Vita Course Technologies DISCOVERY User Manual

Non-invasive Hemodynamic Blood
Pressure Monitor
Discovery 1
User Manual
Vita-Course Technologies Co., Ltd.

User Notes:
Thank you for using the Non-invasive Hemodynamic Blood Pressure Monitor.
Please read the instruction manual carefully before using the product.
Vita-Course shall not take corresponding responsibility for any abnormality or any personal or
machine damage caused by the irregular use, maintenance and reposition without the
guidance of the instruction, and shall not provide free maintenance for such failures.
This manual is only applicable to Discovery 1 type Non-invasive Hemodynamic Blood Pressure
Monitor (hereinafter referred to as "equipment")
Contraindications: unclear

Copyright and Disclaimer
The copyright of the instruction belongs to Vita-Course Technologies Co., Ltd. (hereafter
referred to as “Vita-Course Technologies”). All rights reserved. No unit or individual could
copy or disseminate any part or all of the instruction without prior written permission in any
form or by any means. Otherwise, the legal liability will be held accountable.
Vita-Course Technologies has the final interpretation of the instruction and reserves the right
to upgrade and alter specifications as well as contents of the products. If there are upgrade and
change, we won’t track users’ upgrade.
Vita-Course Technologies tries its best to make contents of the instruction be complete and
accurate, but cannot guarantee that the instruction is free from any errors or omissions.
Vita-Course Technologies shall not bear any responsibility for any consequences arising from
the irregular execution of the contents of this instruction.
All the copyrights, trademarks, patent applications and patent rights that may arise from the
instruction or relate to the products of Vita-Course Technologies are owned by Vita-Course
Technologies. Without the written permission agreement, this instruction does not grant any
license of any of the above rights and the instruction itself cannot be used as a license
agreement to authorize any of the above rights.
If other units or individuals, without the written permission, use or disseminate the contents of
the instruction without authorization, which accordingly affects the interests of themselves or
the third parties, Vita-Course Technologies shall not shoulder any responsibility
File Updating
This instruction was finally revised by Vita-Course Technologies on February 22, 2017.
1. Before the use of the equipment, users need to check the equipment so as to ensure its safe use
and normal work.
2. Do not use the equipment in these places full of the flammable gas and explosion.
3. Allergic reactions may occur in very few other users. If it happens, please stop using the
equipment immediately.
4. When users connect other equipment to their bodies, please be cautious: the total leakage
current may exceed the permissible limitation, causing potential damage to the user.
5. Equipment accessories, such as pulse wave probes and cables whose materials have been tested
for biocompatibility, cannot be replaced at will. Please use the accessories provided by our
company in case it may lead to some adverse consequences related to safety and biocompatibility
etc.
6. All the connections of applications should be away from the user's neck so as not to choke the
user's neck.
7. Before carrying out maintenance, please turn off the equipment.
8. The scrap of this equipment and parts shall comply with local laws and regulations.
9. This equipment belongs to professional medical equipment, and only the professionals
appointed by the factory are responsible for the maintenance.
10. Apply to those who are 16~70 years old.
11. Please close the equipment and evacuate the sensor during magnetic resonance imaging MRI
scanning; if not, it may cause burns or affect the accuracy of MRI images as well as equipment.
12. Please make sure children can't touch it when you keep it.
13. Please do not use the high frequency operation equipment at the same time so as not to affect
the measurement accuracy of the equipment.
Warning
Safety Announcements:
Warning: The patient or the operator may die or be seriously injured when the equipment
is operated mistakenly.
Caution :The damage may happen to people or equipment when the equipment is
employed incorrectly.
Note:Important tips on operation and use

1. Please keep and use the equipment within the regular range of temperature, humidity and
atmospheric pressure; if not, it would lead to damage of the equipment and inaccurate measurement
results.
2. Please do not directly open the equipment after it is affected with damp to avoid damaging the
equipment, and use it after it is dried.
