VALES and HILLS BIOMEDICAL TECH ICV200S User Manual

®
CardioView
iCV200S User Manual
VH-JS-08(63F) Version A/0
VALES & HIL LS BIOMEDICA TECH. LTD.
®
Family
Tel: 0086-010-51665548
Fax: 0086-010-67856343
E-mail: sales@vhmedical.com
Website: http://www.vhECG.com
Address: Bldg 46-1, No2 North Street Jingyuan, Beijing Economic-technological Development
Area, 100176 Beijing, China
CARDIO SYSTEMES 6 rue Descartes 78370 PLAISIR France
1

®
CardioView
iCV200S User Manual
®
Family
Table of Contents
Preface ................................................................................................................................................................ 3
1 iCV200S.............................................................................................................................................. 7
1.1About the iCV200S...................................................................................................................... 7
1.2List of packing ............................................................................................................................ 8
1.3Accessories ................................................................................................................................. 8
2.Installation........................................................................................................................................... 9
2.1 iCV200SECG Acquisition Recorder ........................................................................................... 9
2.2 Down Load vhECG HD from Apple App Store ....................................................................... 10
3.Electrodes Hookup ............................................................................................................................ 11
3.1Hoopup ...................................................................................................................................... 11
4.Getting Start ...................................................................................................................................... 12
4.1Record er Sett ing ........................................................................................................................ 12
4.2Communication Between the Recorder and iPad ........................................................................ 13
5.vhECG Resting EC G System ............................................................................................................. 14
5.1Syste m S e tt ing ........................................................................................................................... 15
5.2Patient Records Archive............................................................................................................. 16
5.3Monitoring and Recording ......................................................................................................... 16
5.4Recor d s Review ......................................................................................................................... 17
5.4 .1 Rec or ds State ....................................................................................................................... 17
5.4 .2 EC G Rev ie w ........................................................................................................................ 18
5.4 .3 Average ECG ....................................................................................................................... 19
5.5 VHCloud ........................................................................................................................................ 20
6.Cleaning and Maintenance ................................................................................................................. 21
7.Guidance and Manufacture’s Declarations ......................................................................................... 23
Annex I : iCV200S ECG Recorder Technic al S pec ificatio ns........................................................... 26
Annex II : Defin itions and Rules For E C G Me asu rements .............................................................. 27
2

®
CardioView
iCV200S User Manual
®
Family
Preface
Copyright
Copyright 2014, Va les and H il ls ( V &H ) . Al l rights reserved. No o ne is permitted t o translat e, r eproduce
or duplicate this manual or any part t her eo f, in a ny fo r m, w ith ou t pr ior wr itt en per mis sio n fro m V& H,
V&H assumes no responsibility for any injury to anyone, or for any illegal or improper use of the
product, that may result from failure to use this product in accordance with the instructions, cautio ns,
warnings, or statement of intended use published in this manual. Unauthorized copying of this
publication may not only infringe cop yright but also r educe the ability of V&H t o provide accurat e and
up-to-date information to users and operators alike.
The information contained in this manual is subject to change without notice. All changes will be in
compliance with regulations governing manufact ur e of medical equipment.
General Description and Inte nde d Use
iCV200S is a po rtable ECG system with CardioView family. It includes a data acquisition reco rder and
iPad with vhECG App. The system is designed and manufactured by V&H for patient ECG recording
with automatic measurements and inte r pr e t at io ns .
This product is not intended for use as sustain or support life devices.
This product is not intended for introcardiac use.
This product is not intended for use in operating room or ICU
This product is not intended for infant.
The device is intended to provide reference for medical diagnosis, not intended for a replacement of
diagnosis c linic ians.
Precautions
Radio Frequency (RF) interference between the iCV200S recorder or electrocardiograph and any
existing RF transmitting or receiving equipment at the installation site, including electrosurgical
equipment, in close proximity to the electrocardiograph should be evaluated before the equipment is
operated as they may serious ly degrade performance.
The iCV200S is susceptible to interference from RF energy sources (lowered RF immunity) which
exceed the IEC 60601-1-2 limits, such as power line bursts, ot her medical equipment, cellular pro ducts,
information technology equipment and radio/television transmission.
To reduce EMC interference t he cardiograph shall be separated fro m t he emitt ing sources as much as
possible. If assistance is needed, call V&H service.
Artifact on the ECG caused by electromagnetic interference should be evaluated by a physician or
physician authorized personnel to deter mine if it will negatively impact diagnosis or treatment.
Like all elect ronic devices, t his electrocardiograph is suscept ible to electro static discharge (ESD ). ESD
typically occurs when electrostatic energy is transferred to the patient, the electrodes, or the
electrocar diogr aph. E S D may result in ECG artifact that may appear as narrow spikes on the cardiograph
display or on the printed report. When ESD occurs, the electrocardiograph’s interpretation may be
inconsistent with the physician’s interpretation.
3

