Verity Medical NeuroTrac Continence, NeuroTrac ECS400A Operation Manual

NeuroTrac®Continence Operation Manual
1
DUAL CHANNEL STIM UNIT
Operators Manual
Visit our website: www.veritymedical.co.uk
for detailed application protocols
English
NeuroTrac Continence
®
NeuroTrac® Continence Operation Manual
2
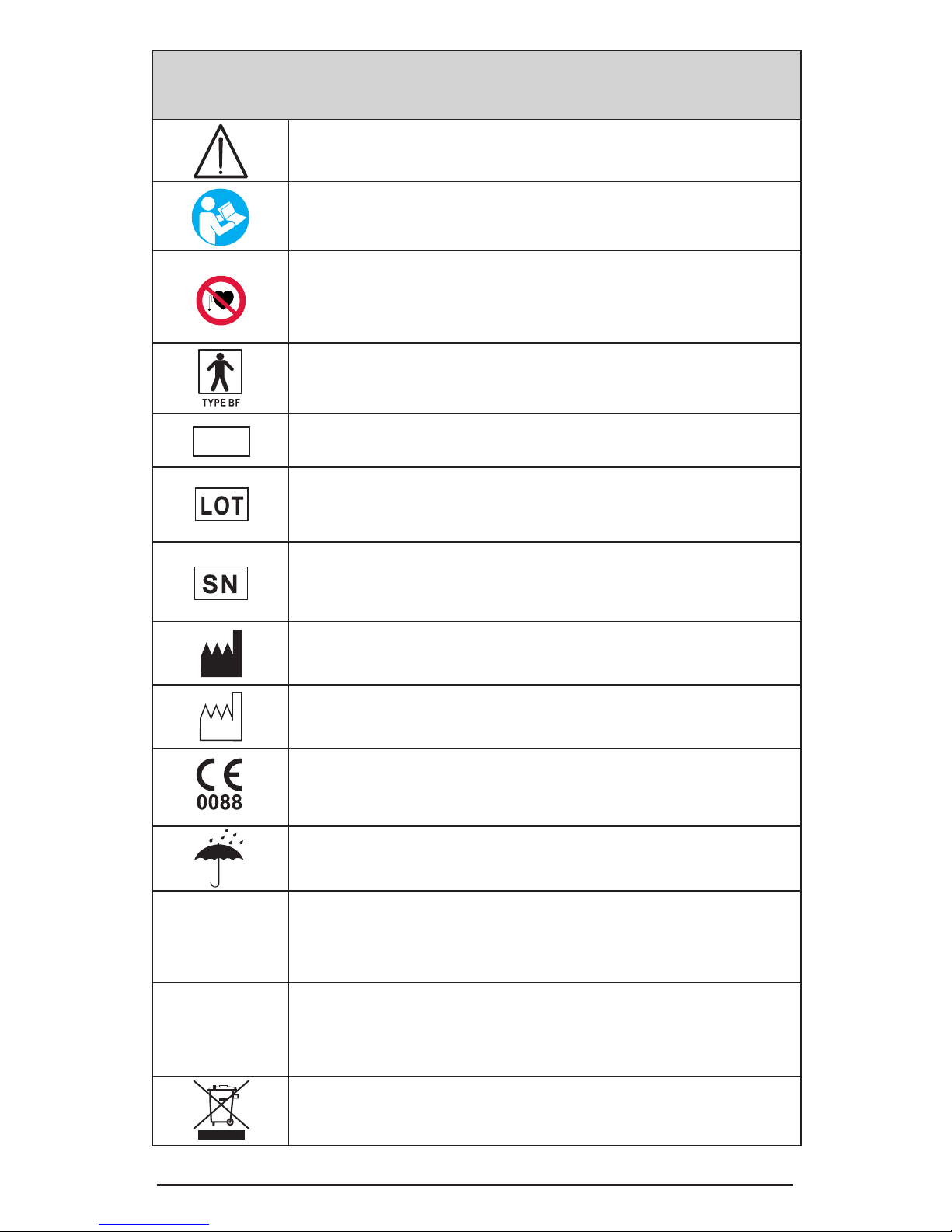
Symbols on the unit and case
Caution! (electrical output).
Follow operating instructions! Failure to do so could place
the patient or operator at risk.
Neuromuscular Stimulation (STIM) and EMG Triggered
Stimulation (ETS) should not be used by Patients tted with
demand style cardiac pacemakers. Please seek advice from
your health supervisor.
Patient’s shock protection type: BF (Body Floated) Equipment. This equipment is not earthed but contains a battery
within an insulated unit.
Indicates the manufacturer’s catalogue number so that the
medical device can be identied.
Manufacturer's LOT/Batch number. Present it together
with SN number when you report a technical fault or
claim a warranty return.
Manufacturer's serial number of the unit. Present it
together with LOT number when you report a technical
fault or claim a warranty return.
Name and address of Manufacturer.
Date of manufacture.
Conformity indication with the essential health and
safety requirements set out in European Directives.
0088 - Notied body identication (LRQA Ltd.)
This product should be kept dry.
IP20
on the unit
This is an indication for protection against ingress of water
and particulate matter. The mark IP20 on your unit means:
your unit is protected against solid foreign objects of 12.5mm
dia and greater. Not protected against water.
IP02
on the case
IP02 on the carrying case means: Protected from the ingress
of water droplets from a shower of rain.
Do not dispose in normal dustbin
(see page 14 for the disposal instructions).
REF

