Volu-Med HairMax LaserComb Premium User Manual

Volu-Med (Ireland)
Ltd.

Contents
1 Introduction
I Introduction to Laser Phototherapy
II Introducing the HairMax
III Two Types of Lasercombs
2 FDA Clearance
I Breaking News
II What it means to be FDA cleared
III Hairmax Clinical trials Overview
3 About HairMax Lasercomb
I HairMax Questions and Answers
II Users guide
III Technical guide and how to look after your lasercomb
4 HairMax Orders, Delivery and Guarantees
I How to Take an Order
II Delivery Information
III Price Information
IV Guarantee, Warranty and Money Back Guarantee

5 Testimonials and Pictures
I Users Experiences
II Before and Afters Pictures
6 About Hairloss and Other Hairloss Remedies
I About Hairloss
II Other Remedies
7 Keywords and Phrases to Keep at Hand
8 Current Prices and Delivery Charges
1. Introduction
I Introduction to Laser Photo-Therapy
Laser Phototherapy is a form of laser technology also referred to as Low Level Light Therapy. Laser
Phototherapy has been used clinically for numerous medical purposes in Europe for over thirty years and has
been the subject of over 2,500 scientific papers published worldwide. There are no reported side effects to the
therapy, which is painless, non-toxic and complements many traditional therapies.
Until recently, Laser Phototherapy has been relatively unknown. Today, however, there is increasing
awareness and acceptance of Laser Phototherapy among healthcare practitioners. The FDA has just cleared
three other Laser Phototherapy devices for Carpal Tunnel Syndrome and chronic pain relief.
For many years, leading hair clinics and salons around the world have also been using Laser Phototherapy for
individuals suffering from thin lifeless hair. These clinics have been very successful in improving the health
and condition of hair.
White Cliffs has been combining the use of this laser technology with proven scalp and hair regrowth
products for a number of years.
The Benefits:
• Stops excessive hair loss
• Stimulates hair follicles to help re-growth
• Produces fuller, shinier and thicker hair
• Repairs and improves quality of hair shafts
• Relieves irritation scalp conditioning
II Introducing the HairMax
The HairMax Lasercomb is a breakthrough in hair care because it is the first Laser Phototherapy device

designed for the general public to use at home. Developed in Sydney, Australia from over 18 years of clinical
experience, the HairMax Lasercomb is a high-quality device using precision components. It is manufactured
and patented in the USA.
The HairMax Lasercomb is the first product of its kind, a unique hand-held, Laser Phototherapy device that
delivers the healthy and nourishing effects of low-level laser to improve your hair.
The HairMax LaserComb is an effective therapy for thin lifeless hair. The LaserComb works through the
scientific principle of PhotoBioStimulation, which is the natural process by which light energy is converted
into cellular energy.
Science has shown that skin cells thrive in light and your hair is no different. Through the scientific
principles of 'Photo-Bio-Stimulation', Laser Photo-Therapy progressively improves the quality, strength and
health of your hair over time. Results vary from person to person, but you should notice early, general
improvements and activation of your hair within 8 to 12 weeks. These improvements include a normalizing
of scalp condition as well as shinier and thicker hair. Gradually, over the next few months, you should notice
your hair becoming fuller, healthier and denser as your hair responds to the rejuvenating effects of the laser
light.
Users of the HairMax LaserComb experience high user satisfaction. Over 93% of the people who use the
HairMax LaserComb experience benefits. Our goal is to provide a sincere service to the public, conveying
realistic expectations and providing positive user experiences and satisfaction.
The information contained in these pages have been compiled to help you answer customer questions
effectively and give you a better understanding of the recently FDA cleared HairMax lasercomb.
