Page 1

CorVitals, Distributor
IFU-02 Rev. J Page ! of ! 1 17
DCO-M-1556 | Date: 06Mar2018
Page 2

CorVitals, Distributor
Device Description
The VitalPatch® device (VitalConnect Sensor) is a component of the VitalConnect Platform. The
VitalPatch device is a wireless, battery-operated wearable biosensor, worn on the torso to record heart
rate, electrocardiography (ECG), heart rate variability, R-R interval, respiratory rate, skin temperature, fall
detection, activity (including step count) and posture (body position relative to gravity including fall
detection). The VitalPatch device continuously gathers physiological data from the person being
monitored and then transmits encrypted data via bi-directional communication to the Relay device when
in range of the Relay device. The encrypted wireless data provided by the VitalPatch device may be
downloaded from the Relay device for storage, or integrated into a Third-Party Relay Application via the
APIs of the Relay Software Library. Additionally, wireless data may be transferred to and stored on an
optional Secure Server for future analysis if there is an active server connection. The data provided by the
VitalPatch device is intended to aid caregivers in making diagnoses by providing additional information to
standard of care patient monitors.
During normal operation, data is collected by the VitalPatch device and transmitted immediately to the
Relay device. A continuous connection is needed between the VitalPatch device and the Relay device in
order to facilitate continuous data transmission. The continuous wireless transmission of data occurs with
a latency of seconds between data collection and transmission.
Indications for Use
The VitalConnect Platform is a wireless remote monitoring system intended for use by healthcare
professionals for continuous collection of physiological data in home and healthcare settings. This can
include heart rate, electrocardiography (ECG), heart rate variability, R-R interval, respiratory rate, skin
temperature, activity (including step count), and posture (body position relative to gravity including fall).
Data are transmitted wirelessly from the VitalConnect Sensor for storage and analysis. The VitalConnect
Platform can include the ability to notify healthcare professionals when physiological data fall outside
selected parameters.
The device is intended for use on general care patients who are 18 years of age or older as a general
patient monitor, to provide physiological information. The data from the VitalConnect Platform are
intended for use by healthcare professionals as an aid to diagnosis and treatment. The device is not
intended for use on critical care patients.
Contraindications
•
The VitalPatch device is not intended for use on users who have implanted defibrillators or
pacemakers.
•
The VitalPatch device is not intended as a stand-alone diagnostic monitor, but the data may be
applicable for use in diagnosis.
Warnings
•
The VitalPatch device is a secondary, adjunct patient monitor and is not intended to replace existing
standard-of-care patient monitoring practices.
•
Depending on wireless connectivity, a temporary interruption of data transmission is possible, which
may impact continuous or real-time monitoring. Data will be stored on the device for transfer once
connectivity is reestablished.
IFU-02 Rev. J Page ! of ! 2 17
DCO-M-1556 | Date: 06Mar2018
Page 3

CorVitals, Distributor
•
The nature of hydrocolloid adhesives may cause adverse skin reactions. Healthcare providers should
advise patients to seek medical attention if either of the following occurs:
o
A severe adverse event
o
An allergic reaction persisting beyond 2-3 days
•
Histories of skin irritations should be considered before placing the VitalPatch device on a patient.
•
Do not use the VitalPatch device during an MRI scan or in a location where it will be exposed to
strong electromagnetic forces.
•
Only place the VitalPatch device on intact skin.
•
Clinical validation has not been performed on patients who are pregnant or breastfeeding.
Precautions
•
For data to be sent to a healthcare professional for review:
o
The VitalPatch device must be properly adhered to the patient.
o
The patient must remain in range of their Relay device.
o
The VitalPatch device must have adequate power for data transmission. Notification of the
VitalPatch device battery level will indicate when the battery power is low.
o
The Relay device must remain charged and functional for data transmission. Wireless
connectivity must be active for transmission of data from the Relay device to the server.
•
If uninterrupted continuous data monitoring is necessary for patient safety, remote monitoring in home
settings using the VitalPatch device may not be appropriate.
•
Data collected by the VitalPatch device for patients experiencing cardiac arrhythmia may indicate
slightly higher or lower respiratory rate values, compared to visual observation, for the duration of the
active arrhythmic episode.