®
CardioView
®
Family
iCV200S User Manual
V&H ass u me s no lia b il it y for fa i lur es res u lt in g fro m RF or ESD interference between V&H CardioV iew
series and any RF or ESD generating equipment when these levels exceed those established by
applicable standard.
Warnings
The electrocard iograph has not been designed for use with high frequency (HF) surg ical equipment a n d
does not protect against hazards to the patient.
Ensure the location of the elect rode and associated cables prov ides maximum separation away from al l
sources of HF energy. The best way to ensure patient sa fety is to comple te ly remo ve all e lect ro des and
cables from the pat ient when exposed to HF energy.
Ot her med ical e quip ment , in clud ing bu t no t limited to defibrillators, ultrasound machines, pacema kers,
and other simulat ors, may be used simultaneous ly with the elect rocardiograph. However, suc h devices
may disturb the electrocardiograph signal.
Cannot touch the patient during Defibrillation.
This equipment has been tested and found to comply with the limits for a Class B digital device,
pursuant to part 15 of the FCC Rules. These limits are des igned to provide reasonable prot ection against
harmful interference in a residential installation. This equipment generates uses and can radiate radio
frequency energ y and, if not installed and used in accordance with the instru ctions, may cause harmfu l
interference to rad io communications. However, there is no guar antee that interference will not occur in
a particular installation. If this equipment does cause harmful interference to radio or television
reception, which can be determined by turning the equ ipment off and on, the user is encouraged to try t o
correct the interference b y o ne or more of the following measures:
- Reorient or relocate the receiving ant en na.
- Increase the separation between t he equ ipment and receiver.
- Connect the equipment into an outlet o n a circuit different from that to which the receiver is
connected.
- Consult the dealer or an experienced radio/TV technician for help
Changes or modifications not expressly approved by the party responsible for compliance could void the
user's author it y to operate the equipment. This device complies with Part 15 of the FCC Rules.
Operation is subject to the following two conditions:
(1) This device may not cause harmful interference, and (2) t his device must accept any interference
received, including interference that may cause undesired op er ation.
Warranty and Service
V&H provides a one-year warranty and life-time ser v ice t o all CardioView series products. Repairs on
products under warranty must be performed or approved by V&H. Unauthoriz ed repairs will void the
war ranty. In addition, whether or no t covered under warranty, a ny produ ct repair sha ll exclusively be
performed by V&H certified service personnel.
Limited w ar r anty state ment
The warranty will not co ver the damages caused by:
1) Use o r mainte nance contr ary to labeled instructions
2) Alteration or repair by anyone no t aut ho r ized by V&H
3) Accidents.
Troubleshooting
4

