VitalWear VitalWrap System User Manual

VitalWrap™ System
Users Manual

Thank you for purchasing our VitalWrap System.
Dedicated to becoming the leader in durable medical
equipment, VitalWear, Inc. develops and markets
affordable thermal and compression therapy systems
for use in accelerating the recovery of anyone who has
experienced an injury and wants more control in their
personal treatment.
Please read this instruction manual completely prior to
using the unit to ensure reliable and safe operation.
VitalWrap System Introduction 2
VitalWrap System Specifications 3
Warranty 4
System Operation 5
Notes, Cautions And Warnings 6
Safety Symbols And Warnings 6
Unpackaging Information 8
Indications & Contraindications 8
Operating Instructions 11
Available VitalWraps for Your System 12
How To Use Your VitalWrap System 13
Wrapping Other Body Parts 14
Tubing Set Instructions for Use 19
Troubleshooting 19
Cleaning The Control Unit 21
Cleaning The Wrap 21
Storage 22
Notes 23
1

VitalWrap System Specifications
Physical
Size (approximately) 8”diameter x 13.5”H (20.3cm x 34.3cm)
Weight (dry) 5 lbs. (2.27 kg)
Single Tubing Set 10.5 ft. (3.2m)
Control System
Type Manual user control
Thermal System
Range 40°F to 105°F (4.4°C to 40.6°C)
Contrast therapy transition time 1 minute typical from extremes in temperature range
Operating period 2-6 hours typical continuous use
Thermal Cutout 45°C
Circulating System
Reservoir Capacity 1.1 gallons (4.1 liters)
Reservoir Fluid Ice water
Flow Rate (through VitalWrap) 5.5 gph (21 lph)
Maximum Pressure 18 psi
Electrical System
Voltage 120Vac, 60Hz, model VIT-00002 or 220-240Vac,
50Hz, model VIT-00123
Internally fused 120 Vac, 60Hz, 300W F1, F2: 125V, 4A
F3: 125V, 300mA
220-240Vac, 50Hz, 3.15A F1, F2: 250V, 2.5A
F3: 250V, 300mA
Leakage Current Under 300µA
Operating Environment
Atmospheric Pressure 525 to 795 mmHg
Humidity 30% to 70% relative non-condensing
Temperature 10°C to 40°C
Transport & Storage Environment
Atmospheric Pressure 179 to 760 mmHg
Humidity 10% to 95%
Temperature 0°C and +50°C
Regulatory
Classification Class I Equipment
Type of Equipment Type BF
Regulatory Approvals
Compliance UL2601-1: Standard for Medical Equipment
Part 1: General Requirements for Safety.
CAN/CSA-C22.2 no. 601.1-M90: Medical Electrical
Equipment Part 1: General Requirements for Medical
Electrical Equipment.
IEC 60601-1: Part 1: General Requirements
for Safety.
The Thermal Wrap System, Model VIT-00002 has also
been evaluated to and found to meet the requirements of
Clause 42, found in IEC 60601-2-35, Medical Electrical
Devices, Particular requirements for the safety of blankets, pads and mattresses, intended for heating in medical
use.
VITALWEAR™ and VITALWRAP™ are registered trade-
marks of VitalWear, Inc.
Covered by US Patents: 7,211,104 & 7,191,798
VitalWrap System Introduction
A significant number of Americans suffer from some type of
persistent or recurrent acute pain sufficient to significantly impact their
lives. The VitalWear VitalWrap System is a patented technology that applies
a unique combination of cooling, heating and compression to the human
body, providing a range of key benefits:
• Combination of compression, heating, moist heat, and/or cooling therapy
to the affected body part at any desired temperature between 40ºF and
105ºF,
• Compression via a proprietary VitalWrap providing for greater fluid
management in the injured area,
• Continuous thermal therapy to minimize tissue damage and scarring, to
reduce pain and discomfort, and to reduce dependency on pain medication,
• Variable and rapidly changeable temperature control allows for extended
application minimizing the risk of skin/nerve damage and for uniquely
beneficial treatments such as Contrast Therapy,
• Safe and effective for both the professional practitioner and individual
user,
• A portable injury therapy system that is affordable, easy to use at a clinic,
home or at work,
• Universal personal wraps convenient, familiar, and easy to apply to the
body, conforming comfortably to the major joints and muscles,
• Applications cover treatment of chronic pain or injury of back, knee, hip,
foot and ankle, hand and wrist, shoulder, elbow, chest, breast, face and
jaw, head, neck, and other specific applications.
Applications for the VitalWrap System:
Orthopedics and Obstetrics General Procedures
Chiropractics Chronic Joint Pain
Physical Therapy Emergency Medicine
Plastic and Reconstructive Procedures Arthritis
Sports Medicine/ Athletics
Pregnant Women and New Mothers
Designed to help reduce pain and swelling, the VitalWrap cooling, heating
and compression therapy system can be used from the earliest stages of
post-operative recovery, through rehabilitation, to postrecovery home use for
treatment of pain and residual swelling.