•
The VitalPatch device is Single Use Only. Do not reapply the device once it is removed.
•
Wireless electronic devices may cause signal interference during data transmission. Avoid close
proximity with interfering devices.
•
Medical electrical equipment or electrical stimulators attached to the patient’s body may degrade
VitalPatch signal quality or produce erroneous results from the biosensor. The potential interaction
must be evaluated and authorized by the responsible organization.
•
Do not use the VitalPatch device if the package has been opened, or appears used, damaged, or
expired.
•
The VitalPatch device may be used while showering. Minimize exposure directly under the shower
head, excessive contact with soap, or scrubbing. Gently dry the device after showering. Do not
submerge the device or use in a sauna.
•
Wear only one VitalPatch device at a time.
•
If discomfort or irritation occurs, the VitalPatch device should be removed. If mild soreness or redness
is experienced after removing the device, do not apply a new device in the same location. Choose
another recommended location.
IFU-02 Rev. J Page ! of ! 3 17
DCO-M-1556 | Date: 06Mar2018
Page 4
CorVitals, Distributor
•
Incorrect handling, excessive force, or dropping the VitalPatch device may cause malfunction or
permanent damage.
•
Keep the VitalPatch device away from children and pets. The device may be a choking hazard, and
may be harmful if swallowed.
•
If VitalPatch fails to operate, contact your healthcare provider immediately.
•
Dispose of the VitalPatch device per local laws, care facility laws or hospital laws for routine/nonhazardous electronic waste.