3. The equipment is limited to one user at the same time.
4. Please be away from equipment with strong electromagnetic interference, such as microwave
ovens, large printers, induction cooker, etc. when using it.
5. When using high frequency surgical equipment, close the equipment and evacuate the sensor.
1. When common arrhythmias, such as atrial fibrillation, premature ventricular fibrillation, and
atrial fibrillation, occur, they may affect the accuracy of output results.
2. Professionals shall interpret the measuring results of the equipment.
3. The value of blood pressure measured by this equipment is equivalent to that measured by
auscultation, and the error is in line with the requirements specified in YY 0667-2008.
4. If those who are equipped with heart pacemaker, a stent and beyond the measurement range
use the equipment, the accuracy of the output results will be affected.
5. Please remove your callus of your hands and nail polish before using the equipment.
6. Check the battery power after each use. When the battery is less than 1 cell, you must charge the
battery, and ensure sufficient storage of electricity.
7. Please stop using the equipment if there is any indication of malfunction.
8. Signal input and output interfaces can only be connected to the required devices.
Caution
Note

Table of Content
Chapter 1 Summary ......................................................................................................................................... 1
1.1 Characteristics of products .................................................................................................................................................... 1
1.2 Intended Use/Instruction for use .......................................................................................................................................... 1
1.3 The scope of products ........................................................................................................................................................... 1
1.4 Structure ............................................................................................................................................................................... 1
1.5 Product classification ............................................................................................................................................................ 1
1.6 Operating environment ......................................................................................................................................................... 2
1.7 Safety .................................................................................................................................................................................... 2
Chapter 2 Wearing and Connecting ...................................................................................................................... 3
2.1 Out of box audit .................................................................................................................................................................... 3
2.2 Appearance presentation ...................................................................................................................................................... 3
2.3 Marks .................................................................................................................................................................................... 4
2.4 Wearing ................................................................................................................................................................................. 4
Chapter 3 Interface Introduction and Operation .................................................................................................. 7
3.1 Boot ....................................................................................................................................................................................... 7
3.2 Connecting APP ..................................................................................................................................................................... 7
3.3 Calibration ............................................................................................................................................................................. 8
3.4 Measurement ........................................................................................................................................................................ 9
3.5 Measuring result ................................................................................................................................................................... 9
3.6 Prompt in measurement ..................................................................................................................................................... 10
3.7 Battery level ........................................................................................................................................................................ 11
Chapter 4 Structural Features and Working Principle .......................................................................................... 13
4.1. Overall structure framework chart ..................................................................................................................................... 13
4.2 Principle of blood pressure measurement .......................................................................................................................... 13
Chapter 5 Technical Characteristics ..................................................................................................................... 14
5.1 Blood pressure .................................................................................................................................................................... 14
5.2 Pulse wave ........................................................................................................................................................................... 14
5.3 Size and weight ................................................................................................................................................................... 14
Chapter 6 Maintenance, Preservation, Storage and Transportation .................................................................... 15
6.1. Cleaning of equipment ....................................................................................................................................................... 15
6.2 Cleaning of accessories ....................................................................................................................................................... 15
6.3. Maintenance test ............................................................................................................................................................... 16
Chapter 7 Storage and Transportation of Equipment .......................................................................................... 17
7.1 Storage ................................................................................................................................................................................ 17
7.2 Transportation..................................................................................................................................................................... 17
Chapter 8 Troubleshooting .................................................................................................................................. 18
8.1 Check the equipment and accessories ................................................................................................................................ 18
8.2 Check cable and charging line ............................................................................................................................................. 18
8.3 Troubleshooting .................................................................................................................................................................. 18
8.4 Disposal of equipment ........................................................................................................................................................ 19
Chapter 9 Appendix ............................................................................................................................................. 20
The warranty ordinance of the product ............................................................................................................... 25

Chapter 1 Summary
1.1 Characteristics of products
Noninvasive and cuff-less equipment
The self-contained screen to monitor blood pressure measurement data; real-time clock to
display the time.
