Page 1
Conductivity
Probe
(Order Code CON-BTA)
The Conductivity Probe can be used to
measure either solution conductivity or
total ion concentration of aqueous samples
being investigated in the field or in the
laboratory. Conductivity is one of the most common environmental tests of aquatic
samples. Even though it does not tell you specific ions that are present, it quickly
determines the total concentration of ions in a sample.
Note: Vernier products are designed for educational use. Our products are not
designed nor are they recommended for any industrial, medical, or commercial
process such as life support, patient diagnosis, control of a manufacturing
process, or industrial testing of any kind.
What's Included
Conductivity Probe
90 mL bottle of 1000 µS/cm NaCl standard solution
Compatible Software and Interfaces
See www.vern ier. com/ manu als/con- bt a for a li st o f interfa ces an d so ftware
compatible with the Conductivity Probe.
Getting Started
1. Connect the sensor to the interface (LabQuest Mini, LabQuest 2, etc.).
2. Start the appropriate data-collection software (Logger Pro, Logger Lite,
LabQuest App) if not already running, and choose New from File menu.
The software will identify the sensor and load a default data-collection setup. You
are now ready to collect data.
If you are collecting data using a Chromebook™, mobile device such as iPad
®
or
Android™ tablet, or a Vernier wireless sensor or interface, please see the following
link for up-to-date connection information:
www.vern ier.co m/sta rt/ con -b ta
Using the Product
1. Set the range switch on the sensor.
2. Thoroughly rinse the lower section of the probe using distilled or deionized
water.
3. Connect the sensor following the steps in the Getting Started section of this
user manual.
4. When you are finished making measurements, rinse the electrode with distilled
water. Store d ry.
Videos
View videos related to this product at www.vern ier.co m/con -b ta
Calibration
For many experiments, calibrating the Conductivity Probe is not required. A
calibration equation is stored on each probe before they are shipped, which is used
as a default by Vernier software.
For the most accurate measurements with this sensor, we recommend calibration. It
is a simple process that takes only a few minutes.
For instructions for Conductivity Probe calibration using Logger Pro computer
soft ware, see
www.vern ier.co m/til/2341
For instructions for Conductivity Probe calibration using LabQuest App, see
www.vern ier.co m/til/3394
For instructions for Conductivity Probe calibration using Graphical Analysis
with a Chromebook, see
www.vern ier.co m/til/3631
For instructions for Conductivity Probe calibration using Graphical Analysis
with an iOS or Android device, see
www.vern ier.co m/til/3630
In order to calibrate a Conductivity Probe, or to confirm that a saved calibration is
accurate, you should have a supply of conductivity standard solutions that cover
the range of the conductivity values you will be measuring. For more information
about conductivity standard solutions, including recipes for preparation, see
www.vern ier.co m/til/760
Specifications
Low range 0 to 200 µS/cm (0 to 100 mg/L TDS)
Mid range 0 to 2000 µS/cm (0 to 1000 mg/L TDS)
High range 0 to 20,000 µS/cm (0 to 10,000 mg/L TDS)
Type ABS body, parallel graphite electrodes
Response time 98% of final reading in 5 seconds
Temperature compensation automatic from 5 to 35°C
Temperature range 0 to 80°C
Accuracy using factory
calibration
±8% of full-scale reading for low range
±3% of full-scale reading for mid range
±4% of full-scale reading for high range
Accuracy using custom
calibration
±2% of full-scale reading for each range
Shaft diameter 12 mm OD
Care and Maintenance
When you have finished using the Conductivity Probe, simply rinse it off with
distilled water and blot it dry using a paper towel or lab wipe. The probe can
2
Page 2
then be stored dry.
If the probe cell surface is contaminated, soak it in water with a mild detergent
for 15 minutes. Then soak it in a dilute acid solution (0.1 M hydrochloric acid
or 0.5 M acetic acid works well) for another 15 minutes. Then rinse it well
with distilled water. Imp ortant: Avoid scratching the inside electrode surfaces
of the elongated cell.
Important: Do not place the electrode in viscous, organic liquids, such as
heavy oils, glycerin (glycerol), or ethylene glycol. Do not place the probe in
acetone or other organic solvents, such as pentane or hexane.
How the Sensor Works
The Vernier Conductivity Probe measures the ability of a solution to conduct an
electric current between two electrodes. In solution, the current flows by ion
transport. Therefore, an increasing concentration of ions in the solution will result
in higher conductivity values.