Product Storage
•
Storage temperature range: 0 – 40o C
•
Storage relative humidity range: 10 – 95% RH
System Interoperability
The VitalPatch device is compatible with Relay devices and software developed with the VitalConnect
Application Programming Interface (API). Please contact VitalConnect, Inc. to obtain implementation
information, including the MAN-001, VitalConnect Platform Integration Manual – Developer Guide.
VitalPatch Operating Instructions
Note: It is recommended that healthcare providers advise users to replace the VitalPatch device after 120
hours (5 days) of use. To preserve data, the VitalPatch device must be connected to the Relay device
prior to the end of battery life (120 hours). The device will no longer be usable after 120 hours.
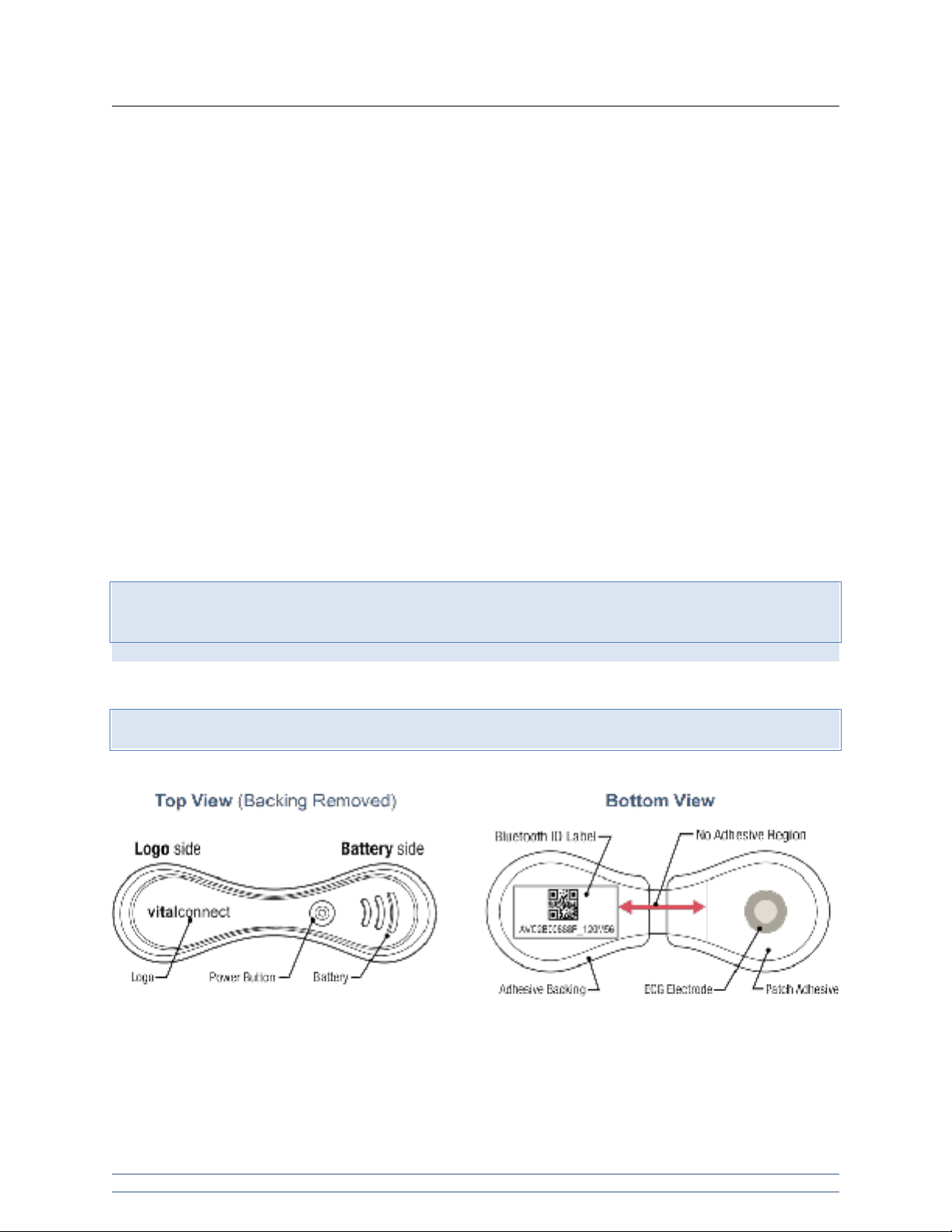
VitalPatch Overview
Note: Orientation of the Logo Side and Battery Side are important when placing the device on the patient.
See image below for a view of the VitalPatch device, showing the logo side and battery side.
!
Product Handling
Ensure hands are clean and dry before handling the VitalPatch device. Gloves are recommended when
handling the device.
IFU-02 Rev. J Page ! of ! 4 17
DCO-M-1556 | Date: 06Mar2018
Page 5
CorVitals, Distributor
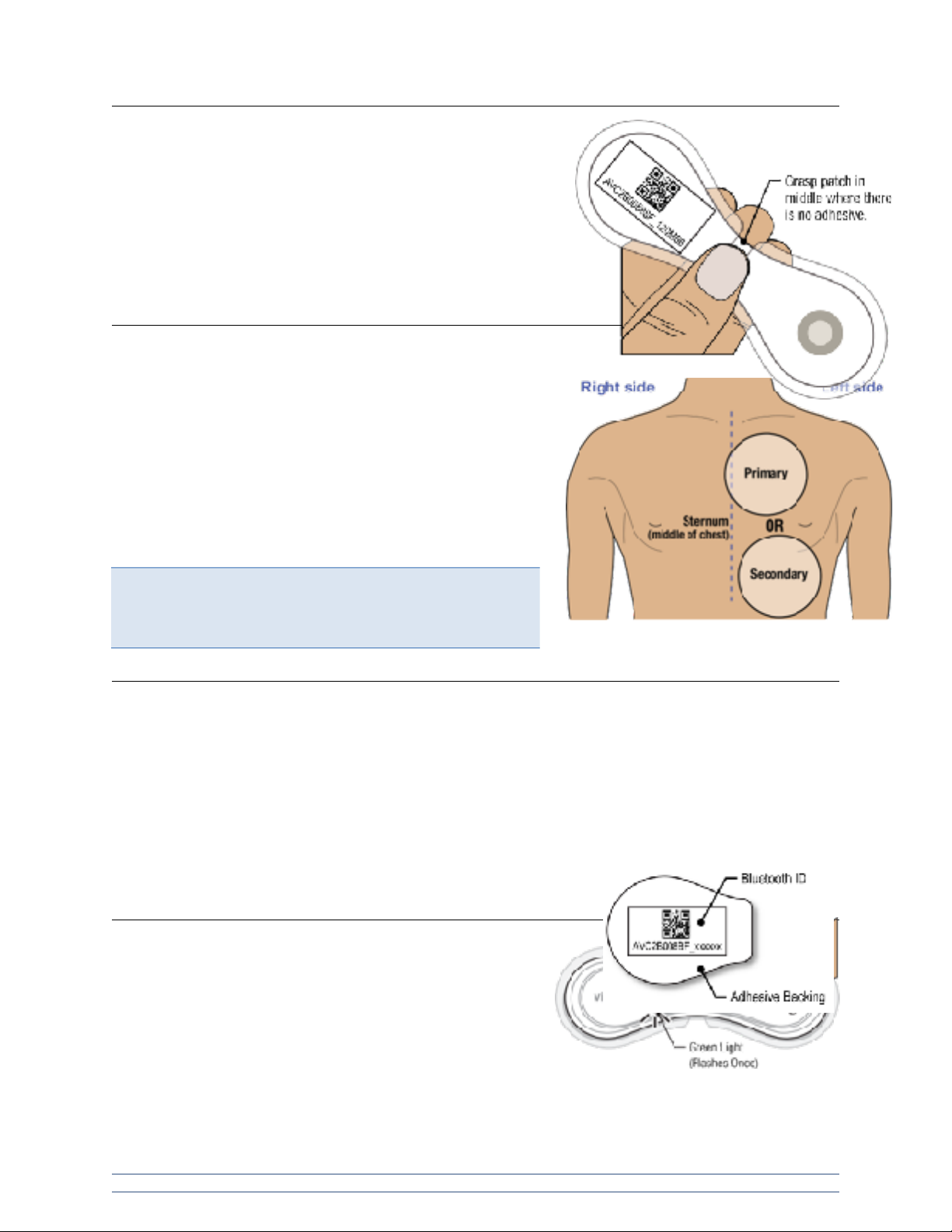
When handling the VitalPatch device, do not touch the adhesive.
The steps below should minimize the chance of touching the
adhesive. If the liners have been removed it is best to hold the
device in the center with your thumb and fingers. Contact with the