2
3
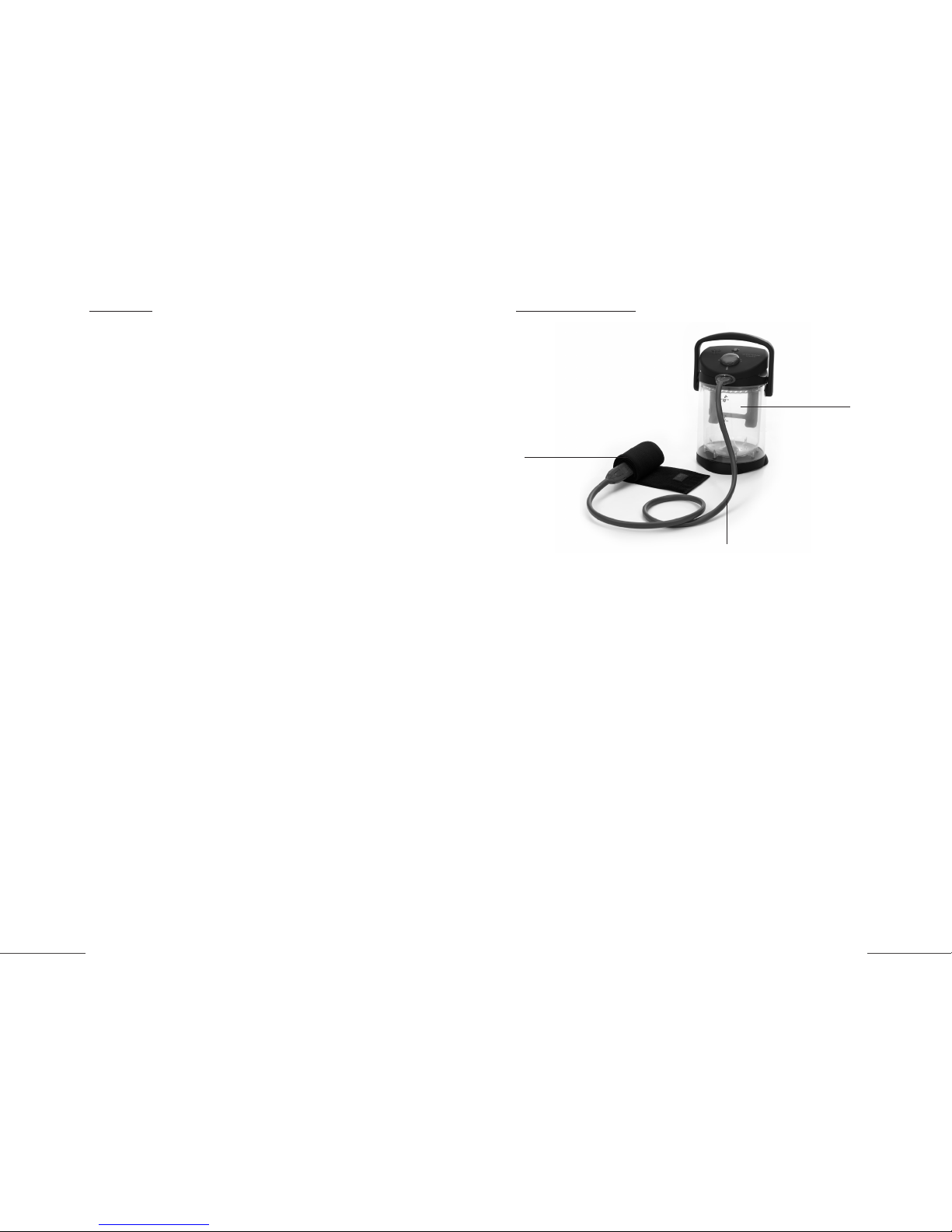
System Operation
The VitalWear VitalWrap System is comprised of a control unit, a tubing set
and a thermal wrap. This VitalWrap is the part of the system that you actually apply to the body to provide cooling and compression therapy. The wrap
can be applied to virtually any area of the body that can be treated with a
compressive bandage.
Setting up the system is a simple three-step process. (1) The user first fills
the portable, leak-proof container of the control unit with ice and water, (2)
then applies the VitalWrap around the injured area and secures it with its
Velcro™ closure, and (3) finally, attaches the control unit to the wrap via the
tubing set.
Once the control unit is turned on, cooled or warmed fluid is passed through
the tubing set from the control unit to the wrap. The fluid is then circulated
through the wrap, drawing heat from or applying to the body and deeply
cooling or heating the body part. The fluid is then passed back through the
tubing set to the control unit where it is re-cooled or re-warmed and then
re-circulated back to the wrap.
When using this system, please check the skin where the wrap is
applied periodically. Discontinue use if continued numbness, skin
discoloration, blisters, etc. are present. Please refer to your health
care professional prior to applying this system and/or using the
system for extended periods of time.