III Two Types of HairMax Lasercombs
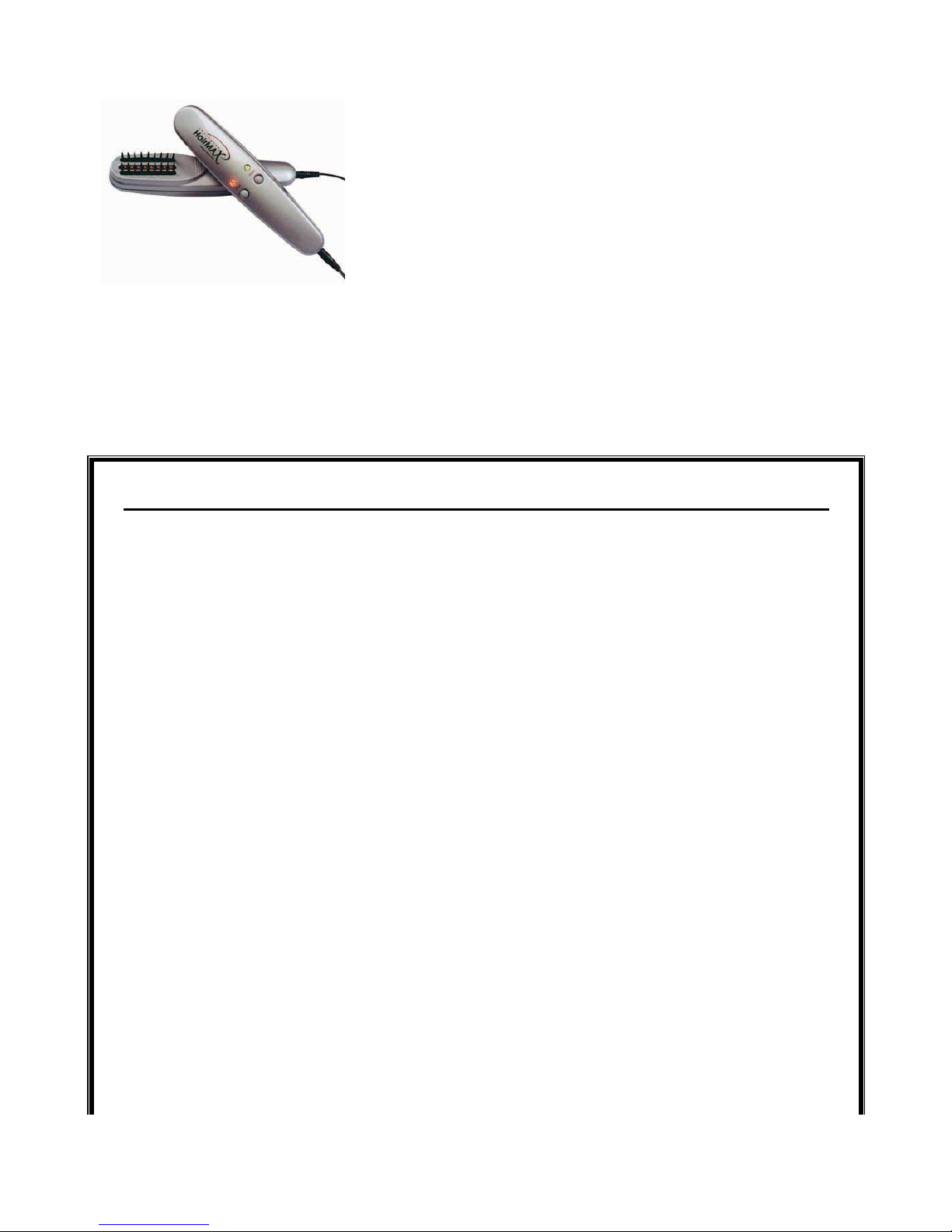
1 HairMax LaserComb® Premium
Ultimate performance and quality for maximum results
.
With the LaserComb Premium, experience real hair growth with minimal effort. Rest easy knowing you have
invested in a precision device, manufactured with the highest quality components and designed to last a
lifetime.