adhesive prior to application to the patient will deteriorate the
adhesive and compromise wear duration. See image to the right.
Skin Preparation and Application
Step 1: Prepare skin.
The primary application site is located on the upper left chest. If
the device cannot be placed on the primary application site, use
the secondary application site instead. The secondary
application site is located just left of the centerline, below the
chest on the rib cage. For a good connection and proper
operation, the VitalPatch device should NOT be worn over areas
with a high concentration of body hair. Remove body hair in the
area of device placement before applying the device. See image
to the right.
Step 2: Remove VitalPatch from pouch.
Tear open the pouch using the notch mark and remove the VitalPatch device carefully, to avoid pressing
the Power Button.
Retain the pouch or the adhesive backing with the device Bluetooth ID number. You will need this
information to connect to your software application after the VitalPatch device is applied to the patient.
The Bluetooth ID number can be found on the pouch label or on the adhesive backing in both human
readable and barcode formats. See image to the right.
Step 3: Power-on VitalPatch.
Locate and press the Power Button. Look for a green light
illuminating temporarily to confirm the device is powered on.
See image to the right.
IFU-02 Rev. J Page ! of ! 5 17
DCO-M-1556 | Date: 06Mar2018
Note: For all patients, use an alcohol wipe to clean skin where
the entire device will contact skin and allow site to dry. The
application site should be free of oils and lotions to maximize
adhesion.