Rapid measurement of blood pressure and pulse rate.
Automatic measurement.
Dynamic continuous measurement can keep 24 hours.
Real-time power display, low battery alert and auto sleep for power save.
Wireless connection via display terminal, such as Bluetooth and intelligent mobile phone,
1.2 Intended Use/Instruction for use
etc.
The Discovery 1 - Non-invasive blood pressure trending device is a small, lightweight, handheld,
device intended for measuring and display of Blood Pressure trending (systolic and diastolic) and
spot-check of Peripheral pulse rate (PPR) and Peripheral pulse wave (PPW). Measurement is
performing on capillary fingertip tissue (other than the thumb). The ring finger is the recommended
site. The results of each measurement are stored in the system memory.
It is intended to be used by any person aged above 18 years old.
1.3 The scope of products
Apply to medical institutions or families to monitor the blood pressure and pulse rate of the human
body.
1.4 Structure
The cuff-less equipment consists of a host, mobile APP software and accessories.
Accessories include :Pulse wave probe and cable, arm band (optional), power adapter, data
transmission line / charging line.
1.5 Product classification
According to the classification of medical device management categories: Class II

According to the type of protection against electric shock: class II internal power supply equipment
According to the degree of protection against electric shock: BF-type equipment
According to the degree of protection against harmful liquid into: ordinary equipment (the closed
equipment that cannot stop the liquid into)
According to the safety degree related to a mixture of flammable anesthetizing gas and air or
oxygen or Nitrous Oxide: not applicable in the place existing flammable anesthetizing gas.