The benefit of compression can continue even if the user chooses to disconnect the tubing set from either the control unit or the wrap.
Warranty
VitalWear, Inc. guarantees this product free from defects in material and
workmanship for a period of 1 year from the date of purchase except as noted
below. This warranty extends only to the original retail purchaser.
This VitalWear product warranty does not cover damage caused by misuse or
abuse; accident; the attachment of any unauthorized accessory; alteration to
the product; or any other conditions whatsoever that are beyond the control
of VitalWear. VitalWear shall not be responsible for any type of incidental,
consequential or special damages. All implied warranties, including but not
limited to those implied warranties of fitness and merchantability, are limited
in total duration of 1 year from the original purchase date.
To obtain warranty service on your VitalWear VitalWrap System, either hand
deliver or mail the unit and your dated sales receipt (as proof of purchase)
to VitalWear, Inc. (384 Oyster Point Blvd., Suite 16, South San Francisco,
CA 94080-6523). Be sure to include the serial number of your unit and your
phone number on any correspondence. If you have any questions, please call
VitalWear Inc. at 1-800-553-4081.
Upon receipt, VitalWear will repair or replace, as appropriate, your VitalWrap
System and return it to you, postpaid. Warranty is solely through VitalWear
Service Center. Service of this product by anyone other than VitalWear
Service Center voids warranty.
This warranty provides you with specific legal rights. You may have additional
rights, which may vary from state to state. Because of individual state regulations, some of the above limitations and exclusions may not apply to you.
4
5
control unit
thermal wrap
tubing set

Periodically check skin where wrap is applied for skin discoloration, numbness, blisters, etc., which can indicate a potential burn.
If detected, discontinue use and consult a physician.
Close supervision is necessary when this appliance is used by,
on, or near children, invalids, or disabled persons. This appliance
should not be used by or on children without parental supervision.
Use this appliance only for its intended use as described in this
manual. DO NOT use attachments not recommended by the manufacturer.
Never operate this appliance if it has a damaged cord or plug, if
it is not working properly, if it has been dropped or damaged, or
dropped into water.
Type BF Applied part complying with IEC 60601-1 and UL 60601-1
to provide protection against electric shock.
Alternating current.
“ON” (power: connection to the mains).
“OFF” (power: disconnection to the mains).
Notes, Cautions And Warnings
Notes, cautions and warnings are used to highlight certain operating procedures
and recommendations. A Note indicates a special procedure, an exception to normal operation or something else of specific interest to the reader. Notes are preceded
by the word “Note” in italics.
The Caution or Warning symbol precedes an operational step that could
damage the instrument if the user does not take certain precautions.
Cautions or Warnings are located in the main text, are preceded by a
Caution or Warning statement and are accompanied by this symbol in
the left margin.
Caution: risk of electric shock.
Important Safety Instructions
Read all instructions before using.
This equipment has been tested and found to comply with the limits for medical
devices to the IEC 60601-1.
Follow local governing ordinances and recycling plans regarding disposal or recycling of device components.
Grounding reliability can only be achieved when the equipment is connected to an
equivalent receptacle marked ‘hospital only’ or ‘hospital grade’.
When using an electrical appliance, especially when children are present, basic
safety precautions should always be followed.
Safety Symbols And Warnings
DANGER –To reduce the risk of electric shock:
1. Always unplug this appliance from the electrical outlet immediately
after using and before cleaning.
2. DO NOT use while bathing or in a shower.
3. DO NOT place or store appliance where it can fall or be pulled into a
tub or sink. DO NOT place in or drop into water or other liquid.
4. DO NOT reach for a product that has fallen into water. Unplug immediately.
An appliance should never be left unattended when plugged in. Unplug
from outlet when not in use, and before putting on or taking off parts.
6
7
1. Ensure the top of the appliance is properly installed and the handle is fully
engaged prior to carrying the appliance.
2. DO NOT carry this appliance by supply cord or use cord as a handle.
3. Keep the cord away from heated surfaces.
4. Never operate the appliance without the Fluid Canister, the Top
Assembly, the Tubing Set, and the Wrap completely connected.
5. Never operate the appliance with any foreign objects in the Fluid Canister.
6. Never drop or insert any object into any opening.
7. To disconnect, turn off power (“O” position), then remove plug from outlet.
8. Use cooling surfaces carefully. Do not use over insensitive skin areas or in
the presence of poor circulation. The unattended use of cooling by children
or incapacitated persons may be dangerous. Consult your physician.
9. DO NOT stand on or in the appliance.
10. Do not use while sleeping or drowsy.
11. Do not place the appliance or operate the appliance while it is on a surface
more than 3 feet above the floor.
12. Unplug this product before filling. Fill with water and ice only.
Do not overfill.
13. Do not operate without water.
14. Do not operate with hot water.
~
 Loading...
Loading...