Page 6
CorVitals, Distributor
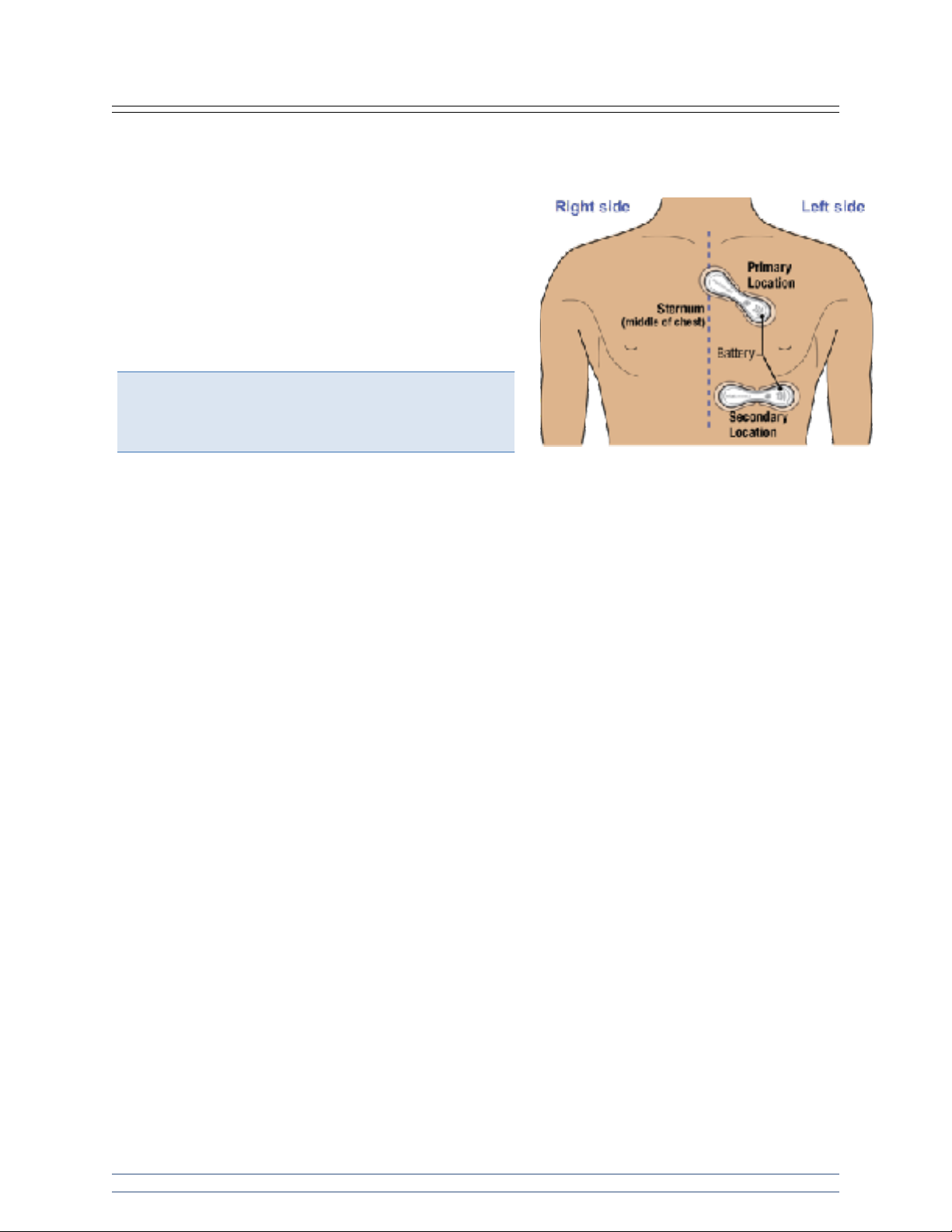
Step 4: Position VitalPatch on the body.
With the adhesive backings still adhered, place the VitalPatch device on a flat body surface on the left
chest with one electrode two fingers below the suprasternal (jugular) notch, and angled diagonally toward
the heart. The exact angle is not critical; it is more important
to locate the flattest surface of the chest for the VitalPatch
device placement in order to minimize movement during the
monitoring session. The battery side of the VitalPatch device
should be pointed to the left side of the patient's chest. See
image to the right.
If the device cannot be placed in the Primary Location use
the Secondary Location instead. The Secondary Location is located just left of the centerline, below the
chest on the rib cage, positioned horizontally in an area with minimal body curvature. This location is not
recommended for obese persons.
IFU-02 Rev. J Page ! of ! 6 17
DCO-M-1556 | Date: 06Mar2018
Note: The VitalConnect logo should be oriented such that
it is readable by someone facing the patient when it is
applied. The battery will be closest to the left side of the
chest.
Page 7

CorVitals, Distributor
Step 5: Apply VitalPatch to body.
Hold one end of the VitalPatch against the chest. Lift the other
side and grab the adhesive backing tab located near the center
of the VitalPatch. Without touching the adhesive, pull the tab to
remove the adhesive backing and press the VitalPatch down to
apply. Repeat this process to apply the other side of the
VitalPatch.
Press down on both ends of the device to ensure it is well
adhered to skin. Avoid exercise for at least 30 minutes after
application.