According to the work system: continuous operation of equipment
1.6 Operating environment
1.Operating temperature:5 ℃~45 ℃;
Relative humidity:10%~95%( non-condensing);
Atmospheric pressure:70.0 kPa~106.0 kPa;
2.Working mode: internal power supply: DC 3.7, V/2200, mAh;
Charging mode: power adapter input voltage: 220 V, 50Hz.
1.7 Safety
1. Comply with IEC 60601-1:2012 “Medical electrical equipment; Part 1: General requirements for
safety”
2. Comply with IEC 60601-1-2:2014 “Medical electrical equipment – Part 1-2: General requirements
for basic safety and essential performance – Collateral Standard: Electromagnetic disturbances –
Requirements and tests”
3. Comply with ISO 80601-2-61 “Particular requirements for basic safety and essential
performance of pulse oximeter equipment”
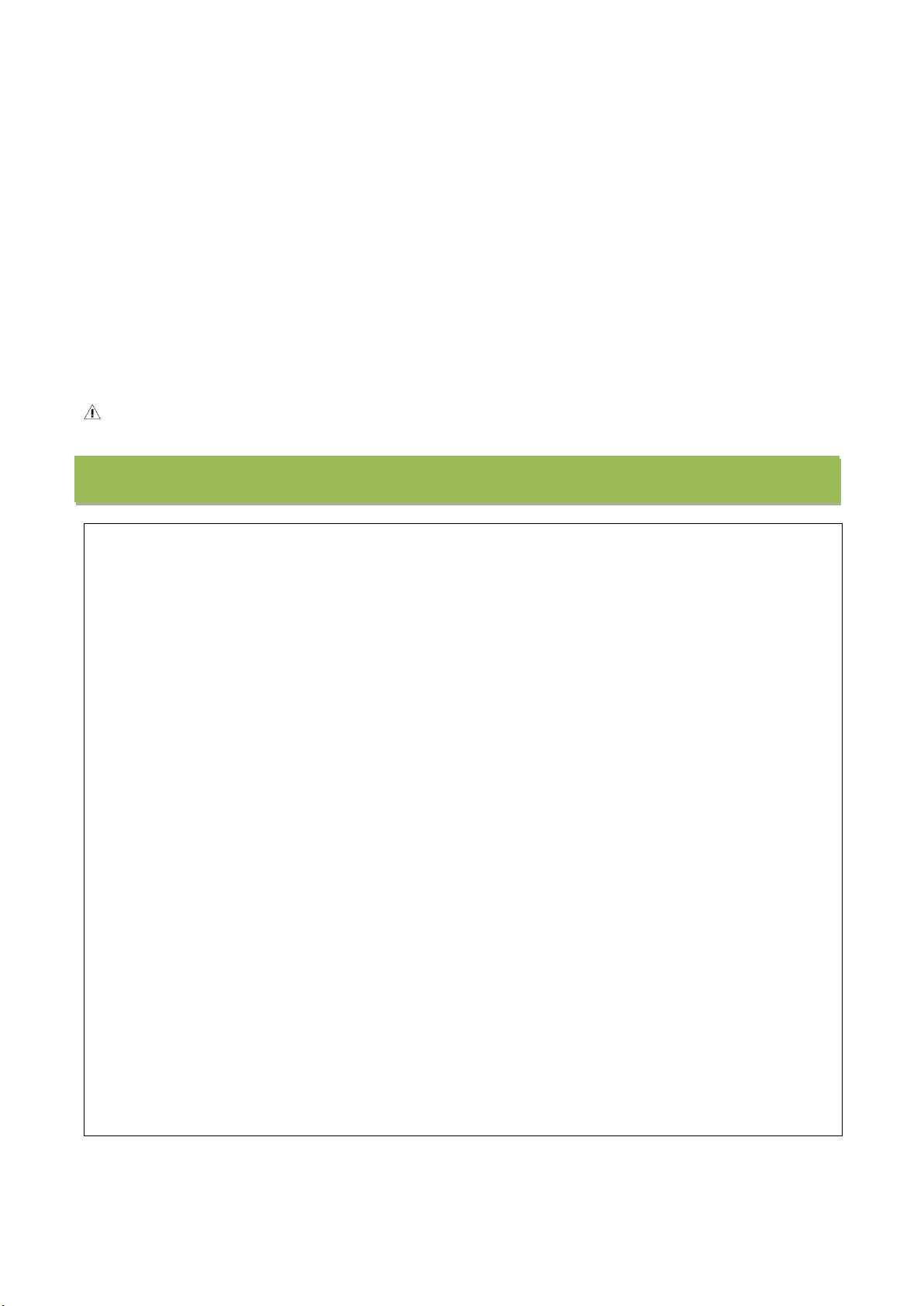
Content
Description
Non-invasive Hemodynamic Blood
Pressure Monitor
Non-invasive Hemodynamic Blood
Pressure Monitor
Pulse wave probe
Pulse wave probe interface
Screen
On/off key
Charging interface
USB connection port
Chapter 2 Wearing and Connecting
2.1 Out of box audit
1、Open the packing box and take out the equipment and accessories.
2、Open the random file and check accessories according to the packing list:
1) Check whether the equipment has any mechanical damage.
2) Check whether all accessories including plug, wires and sensor have scoring or shortness.
3) Before using the equipment, check whether the equipment and accessories are in danger or have
abnormality; if it has abnormality (such as patients’ cable rupture, shell cracking, etc.), do not carry
out measurement.)
If you have any questions, please contact the seller or contact us. We will provide you with
satisfactory service in time.