NeuroTrac®Continence Operation Manual
3
* This unit must be used with the guidance of a Physiotherapist or Doctor.
* Type BF equipment, Continuous Operation.
* Do not insert lead wires into a mains power supply.
* Do not immerse unit into water or any other substance.
* The unit is not protected from the ingress of shower of rain if used outside
the carrying case.
* Do not use the NeuroTrac® Continence unit in the presence of a
ammable anaesthetic gas mixture.
* Please use 9 Volt alkaline battery (IEC code: 6LR61, size: PP3). If using
rechargeable 9 Volt battery, be sure to use Nickel Metal Hydride type
batteries (IEC Code 6HR61).
We advise not to use Ni-Cad rechargeable batteries.
Caution: Do not use lithium batteries unless they comply with
IEC60086-4.
* Patient Electrodes are for single patient use only.
* Keep out of reach of children.
* Do not use this stimulator on your facial area unless you are under strict
guidance from a qualied Clinician.
* Application of electrodes near the thorax may increase the risk of cardiac
brillation.
* Operation in close proximity to shortwave or microwave therapy
equipment may produce instability in the stimulator output.
* Simultaneous connection of a patient to high frequency surgical equipment
may result in burns at the site of the stimulator electrodes and possible
damage to the stimulator.
* This device can deliver current densities in excess of 2mA/cm2 when used
at a high intensity with small electrodes.
* No modication of this equipment is allowed.
Warnings

NeuroTrac® Continence Operation Manual
4
Contents Page
Unit symbols explanation 2
Warnings 3
What is STIM? 5
Contraindications & precautions 6
Description of STIM unit & functions 7
Quick start instructions 8-9
Lock button 10
Setting up customised programmes 10
Continence treatment programmes 12
Electrode types & tips 13
Care, maintenance, accessories and disposal 14
Indications for use 15
Technical specications 16
Troubleshooting 17
Warranty 18
Clinical references 19
Contents

NeuroTrac®Continence Operation Manual
5
Neuromuscular Stimulation has been used for many years to stimulate muscle
and nerve bres to treat a number of muscle and nerve related conditions.
Over the last 30 years numerous clinical trials and papers have been written.
The NeuroTrac® Continence is one of a new breed of modern neuromuscular
stimulators which Verity Medical have developed with the therapist and patient
in mind. Our principle aim is to design products that have high levels of
functional use, are sensibly priced, compact and user friendly.
The NeuroTrac® Continence is a dual channel device combining several
treatment programmes into one unit. Neuromuscular stimulation is increasingly
understood by therapists and doctors. There is a better understanding of the
mechanisms which exist between nerves and muscles that makes it possible
to stimulate the neuromuscular system with precise electrical signals. The
NeuroTrac® Continence offers precision giving full control of pulse widths,
rates, ramp up times, work / rest cycles as well as alternating or synchronous
application if two channels are being applied.
Customer Care
We welcome constructive comments regarding our equipment, particularly
those that might help us to improve existing features, add new ones or develop
new products for the future.
What is STIM?

NeuroTrac® Continence Operation Manual
6
Before using this equipment you must rst seek the advice of your
physiotherapist or doctor.
Read this operating manual before using the STIM unit.
STIM should not be used:
* By patients tted with a demand style cardiac pacemakers unless so
advised by their doctor.
* During pregnancy [unless medically advised].
* By patients with undiagnosed pain conditions.
* By patients with undiagnosed skin, vaginal or rectal conditions.
* With patients who have diminished mental capacity or physical competence
who cannot handle the device properly.
* On anaesthetised or desensitised skin.
* When driving a vehicle or operating potentially dangerous equipment.
* Do not place electrodes:
> Over carotid sinus nerves.
> Over larynx or trachea.
> Inside mouth.
> Over the area of the heart unless so advised by your doctor.
> On your facial area unless under strict guidance from a qualied clinician.
> Do not apply stimulation across or through the head, directly on the eyes,
covering the mouth, on the front of the neck (especially the carotid sinus) or
via electrodes placed on the chest and upper back or crossing over the heart.
* The patient should use the unit only as prescribed.
* Do not immerse the unit in water or any other liquid.
* Keep unit out of reach of children.
* If in doubt about the use of the STIM unit, call your doctor, therapist,
clinician or your distributor for advice.
* Only use CE approved skin electrodes.
* Only use CE approved vaginal or rectal probes.
Contraindications & Precautions
 Loading...
Loading...