The Conductivity Probe is actually measuring conductance, defined as the
reciprocal of resistance. When resistance is measured in ohms, conductance is
measured using the SI unit, siemen s (formerly known as a mho). Since the siemens
is a very large unit, aqueous samples are commonly measured in microsiemens, or
µS.
Even though the Conductivity Probe is measuring conductance, we are often
interested in finding conductivity of a solution. Conductivity, C, is found using
the following formula:
C=G•k
c
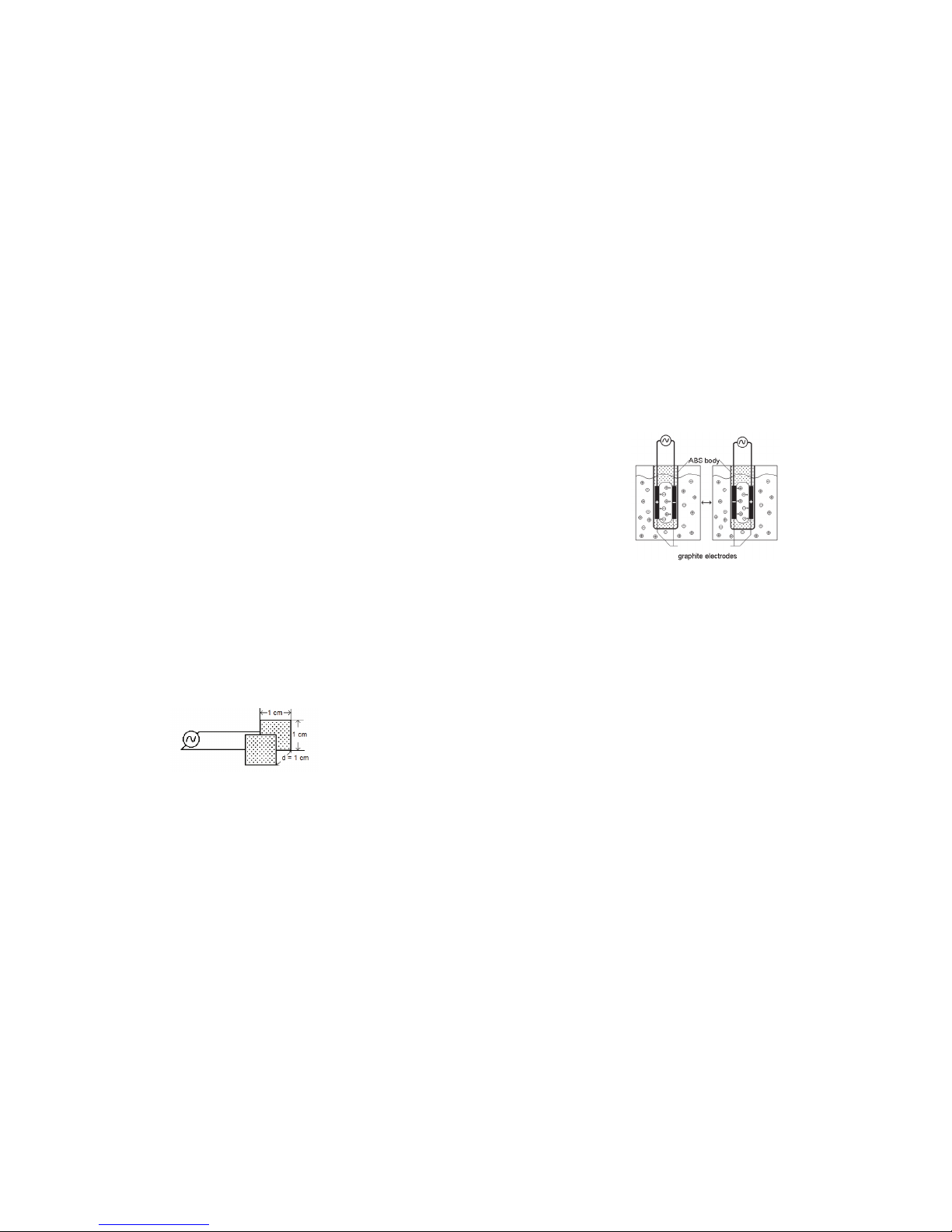
where G is the conductance, and kc is the cell constant. The cell constant is
determined for a probe using the following formula:
k
c
=d/A
where d is the distance between the two electrodes, and A is the area of the
electrode surface.
For example, the cell in Figure 1 has a cell constant:
k
c
=d/A=1.0cm/1.0cm2=1.0cm
-1
The conductivity value is found by multiplying conductance and the cell constant.