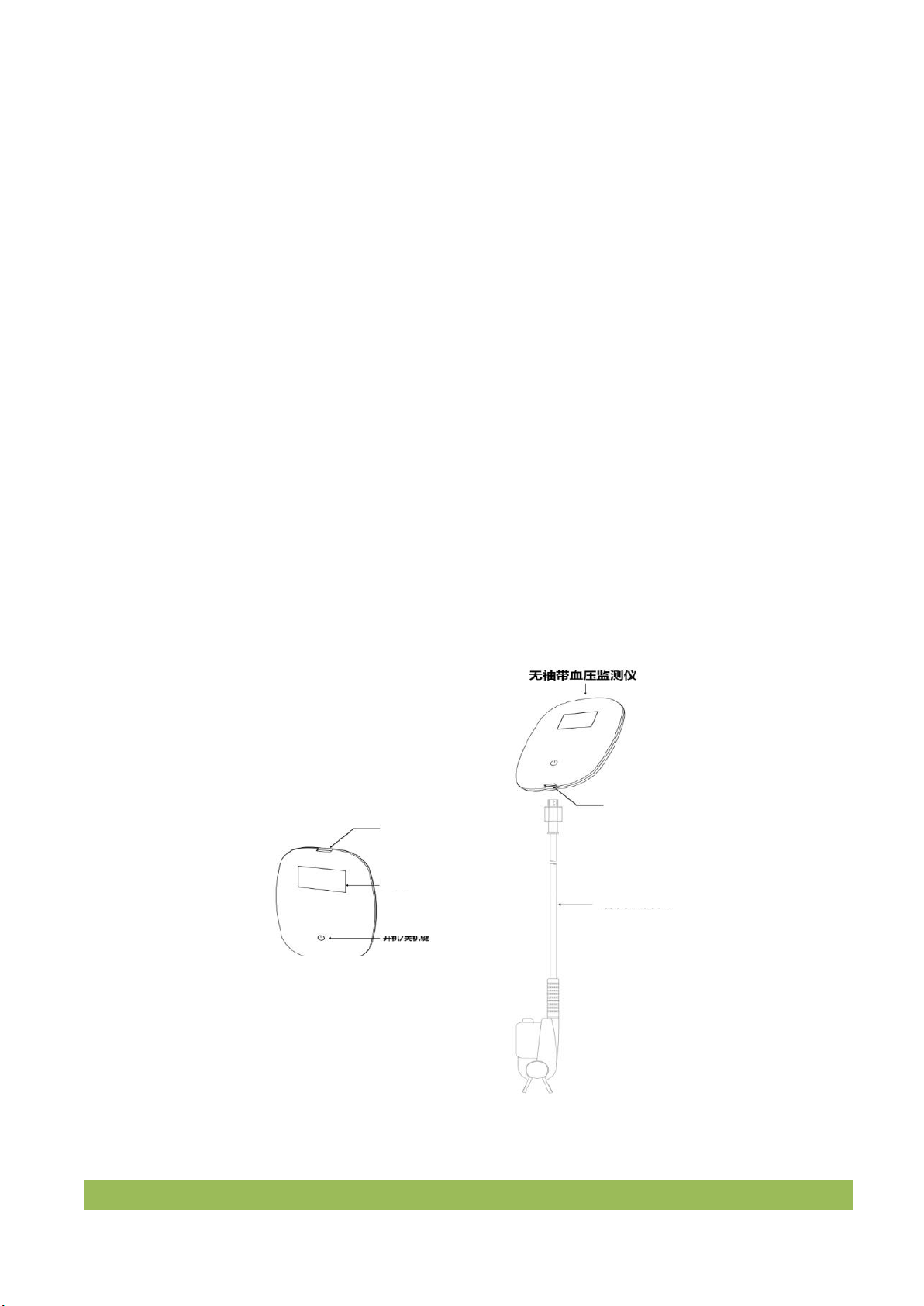
2.2 Appearance presentation
Figure 2.1 product drawing
On/off key
Press the power on / off button for 1 second to turn on the equipment
Press the power on / off button for 4 seconds to turn off the battery
Screen
Display interface content
USB connection port
Connect line USB to charge upgrade the program and export
data
