing of range of motion
Contraindication
Stimulators should not be used on patients with cardiac demand pacemakers.
Warnings
• The long-term effects of chronic electrical stimulation are unknown.
• Stimulation should not be applied over the carotid sinus nerves, particularly in
patients with a known sensitivity to the carotid sinus refl ex.
• Stimulation should not be applied over the neck or mouth; severe spasm of the
laryngeal and pharyngeal muscles may occur and the contractions may be strong
enough to close the airway or cause diffi culty in breathing.

5
• Electrodes should not be placed on the chest in such a confi guration that would
allow electrical current delivered by the device to fl ow through the chest because
it may cause a cardiac arrhythmia.
• Stimulation should not be applied transcerebrally.
• Stimulation should not be applied over swollen, infected, or infl amed areas or skin
eruptions, e.g. phlebitis, thrombophlebitis, varicose veins, etc.
• Stimulation should not be applie d over, or in proximity to, cancerous lesions.
• Due to the potential for causing skin burns, this device should only be used with
electrodes which limit the current density to 0.25W/cm
2
• Stimulation should not be applied transthoracically in that the introduction of electrical
current may cause cardiac arrhythmias.
Precautions
• Safety of stimulators for use during pregnancy has not been established.
• Caution should be used for patients with suspected or diagnosed heart problems.
• Caution should be used for patients with suspected or diagnosed epilepsy.
• Caution should be used in the presence of the following:
- When there is a tendency to hemorrhage following acute trauma or fracture
- Following recent surgical procedures when muscle contraction may disrupt
the healing process
- Over the menstruating or pregnant uterus
- Over areas of the skin which lack normal sensation
• Some patients may experience skin irritation or hypersensitivity due to electrical
stimulation or electrical conductive medium. The irritation can usually be reduced
by using an alternative conductive medium or alternate electrode placement.