Since the Vernier Conductivity Probe also has a cell constant of 1.0 cm
-1
, its
conductivity and conductance have the same numerical value. For a solution with
a conductance value of 1000 µS, the conductivity, C, would be:
C=G•k
c
= (1000 µS) X (1.0 cm-1) = 1000 µS/cm
3
A potential difference is applied to the two probe electrodes in the Conductivity
Probe. The resulting current is proportional to the conductivity of the solution.
This current is converted into a voltage.
Alternating current is supplied to prevent the complete ion migration to the two
electrodes. Each cycle of the alternating current, the polarity of the electrodes is
reversed, which in turn reverses the direction of ion flow. This very important
feature of the Conductivity Probe prevents most electrolysis and polarization from
occurring at the electrodes. Thus, the solutions that are being measured for
conductivity are not fouled. It also greatly reduces redox products from forming on
the relatively inert graphite electrodes.
The Vernier Conductivity Probe is automatically temperature compensated
between temperatures of 5 and 35°C. Note that the temperature of a solution is
being read by a thermistor that extends into the space between the graphite
electrodes. Readings are automatically referenced to a conductivity value at 25°C;
therefore, the Conductivity Probe will give the same conductivity reading in a
solution that is at 15°C as it would if the same solution were warmed to 25°C.
This means you can calibrate your probe in the lab, and then use these stored
calibrations to take readings in colder (or warmer) water in a lake or stream. If the
probe was not temperature compensated, you would notice a change in the
conductivity reading as temperature changed, even though the actual ion
concentration did not change.
Troubleshooting
When testing a Conductivity Probe, it is best to measure a standard solution
because it is easier to determine if the sensor is reading correctly. If your
Conductivity Probe is reading differently from the standard solution, you may
simply need to calibrate the sensor. See the Calibration section for more
information. Here are some other tips to ensure best data collection practices:
Blot the inside and outside of the electrode cell dry to avoid water droplets
diluting or contaminating the sample to be tested.
Be sure the electrode surfaces in the elongated cell are completely submerged
in the liquid and that there are no bubbles around the electrode surface.
Gently swirl the probe, or stir the solution with a stirring bar and stir plate,
during data collection.
Do not completely submerge the sensor. The handle is not waterproof.
4
Page 3

If you are taking readings at temperatures below 15°C or above 30°C, allow
more time for the temperature compensation to adjust and provide a stable
conductivity reading.
When you have finished using the Conductivity Probe, simply rinse it off with
distilled water and blot it dry using a paper towel or lab wipe. The probe can
then be stored dry.
If the probe cell surface is contaminated, soak it in water with a mild detergent
for 15 minutes. Then soak it in a dilute acid solution (0.1 M hydrochloric acid
or 0.5 M acetic acid works well) for another 15 minutes. Then rinse it well
with distilled water and blot dry. Important: Avoid scratching the inside
electrode surfaces of the elongated cell.
Some combinations of sensors interfere with each other when placed in the
same solution. The degree of interference depends on many factors, including
which combination of sensors is being used, which interface is being used, and
others. For more information, see
www.vern ier.co m/til/638/
Sampling in Streams and Lakes
It is best to sample away from shore and below the water surface, if possible. In
free-flowing streams, there will usually be good mixing of the water, so that
samples taken near the current will be quite representative of the stream as a
whole. If you are sampling an impounded stream or a lake, there will be very little
mixing; therefore, it is important to sample away from shore and at different
depths, if possible. Do not drop the Vernier Conductivity Probe so that the entire
electrode is submerged. The electrode is not constructed to withstand higher
pressures, so seepage into electronic components of the electrode will result.
Although it is better to take readings at the collection site, readings of total
dissolved solids or conductivity should not change significantly if you collect
samples and take readings at a later time. However, be sure that samples are
capped to prevent evaporation.
If sample bottles are filled brim full, then a gas such as carbon dioxide, which is
capable of forming ionic species in solution, is prevented from dissolving in the
water sample. Since the probe has built-in temperature compensation, you can do
your calibration in the lab. This means that even though you will be sampling in
water that has a different temperature than your calibration temperature, the probe
will take correct readings at the new sampling temperature.