Pulse wave probe
interface
Connect pulse wave probe
Content
Description
Be careful!Consult the random file
USB connection port
PPG
Pulse wave probe
BF applied part
On /off button
Non-Ionizing Radiation
2.3 Marks
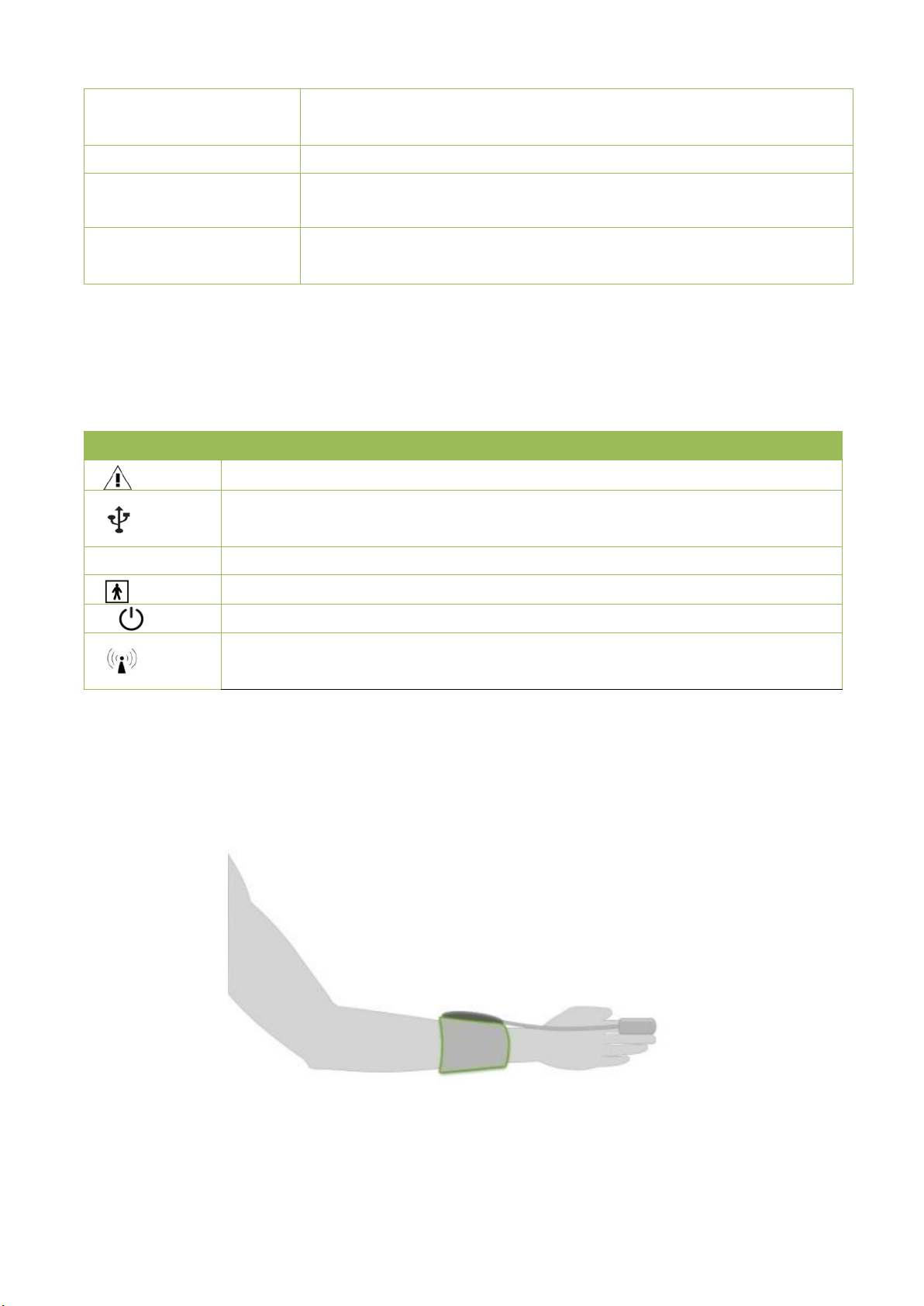
2.4 Wearing
Please wear arm belt as shown in Figure 2.2
Figure 2.2 wearing arm belt
 Loading...
Loading...