®
Degree of protection against electrical shock
Type CF, defibrillation-proof
The power supply of ECG Acquisition box
2XAAABattery
Degree of protection against harmful ingress
Enclosed equipment without
water
Degree of safety of application in the presence
Not suitable for use in the
with oxygen or nitrous oxide.
Method(s) of sterilization or disinfection
recommended by the manufacturer
Sterilization: not applicable
disinfection: see user manual
Mode of operation
Continuous operation
The defibrillation-proof recovery time
10s
CardioView
®
Family
iCV200S User Manual
Most of the problem can be solved by contacting V&H ser vice depart ment support@vhmedical.com. To
get the prompt and efficient response you will provide:
Product name and its serial number.
A complete problem description
The complete name, address a nd phone nu mber of your facility
For out-of-warranty repairs or spare parts order, a purchase order
For parts order, the required spare or replacement part number
Product return
If the product has to be returned for ser vice, V&H recommends these park ing instr u ctions:
Wherever possible, use the original shipping carton and packing mat e r ia ls.
Inc lu ding a packing list .
Including a problem description report.
It is recommended that all returned goods be insured. Claim for loss or damage to the product must be
initia l e d by the sender.
Unit disposal Inst ruction s
D isposal of b attery and the unit after the end of device life in municipa l ly approved recycling area. The
life of device is 4 years.
Classification
of wat er
of a flammable anesthetic mixture with air or
wit h ox y g en or ni t r o us ox ide
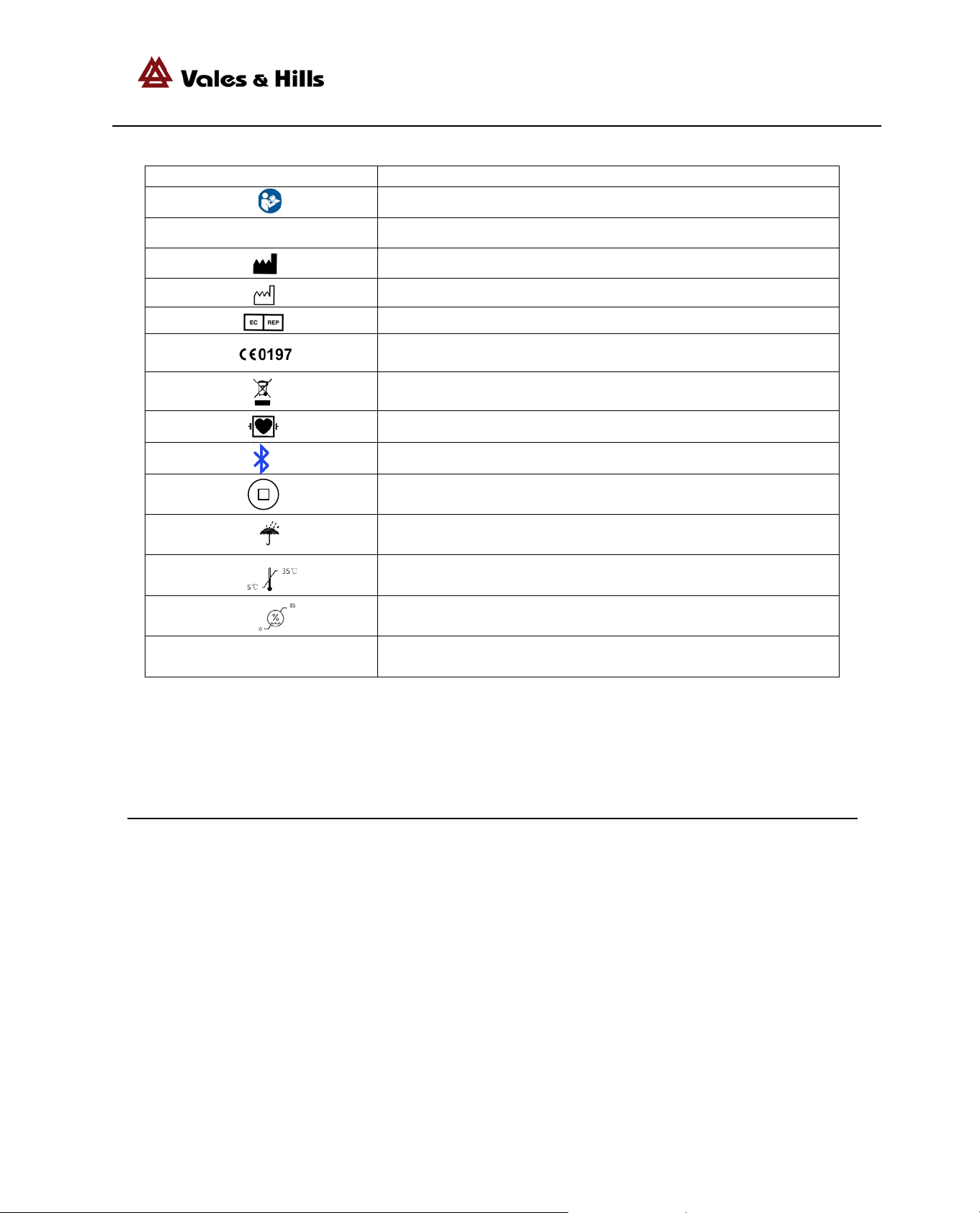
List of S ymbols
protection against ingress of
presence of a flammable
anesthetic mixture with air or
5
®
Symbols
Meaning
Indicates the range of humidity to which the medical device can be
CardioView
®
Family
iCV200S User Manual
Caution, consult accompanying documents.
SN Serial Number(xxxxxxxxxxxxxxx)
Manufacturer
Date of manu fa c ture
Authorized representative in the European community
IPX0 Against ingress of water with harmful effects : non-protected
The C E ma rkin g w i th the Regis tration Number of the Notifi ed Body. This denotes
the compliance of European Medical Device Directive 93/42/EEC
Waste Electrical and Electronic Equipment (WEEE) It is the resp onsi bility of th e en d
user to dispose of this equipment at a designated collection point for recycling.
DEFIBRILLATION-PROOF TYPE CF APPLIED P ART
Bluetooth
Function switch but to n
Keep dry
temperature limits
safely exposed.
---------------------------------------------------------------------------------------------------------------------------Warning: Warning statements describe conditions or actions that can result in personal injury or lost of life.
----------------------------------------------------------------------------------------------------------------------------Caution: Warning statements describe conditions or actions that can result in damage to the equipment of software.
----------------------------------------------------------------------------------------------------------------------------Note: Notes contain additional information on iCV200S usage.
6