Sampling in Ocean Water or Tidal Estuaries: Salinity
Salinity is the total of all non-carbonate salts dissolved in water, usually expressed
in parts per thousand (1 ppt = 1000 mg/L). Unlike chloride (Cl
-
) concentration,
you can think of salinity as a measure of the total salt concentration, comprised
mostly of Na
+
and Cl-ions. Even though there are smaller quantities of other ions
in seawater (e.g., K
+
,Mg2+,orSO
4
2-
), sodium and chloride ions represent about
91 percent of all seawater ions. Salinity is an important measurement in seawater
or in estuaries where freshwater from rivers and streams mixes with salty ocean
water. The salinity level in seawater is fairly constant, at about 35 ppt
(35,000 mg/L), while brackish estuaries may have salinity levels
between 1 and 10 ppt.
5
The salinity range of the Conductivity Probe is 0 to 10 ppt. Seawater has a salinity
of 35 ppt, so any seawater samples will need to be diluted before making
measurements with this sensor. We recommend that you dilute seawater samples
(or other samples that initially give readings above 10 ppt) to 1/4 of their original
concentration, then multiply their measured salinity reading by 4 to obtain a final
salinity value, in ppt. Brackish water in coastal estuaries is often in the range of
0 to 10 ppt, well within the high range of the probe. Note: Vernier also sells a
Salinity Sensor (order code SAL-BTA) with a range of 0 to 50 ppt.
Since there is no stored salinity calibration for a Conductivity Probe, perform a
two-point calibration using 5 ppt and 10 ppt salinity standards. Make sure your
sensor switch is on the high conductivity setting. You will need to prepare two
standard solutions to calibrate for salinity:
A low standard (5 ppt salinity), add 4.60 g of NaCl to enough distilled water
to prepare 1 liter of solution.
A high standard (10 ppt salinity), add 9.20 g of NaCl to enough distilled water
to prepare 1 liter of solution.
Determining the Concentration: Total Dissolved Solids
Because there is a nearly linear relationship between conductivity and
concentration of a specific ion or salt, the Conductivity Probe can be used to
determine the concentration of an ion. A curve can be obtained if you prepare or
purchase standard solutions. Note in this figure the 2:1 ratio between conductivity
in µS/cm and TDS concentration in mg/L.
Even though total dissolved solids is often defined in terms of this 2:1 ratio, it
should be understood that a TDS reading of 500 mg/L can have a different
meaning in a sample that is mostly NaCl than in another sample that is composed
primarily of hard water ions such as Ca
2+
and HCO
3
-
. The relationship between
conductivity and sodium chloride concentration is approximately a 2:1 ratio and
is very nearly a direct relationship. The table shows the relationship for sodium
chloride concentration in mg/L to TDS to conductivity.
6
Page 4

Sodium chloride concentration
(mg/L)
Total dissolved
solids (TDS)
(mg/L)
Conductivity
(µS/cm)
1.0 1.1 2.2
5.0 5.4 10.8
10 10.7 21.4
20 21.4 42.7
50 52.5 105
100 105 210
150 158 315
200 208 415
500 510 1020
1000 995 1990
1500 1465 2930
2000 1930 3860
5000 4482 8963
10250 9000 18000
Repair Information
If you have watched the related product video(s), followed the troubleshooting
steps, and are still having trouble with your Conductivity Probe, contact Vernier
Technical Support at support@vernier.com or call 888-837-6437. Support
specialists will work with you to determine if the unit needs to be sent in for
repair. At that time, a Return Merchandise Authorization (RMA) number will be
issued and instructions will be communicated on how to return the unit for repair.
Accessories/Replacements
Item Order Code
Conductivity Standard Solution (Low, 150 µS/cm), 500 mL CON-LST
Conductivity Standard Solution (Middle, 1413 µS/cm), 500 mL CON-MST
Conductivity Standard Solution (High, 12880 µS/cm), 500 mL CON-HST
7
Warranty
Vernier warrants this product to be free from defects in materials and workmanship
for a period of five years from the date of shipment to the customer. This warranty
does not cover damage to the product caused by abuse or improper use. This
warranty covers educational institutions only.
Vernier Software & T echnology
139 79 SW Millikan Way • Beave rto n, OR 97005-2886
Toll Free (888) 837-6437 • ( 503) 277- 2299 • F AX (503) 277-2440
info@vernier.com• www.vernier.com
Rev. 2/8/16
Logger Pro, Logger Lite, Vernier LabQuest 2, LabQuest Mini, and other marks shown are our trademarks or
registered trademarks in the United States.
All other marks not owned by us that appear herein are the property of their respective owners, who may or may
not be affiliated with, connected to, or sponsored by us.
8
 Loading...
Loading...