• Electrode placement and stimulation setting should be based on the guidance of
the prescribing practitioner.
• Keep out of the reach of children.
• Use only with the lead wires and electrodes recommended for use by
the manufacturer.
• Portable stimulators should not be used while driving, operating machinery, or
during any activity in which involuntary muscle contractions may put the user at
undue risk of injury.
• When the amplitude in the IF mode exceeds 40mA, electrical noise from the
device could interfere with other equipment operating in the nearby area.
• Do not trim electrodes as cut edges may affect even distribution of stimulation.
• Do not immerse electrodes in water.
Adverse Reactions
Skin irritation and burns beneath the electrodes have occasionally been reported
with the use of electrical stimulators. If you experience any symptoms of skin
irritation or any unusual reactions while using the OrthoStim4, discontinue use
immediately and contact VQ OrthoCare’s Patient Care Department at 800.452.7993
or consult your physician.
6
Controls and Features
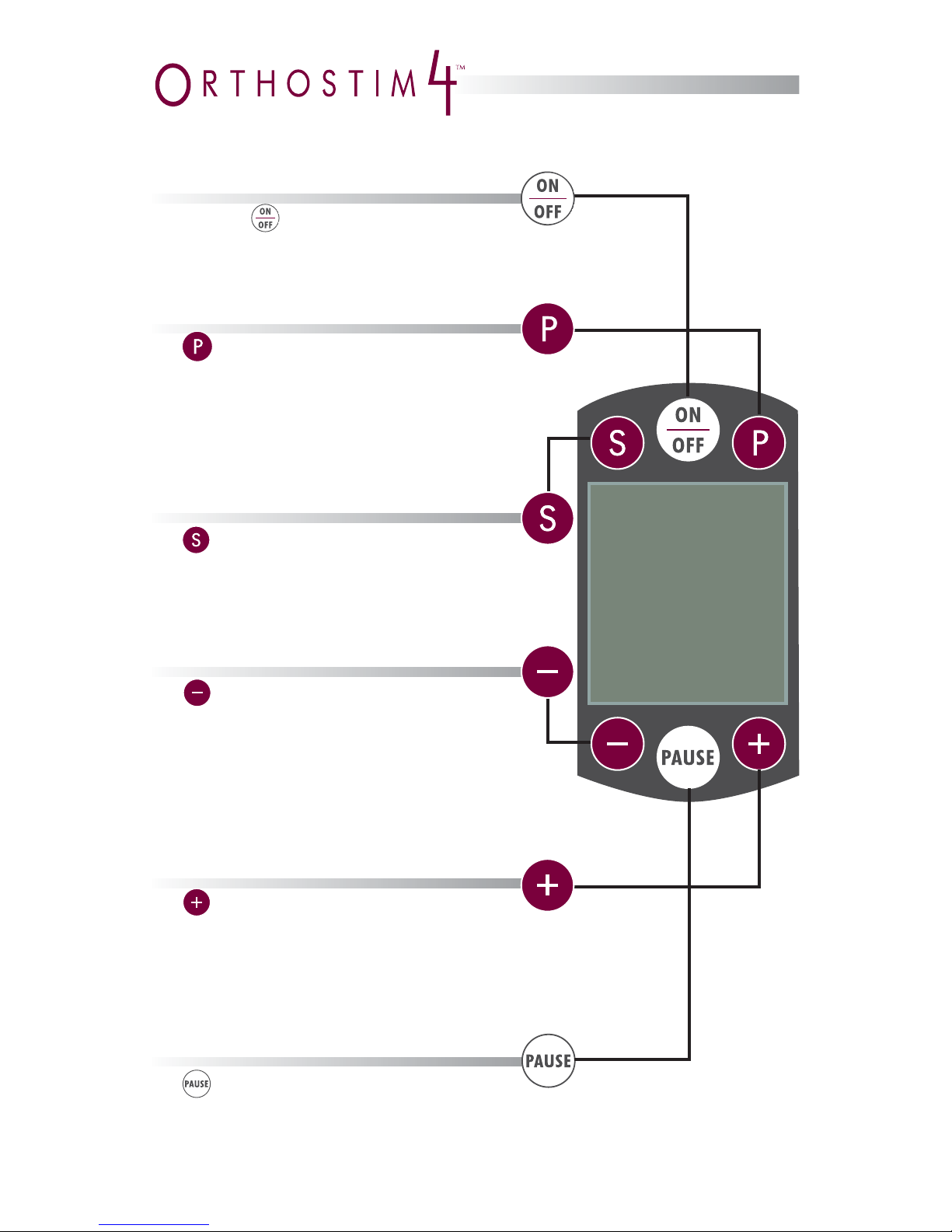
Pressing the button turns on
the OrthoStim4 and performs a brief
system test. Once the OrthoStim4 is
turned on, pressing this button turns it off.
The button pauses treatment at any
time. Pressing this button again resumes treatment.
The (–) button has three functions. It can be
used to:
• Decrease the intensity of stimulation, either
in small increments through single presses
or by holding the button down to implement
a continuous decrease
• Move the cursor down in a selection list
• Rotate stimulation to the left in vector mode
The (+) button has three functions. It can be
used to:
• Increase the intensity of stimulation through
pressing and releasing the button
• Move the cursor up in a selection list
• Rotate stimulation to the right in vector mode
The (S) button has three functions. It can be
used to:
• Select items when options are provided
• Select channels in neuromuscular mode
• Access vector mode in interferential treatment
The (P) button has three functions. It can
be used to:
• Proceed to the next step, according