Step 6: Connect VitalPatch.
Refer to your software application provider’s user manual for more instructions on how to connect to the
VitalPatch device. A connection is required to establish a start time in the data file. For calibrating your
VitalPatch device, refer to your software application provider’s user manual.
Should the software application indicate that a “Patch off” event has been detected but the patch has not
been removed – check if the patch has lifted from the skin. If it has noticeably lifted, remove the patch and
replace with a new one following the Skin Preparation and Application steps described previously.
Additionally, if multiple “Patch Off” notifications are received in a short period of time, remove the patch
and replace with a new one.
VitalPatch Removal and Disposal
Disconnect the VitalPatch device according to your software application provider’s user manual prior to
removing the device from the patient.
IFU-02 Rev. J Page ! of ! 7 17
DCO-M-1556 | Date: 06Mar2018
Page 8

CorVitals, Distributor
When removing the VitalPatch device, use of an adhesive
tape remover is recommended. Gently sweep the remover
pad under the device and pull away from skin. See image to
the right.
Please observe local laws for disposal of battery-operated electronic products.
Troubleshooting
For issues related to a user interface application, refer to separate instructions for use for the interface
and for additional troubleshooting guidance.
IFU-02 Rev. J Page ! of ! 8 17
DCO-M-1556 | Date: 06Mar2018
Note: The VitalPatch device is Single Use Only. Do not
reapply the device once it is removed.
Note: If the VitalPatch device has not been disconnected
prior to removal, it will continue to generate and stream
data until it is disconnected.
Page 9

CorVitals, Distributor
Contact Information
VitalConnect, Inc. !
224 Airport Parkway, Suite 300!
San Jose, CA 95110 USA!
Phone: (408) 963-4600 !
www.vitalconnect.com
CorVitals, Inc. !
990 Stewart Ave.
Suite LL45A
Garden City, NY, 11530
USA
Tel: (888) 401.9998
Fax: (800) 559-3413
IFU-02 Rev. J Page ! of ! 9 17
DCO-M-1556 | Date: 06Mar2018
Page 10

CorVitals, Distributor
Product Specifications
IFU-02 Rev. J Page ! of ! 10 17
DCO-M-1556 | Date: 06Mar2018
Page 11

CorVitals, Distributor
Electromagnetic Emission Declaration
The VitalPatch device is intended for use in the electromagnetic environment specified below. The end user of the
device should assure that it is used in such an environment.
Measurements
ECG Dynamic Range
-10mV to +10mV
Heart Rate (stationary
and ambulatory)
30 – 200 Beats per Minute (<±5 or 10% Beats per Minute,
whichever is greater)
Respiration Rate
o
10-30 Breaths per Minute with a mean absolute error of less
than 3 Breaths per Minute, validated by clinical studies
o
4-42 Breaths per Minute with a mean absolute error of less
than 1.5 Breaths per Minute, validated by simulation studies
Skin Temperature
150C – 500C (≤± 0.30C )
Fall Detection
Fall or No Fall (> 90% Sensitivity and >98% Specificity)
Step Count
< 5% Absolute Error Compared to Manual Count
Step count is reset to 0 after step count 65535 is reached.
Posture Detection
Lying down, Upright, Walking, Running, or Leaning (>70% Accuracy
Compared to Visual)
Communications
Bluetooth (BT4.1)
Max. 10 Meters (30 Feet Line of Sight)
Radio Modulation
FSK (Frequency Shift Keying)
Radio Frequency
2.4 – 2.5GHz
Transmit power
≤10dbm
Security
AES-CCM 128 Bit Encryption (Advanced Encryption Standard-CCM
mode)
Battery
Battery Type
Zinc Air
Battery Voltage
DC 1.4V
Battery Life
120 Hours
Operating
Conditions
Ambient Temperature
10 – 40 o C
Humidity
10 – 95% RH
Altitude
<3000 m
Barometric Pressure
70 kPa to 102 kPa
Emission test
Compliance
Electromagnetic environment
RF emissions
CISPR 11
Group 1
The VitalPatch device uses RF energy only for its internal function.
Therefore, its RF emissions are very low and are not likely to cause
any interference in nearby electronic equipment.