LaserComb Premium Features:
9 Laser Beams
10-15 Minute Treatment Time
10-15 Year Life Span
26 Week Money Back Guarantee (less 20% re-stocking fee)
Your Set Includes:
One HairMax LaserComb®
Premium Leather Travel Case
Worldwide AC Power Adapter 110-240 Volts
Second Set of Comb Teeth

User Manual
DVD or VHS instructional Video
Warranty Card
Quick Start Guide
6 months Money Back Guarantee if unsatisfactory results!
1 Year Product Warranty
2 HairMax LaserComb SE™ Compact
Unmatched LaserComb Technology. Unbeatable Value.
Discover the power of low-level laser therapy to help you grow new hair at an affordable price. The HairMax
LaserComb SE Compact delivers the effective power of laser energy at a reduced size and cost.
LaserComb SE Compact Features:
5 Laser Beams
15-20 Minute Treatment Time
5-7 Year Life Span
12 Week Money Back Guarantee
Your Set Includes:
One HairMax LaserComb SE™
Premium Leatherette Travel Case
Worldwide AC Power Adapter 110-240 Volts
User Manual
DVD or VHS instructional Video
Warranty Card
Quick Start Guide
12 Week Money Back Guarantee!
1 Year Warranty
2. FDA Clearance
I Breaking News
FDA Clears the HairMax LaserComb for the Promotion of Hair Growth
BOCA RATON, FL, February 15, 2007 – Lexington International LLC is proud to announce the landmark
achievement of US Food & Drug Administration (FDA) clearance for their medical laser device, the
HairMax LaserComb. Through years of extensive research and clinical studies in the science of hair growth,
Lexington have developed an affordable and convenient, hand-held laser device, clinically proven to promote
hair growth for men of all ages (Norwood IIA to V).
For the FDA submission, Lexington conducted an extensive clinical study in four different locations around
The United States. The study concluded that 93% of the participants (ages 30-60) using the HairMax
LaserComb had an increase in the number of terminal hairs. The average number of terminal hairs per square
centimeter increased by 19 hairs/cm² over a six-month period. During the study, there were not any reports of
serious adverse events. The number and types of adverse events were similar in both the active and placebo
groups.
David Michaels, Managing Director, shares his thoughts on this momentous announcement, “I believe the
results of this study are far more significant than any other product on the market today. We are very excited
about having reached a major milestone in hair care and science. Laser hair treatment is the talk of the hair
restoration industry and with our FDA clearance behind us, we are now soundly positioned to dominate this
emerging Laser treatment segment.”
It is estimated that 55 million men are suffering from hair loss in the United States alone. Until recently, the
FDA has only approved two other products as solutions to combat hair loss. The clearance of the HairMax
LaserComb offers an exciting new modality for the stimulation of hair growth in males. Compliance is a key
factor in the efficacy of any hair growth treatment. Fortunately, The HairMax LaserComb is very easy to use
in the comfort and convenience of your own home, treatments last 10 to 15 minutes three times per week.
The HairMax LaserComb is also the only natural, drug-free option available thus allowing many who were
previously unable or weary of drug based solutions new found hope in their battle against hair loss.
Dr. Matt Leavitt, Chief Medical Advisor to Lexington International, expresses his enthusiasm on news of the
clearance, “The HairMax LaserComb is a method of treatment that can be of great help to men of all ages
suffering from hair loss. I am impressed with the results from the clinical trials that Lexington has conducted
and believe the LaserComb will offer a third FDA cleared solution for hair growth. We can now deliver a
new confidence to our patients and gain strong customer acceptance.”
Randy Veliky, Lexington COO, comments, “The HairMax LaserComb will revolutionize the hair growth
industry, but we are not stopping there. Lexington is dedicated to continuing research into laser technology.
We will be expanding our offering of laser hair growth solutions to include freestanding clinical units,
affordable laser panel arrays for the home market and broadening the features of our current products.”
For over 20 years, Lexington has been at the forefront of laser hair growth research. Born from this passion
for innovation, The HairMax LaserComb uses a patented technology to part the hair allowing the focused
laser energy to reach the scalp.