to directions on the screen
• Go to the Protocol screen
• Manually start a download when the
OrthoStim4 is in the cradle
7
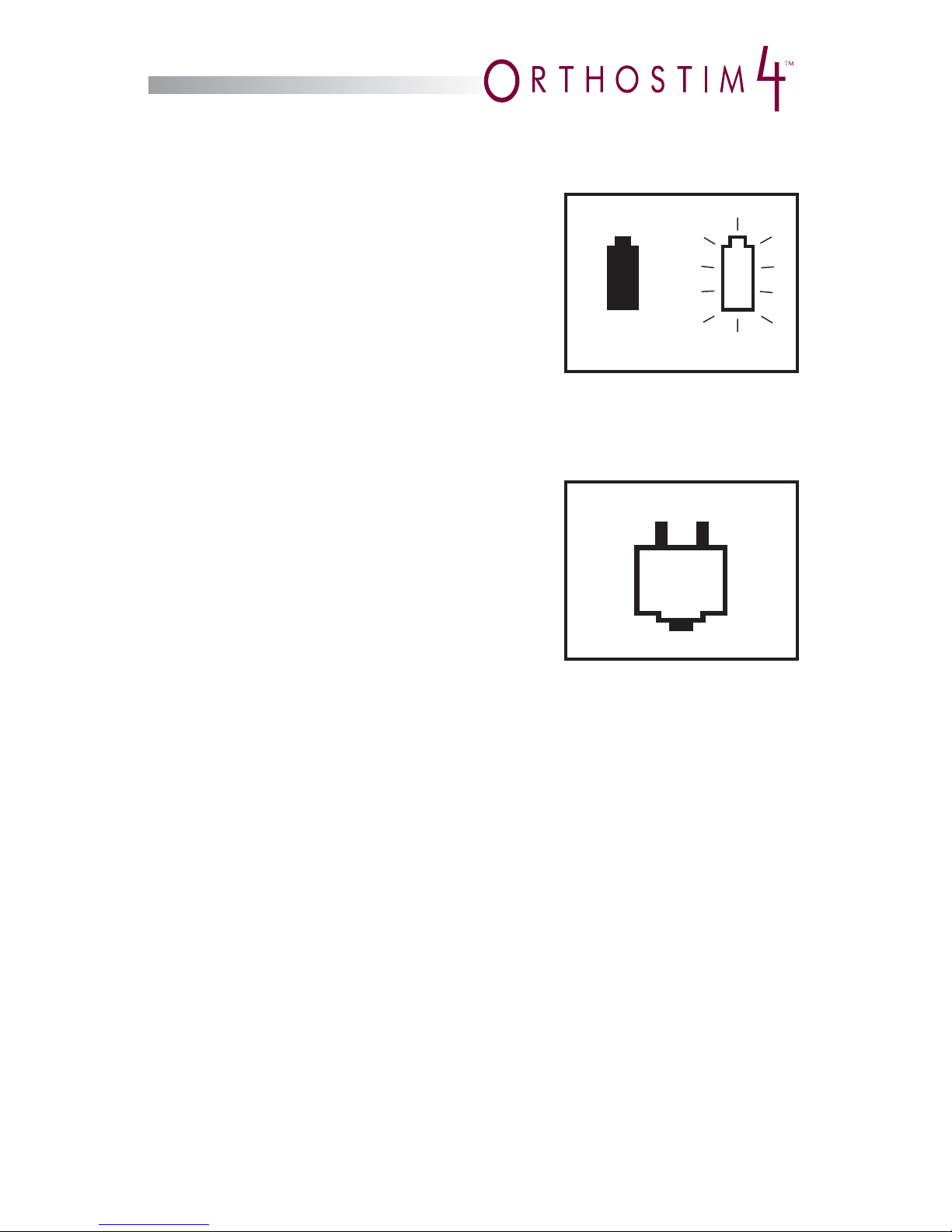
Battery Indicator
A battery symbol in the upper left corner of
the screen will display the battery status.
When the battery is fully charged, the symbol
displays as black. When the battery power
is low, the symbol displays as white, and
starts to fl ash and the device will beep once
a minute. Replace the alkaline Power Packs as
soon as possible. When battery power is suffi ciently depleted, the device will shut
off automatically.
AC Adapter Indicator
When an AC Adapter is plugged into the
OrthoStim4, a plug symbol appears next to
the battery indicator. The AC Adapter bypasses
the Power Packs and supplies the power.
Full Replace

8
Accessories
Lead Wire
The lead wire connects the
OrthoStim4 to the electrodes.
When plugging the lead wire
into the OrthoStim4, make sure it is
pushed fi rmly into the socket, with the
strain relief pointing toward the bottom of
the OrthoStim4 (as shown). To remove the wire,
grip the connector close to the socket and pull
straight out.
Power Supply
The OrthoStim4 can be operated with two non-rechargeable alkaline
Power Packs, the AC Adapter, or by placing the OrthoStim4 in the cradle.
Power Packs
The unit should be turned off when switching from one power source to
the other. Additional Power Packs can be ordered from VQ OrthoCare’s
Patient Care Department by calling 800.452.7993.
Alkaline Power Packs (non-rechargeable)
Insert two alkaline Power Packs in the OrthoStim4
with the ribbon handle facing out. The plus symbol
should be aligned toward the top right of the OrthoStim4 and the minus symbol toward the bottom left,
as indicated on the label. When the display indicates
low battery power, remove and properly dispose of.
Power Packs cannot be re-used or recharged.

9
AC Adapter
If the alkaline Power Packs are in the
OrthoStim4, the AC Adapter will
bypass the Power Packs and provide power. The alkaline Power
Packs are non-rechargeable. Use
only the AC Adapter provided, as
use of other products’ AC Adapters may damage the OrthoStim4.