RF emissions
CISPR 11
Class B
The VitalPatch device is suitable for use in all establishments,
including domestic establishments and those directly connected to
the public low-voltage power supply network that supplies buildings
used for domestic purposes.
IFU-02 Rev. J Page ! of ! 11 17
DCO-M-1556 | Date: 06Mar2018
Page 12

CorVitals, Distributor
FCC Compliance (FCC ID:SPO-VCI-VP2)
The VitalPatch device complies with part 15 of the FCC Rules. Operation is subject to the following two conditions:
(1) This device may not cause harmful interference, and !
(2) This device must accept any interference received, including interference that may cause undesired operation
(FCC Title 47, Subpart A, Part 15.19(3)).
Changes or modifications not expressly approved by the party responsible for compliance could void the user’s
authority to operate the equipment (FCC Title 47, Subpart A, Part 15.21)
Note: This equipment has been tested and found to comply with the limits for a Class B digital device, pursuant to
part 15 of the FCC Rules. These limits are designed to provide reasonable protection against harmful interference in
a residential installation. This equipment generates uses and can radiate radio frequency energy and, if not installed
and used in accordance with the instructions, may cause harmful interference to radio communications. However,
there is no guarantee that interference will not occur in a particular installation. If this equipment does cause harmful
interference to radio or television reception, which can be determined by turning the equipment off and on, the user is
encouraged to try to correct the interference by one or more of the following measures (FCC Title 47, Subpart B, Part
15.105(b)):
•
Reorient or relocate the receiving antenna.
•
Increase the separation between the equipment and receiver.
•
Connect the equipment into an outlet on a circuit different from that to which the receiver is connected.
•
Consult the dealer or an experienced radio/TV technician for help.
Canada License-exempt (IC ID:11013A-VCIVP2)
The device complies with Industry Canada license-exempt RSS standard(s). Operation is subject to the following two
conditions: (1) this device may not cause interference, and (2) this device must accept any interference, including
interference that may cause undesired operation of the device.
Le présent appareil est conforme aux CNR dʼIndustrie Canada applicables aux appareils radio exempts de licence.
Lʼexploitation est autorisée aux deux conditions suivantes: (1) lʼappareil ne doit pas produire de brouillage, et (2)
lʼutilisateur de lʼappareil doit accepter tout brouillage radioélectrique subi, même si le brouillage est susceptible dʼen
compromettre le fonctionnement.
Guidance and Declaration – Electromagnetic Immunity!
(For ME equipment ME system that are not life-supporting)
The VitalPatch device is intended for use in the electromagnetic environment specified below. The end user of the
device should assure that it is used in such an environment.
Immunity test
IEC 60601
test level
Compliance
level
Electromagnetic environment- guidance
IFU-02 Rev. J Page ! of ! 12 17
DCO-M-1556 | Date: 06Mar2018
Page 13

CorVitals, Distributor
Note 1: At 80 MHz and 800 MHz, the higher frequency range applies. Note 2: These guidelines may not apply in all
situations. Electromagnetic propagation is affected by absorption and reflection from structures, objects, and people.!
Note 3: UT is the a.c. mains voltage prior to application of the test level. (a) Field strengths from fixed transmitters,
such as base stations for radio (cellular/cordless) telephones and land mobile radios, amateur radio, AM and FM
radio broadcast and TV broadcast cannot be predicted theoretically with accuracy. To assess the electromagnetic
environment due to fixed RF transmitters, an electromagnetic site survey should be considered. If the measured field
strength in the location in which the device is used exceeds the applicable RF compliance level above, the device
should be observed to verify normal operation. If abnormal performance is observed, additional measures may be
necessary, such as re-orienting or relocating the device. (b) Over the frequency range 150 kHz to 80 MHz, field
strengths should be less than 3 V/m.
Recommended separation distance between portable and mobile RF
communications equipment and VitalPatch!
(For ME equipment ME system that are not life-supporting)
The VitalPatch device is intended for use in the electromagnetic environment in which radiated RF disturbances are
controlled. The end user of the device can help prevent electromagnetic interference by maintaining a minimum
distance between portable and mobile RF communications equipment (transmitters) and the VitalPatch device as
recommended below, according to the maximum output power of the communications equipment.