The laser is most effective when it has unobstructed path directly to the hair follicle. Extensive research has
also allowed Lexington to determine the optimal wavelength and energy level to deliver maximum results.
Lexington’s continued dedication to consistent innovation ensures that users of users are always receiving the
latest in Laser hair technology. About Lexington International, LLC
Based in Boca Raton, Florida Lexington International manufactures and distributes the HairMax
LaserComb® (www.hairmax.com) to more than 80 countries and has provided new hope and satisfaction to
tens of thousands of customers since 2001. The HairMax LaserComb is based on 20 years of international
research and studies on the effects of Low Level Laser use. Both the press and users alike have universally
taken to the HairMax LaserComb. Coverage has included Dateline NBC, TIME Magazine, as one of their
“Inventions of the Year”, as well as the November 2004 issue of Good Housekeeping Magazine.
Lexington is a proud member of “Team Florida” and has participated in high-level, overseas trade missions
with Governor Jeb Bush. Additionally, the company was awarded by the U.S. Department of Commerce for
“Commercial and Export Achievement”.
II What it means to be FDA cleared
The FDA, US’s Food and Drug Administration, is the biggest medical clearance authority in the world. The
responsibility of the FDA is to determine if newly developed medical products are safe and effective.
Whether it is a prescription medication, a medication sold over the counter, a medical device, a vaccine, or
another type of biologic, the product can be marketed for general sale in the United States only if it has FDA
approval.
Certain claims about the medication, medical device and vaccination can only be made if it is FDA approved.
Hair Max has recently been FDA cleared. We can now make claims about regrowth, reduction of shedding or
increasing the strength of the hair in MEN ONLY (the device has not been approved for women yet so we
cannot make this claim for women at present how it is clinically proven and FDA submissions are in
progress).
• Improve the health and condition of hair
• Re-grow your hair

• Makes hair appear thicker, fuller healthier
• Reduce shedding
• Makes hair shinier, more manageable
• Improve blood flow or circulation
• Energize or stimulate follicles
• Improves appearance of hair
• Energizes scalp
• Users experience more “good hair” days
III Hairmax Clinical trials
Here you will find a summary of the clinical trial which lead to the approval by the FDA. Full report is also
available.
Final Clinical Study Report
A Randomized, Double-Blind Clinical Trial to Evaluate
the Safety and Efficacy of the Hairmax LaserComb
for the Treatment of Androgenetic Alopecia in Males
Conclusions
The results of this study showed that the HairMax LaserComb® was effective in the promotion of hair growth
and in the cessation of hair loss in males and females diagnosed with androgenetic alopecia when treatment is
applied 10-15 minutes three non-concurring days a week (i.e. Monday, Wednesday, Friday) for 26 weeks. The
results also demonstrated that the LaserComb is safe in these subjects.
Study Objectives
The primary aim of the study was to assess the safety and effectiveness of the HairMax LaserComb in the
promotion of hair growth and in the cessation of hair loss in males and females diagnosed with Androgenetic
alopecia.
Study Design
The study was designed as a multi center, randomized, placebo-controlled trial conducted at four sites in the
United States. Subjects who met all entry criteria were randomized in a 2:1 fashion to receive either the
HairMax LaserComb or an identical sham device that did not emit a laser. Subjects were to use the device
three times per week on non-concurring days for a total of 26 weeks. Hair density measurements were done at
baseline immediately prior to randomization and again at 26 weeks. Subjects who terminated prematurely
were to have had hair density measurements at their termination visit. Additional clinic visits were scheduled
at 8 and 16 weeks.
Subject Population and Demographics
The study population included males and females between the ages of 30 and 60 years with a diagnosis of
androgenetic alopecia who had been experiencing active hair loss within the last 12 months. They were also
required to have a Norwood-Hamilton classification of IIa to V (for males) or Ebling Rook I to III (for
 Loading...
Loading...