®
CardioView
®
Family
iCV200S User Manual
1. iCV200S
iCV200S Rest ECG for iOS (i Pad, iPad-mini and iPhone)
Thank you for your purchase of iCV200S Resting ECG System and we hope that you will enjoy
working with it. If you have any comments or suggestions, we would appreciate hearing from you.
Please contact support@vhmedical.com
1.1 About iCV200S
iCV200S is a portable ECG system. It includes a data acquisition recor d er and patie nt cable. The s ystem
is designed and manufactur ed by V&H, T he ECG Acquis it ion System is capable of sampling, reco rding
and analyzing patients resting ECG. This system is app licable to t he heart d isease analysis for the
medical treatment institution.
Fig1.1 iCV200S Rest ECG Composition
7
®
iCV200Ssoftware
(Note1)
Item No.
Description
Intende d Use
CV-022010-M0201120
ICV200S patient cable
Cables allow connection to the
signals
CV-021010-M0201044
Standard Patient Cable
CV-040002
Battery
Power for ECG acquisition box
CardioView
iCV200S User Manual
1.2 List of packing
The ECG Ac quisition Syste ms c ons is ts of t he fo llowing items:
Part No. Description QTY
CV060F-010 ECG Acqui sit ion B ox 1
®
Family
M0201120 ECG cable 1
CV060F-060 User manual 1
1
Note1: About the software download please see 2.2 Download vhECG HD from Apple App Store.
1.3 Accessories
electrode leads, which adhere to
the patient for detection of ECG
Note:
1. If any lead wire of lead part is failure, t he user is unab le to replace t he lead wire, please contact with
V&H.
2. If any lead wire of lead part is failure, t he user can rep lace the standard 6511ECG cable, the ECG
cable safety requirement s tested for compliance wit h 93/4 2/ EEC, sho uld have the CE cert ificat e. Vales
& Hil ls recommend s Qingd ao B right med ic al, the mo d el is M 0 201020.If use the manufacturer app roval
attachment may reduce the safety of the products.
3.iCV200Sis powered by two AAA LR03 bat teries.Insufficient of power may affect the
communicat ion between t he recorder and iPad.Check the batteries with adequacy of power befor e using.
If the power is low,the user can rep lace t he new battery, Vales & H ills re co mmend s the battery model is
AAA LR03.
4. For the sake of environment al protection, please recycle disposal of the battery.
5. For product s and accessories that are no longer used, it will be the cause of environment al pollution, it
should be properly treated.
6. Do not include standard Pat ient cable a nd bat tery when we sale.
7. If you find anything above is not included in the packing, please contact us by the following address:
8
Technical Support Dept. of Vales & Hills Biome dical Tech. Ltd.
Tel:0086-010-51665548
E-mail:service@vhmedical.com
Website: http://www.vhecg.com
Fax:0086-010-67856343

®
CardioView
iCV200S User Manual
®
Family
2. Installation
A complete iCV200S syste m consist s of iCV200S dat a acquisit ion recorder and iPa d o r iPa d-min i w ith
Apple App – vh EC G H D. Th is s ec t io n w il l guide you iCV200S reco rder composition and vhECG HD
installation.
2.1Hardware Connection
iCV200S ECG Acq uis iti o n Rec or de r
15-Pin-Share-Socket
iCV200S Pat ient Cable Connector
Battery Compartment Cover
Fig 2.1 iCV200S Recorder
Button
LED
iCV200S Recorder Frontside
iCV200S Recorder Backside
9
 Loading...
Loading...