Radiated RF
IEC 61000-4-3
10 V/m
80 MHz to
2.5 GHz
10 V/m
Portable and mobile RF communications equipment should be
used no closer to any part of the VitalPatch device than the
recommended separation distance calculated from the equation
applicable to the frequency of the transmitter. Recommended
separation distance:
80 MHz to 800 MHz
800MHz to 2.5 GHz
where P is the maximum output power rating of the transmitter in
watts (W) according to the transmitter manufacturer and d is the
recommended separation distance in meters (m).
Field strengths from fixed RF transmitters, as determined by an
electromagnetic site surveya should be less than the compliance
level in each frequency range b.
Interference may occur in the vicinity of equipment marked with
the following symbol:
Electrostatic
discharge (ESD)
IEC 61000-4-2
± 8 kV
contact
± 15 kV air
± 8 kV
contact
± 15 kV air
Floors should be wood, concrete, or ceramic tile. If floors are
covered with synthetic material, the relative humidity should be at
least 30 %.
Power frequency
(50/60 Hz)
magnetic field
IEC 61000-4-8
30 A/m
30 A/m
Power frequency magnetic fields should be at levels
characteristic of a typical location in a typical commercial or
hospital environment.
Rated maximum
output power of
transmitter (W)
Separation distance according to frequency of transmitter (m)
80 MHz to 800 MHz
800 MHz to 2.5 GHz
0.01
0.17
0.23
0.1
0.37
0.74
1
1.17
2.33
10
3.69
7.38
IFU-02 Rev. J Page ! of ! 13 17
DCO-M-1556 | Date: 06Mar2018
Page 14

CorVitals, Distributor
For transmitters rated at a maximum output power not listed above, the recommended separation distance d in
meters (m) can be estimated using the equation applicable to the frequency of the transmitter, where P is the
maximum output power rating of the transmitter in watts (W) according to the transmitter manufacturer.!
The VitalPatch device complies with the applicable requirements and relevant provisions of the Radio Equipment
Directive 2014/53/EU (RED). Note 1: At 80 MHz and 800 MHz, the separation distance for the higher frequency
range applies. Note 2: These guidelines may not apply in all situations. Electromagnetic propagation is affected by
absorption and reflection from structures, objects, and people
100
11. 67
23.33
IFU-02 Rev. J Page ! of ! 14 17
DCO-M-1556 | Date: 06Mar2018
Page 15

CorVitals, Distributor
General Symbols
IFU-02 Rev. J Page ! of ! 15 17
DCO-M-1556 | Date: 06Mar2018
Page 16

CorVitals, Distributor
Symbol
Title
Symbol
Title
Protected against splashing water
Protected against submerging in
water (up to 1 meter for 30 minutes)
Re-use is not allowed
Read usage instructions
Properly dispose of EEE
(Electrical and Electronic
Equipment)
Non-ionizing radiation
Defibrillation proof type CF
applied part
MR Unsafe
CE Marking conformity
Manufacturer
Caution, consult documents
Not to be used in case package is
damaged
Prescription only
Authorized Representative in the
European Community
Catalogue number
Batch code
Use by date
Temperature limits (Storage)
Humidity limits (Storage)
Contents (Numeral represents
quantity of units inside)
Underwriters Laboratories!
MEDICAL — PATIENT MONITORING EQUIPMENT AS TO ELECTRICAL SHOCK, FIRE AND
MECHANICAL HAZARDS ONLY IN ACCORDANCE WITH ANSI/AAMI ES60601-1 (2005),
"Medical Electrical Equipment - Part 1: General Requirements for Basic Safety and Essential
Performance; CAN/CSA-C22.2 No. 60601-1:08; ANSI/AAMI/IEC 60601-2-25, "Medical
Electrical Equipment - Part 2-25: Particular Requirements for the Basic Safety and Essential
Performance of Electrocardiographs" E358758
!
!
!
!
!
!
!
!
!
!
!
!
!
!
!
!
!
!
!
!
!
IFU-02 Rev. J Page ! of ! 16 17
DCO-M-1556 | Date: 06Mar2018
Page 17

CorVitals, Distributor
IFU-02 Rev. J Page ! of ! 17 17
DCO-M-1556 | Date: 06Mar2018
 Loading...
Loading...