Press the (PAUSE) button to pause
stimulation before connecting or disconnecting the AC Adapter.
Press (PAUSE) to resume treatment.
Cradle/Modem
The OrthoStim4 system includes a cradle
that contains a modem that enables the
download of updates and information to/
from the OrthoStim4 through a landline
telephone connection. The modem also
enables VQ OrthoCare to modify the
OrthoStim4 from a remote location per
physician specifi cations. The Cradle has
a plug receptor that will accept a regular
phone line (RJ11 plug) from any standard
home telephone. The Cradle/Modem will
initially be set up by a VQ OrthoCare
Patient Technician. For set-up information, see page 20.

10
Starting Your Treatment Sessions
1. Insert two alkaline Power Packs into the OrthoStim4. Make sure each
is oriented according to the label on the battery pack and the battery
compartment, as the OrthoStim4 will only operate if the Power Packs
are inserted correctly. To use external power instead, plug the AC Adapter
into the OrthoStim4 or place the OrthoStim4 in the cradle.
[NOTE: If electrodes have been placed beneath a brace, cast or
bandages by your healthcare professional, skip to step 5.]
2. Prepare the skin. Electrode application sites must be clean, dry, un-
broken skin surfaces. To reduce the risk of skin irritation, observe the
following instructions for proper skin care before treatment.
Wash site of electrode placement with mild soap and water before
applying electrodes.
Rinse all soap off the skin before continuing.
Dry the skin thoroughly.
Trim excess body hair from electrode sites with scissors, being
careful not to cut (or break) the skin. DO NOT SHAVE THE SKIN
IMMEDIATELY PRIOR TO STIMULATION, because shaving will
create small cuts in the surface layer of skin.
CAUTION:
DO NOT apply lotions, oils or other ointments to the skin prior
to the application of electrodes, unless directed by your clinician or
a VQ OrthoCare representative. Topical agents may increase the risk
of skin irritation.
DO NOT clean the skin with anything other than soap and water.
Other cleaning agents may interfere with proper conduction of
electrical pulses.
In the event of skin irritation, discontinue use immediately and contact
VQ OrthoCare’s Patient Care Department at 800.452.7993 or consult
your physician or therapist.
3. Inspect each electrode and lead wire before application. Do not proceed
if there is any doubt about the integrity or proper function of any
electrode or lead wire. Connect the lead wire to the electrodes, making
sure there is no bare metal exposed at the connection points. Do not use
unnecessary force in connecting the electrodes to the lead wires. If they
do not fi t, use a different set of electrodes and call VQ OrthoCare to re-
11
port the situation and receive a replacement set of electrodes.
Save the non-working electrodes for evaluation by VQ OrthoCare’s
Quality Assurance Department; DO NOT THROW THEM AWAY!
Select
Protocol
1
PAIN
40:00
(S) Select
6. Turn the OrthoStim4 on by pressing the (ON/OFF) button. The serial
number and compliance time will be displayed. After releasing the
(ON/OFF) button, the OrthoStim4 will start its self-test. When the
self-test is completed, you will see Select Protocol at the top of the
screen and a number 1 fl ashing in the middle of the screen, along
with the length and purpose of treatment (e.g., pain; spasm).
4. Gently remove the electrodes from the plastic liner. Place the electrodes
on the sites prescribed by your physician or therapist and press them
fi rmly onto the skin. Electrodes should be placed at least one inch apart
from each other.
5. Insert the lead wire into the
OrthoStim4. For instructions,
see page 8.
12
Starting Your Treatment Sessions
(continued)
CAUTION: If using pain medication and/or other pain management
devices, exercise caution when increasing the amplitude of the
OrthoStim4.
CAUTION: When the device is used in environments where other
medical equipment is in use, the amplitude of Interferential (IF) should
not exceed 40mA or it may interfere with the operation of nearby
equipment.
7. Select the preset as directed by your physician or therapist by pressing
the (+) or (–) button until the number indicated for the protocol is shown
in the middle of the screen. Press (S) to select.
8. Increase the stimulation intensity level by repeatedly pressing and re-
leasing the (+) button. If your protocol involves multiple treatment types,
you will be asked to set the stimulation for each treatment before beginning. Follow the prompts on the screen to set the intensity levels for
each segment of your protocol. The screen will then count down from 5
and treatment will start.
 Loading...
Loading...