Ulco Medical Signet 615 User Manual
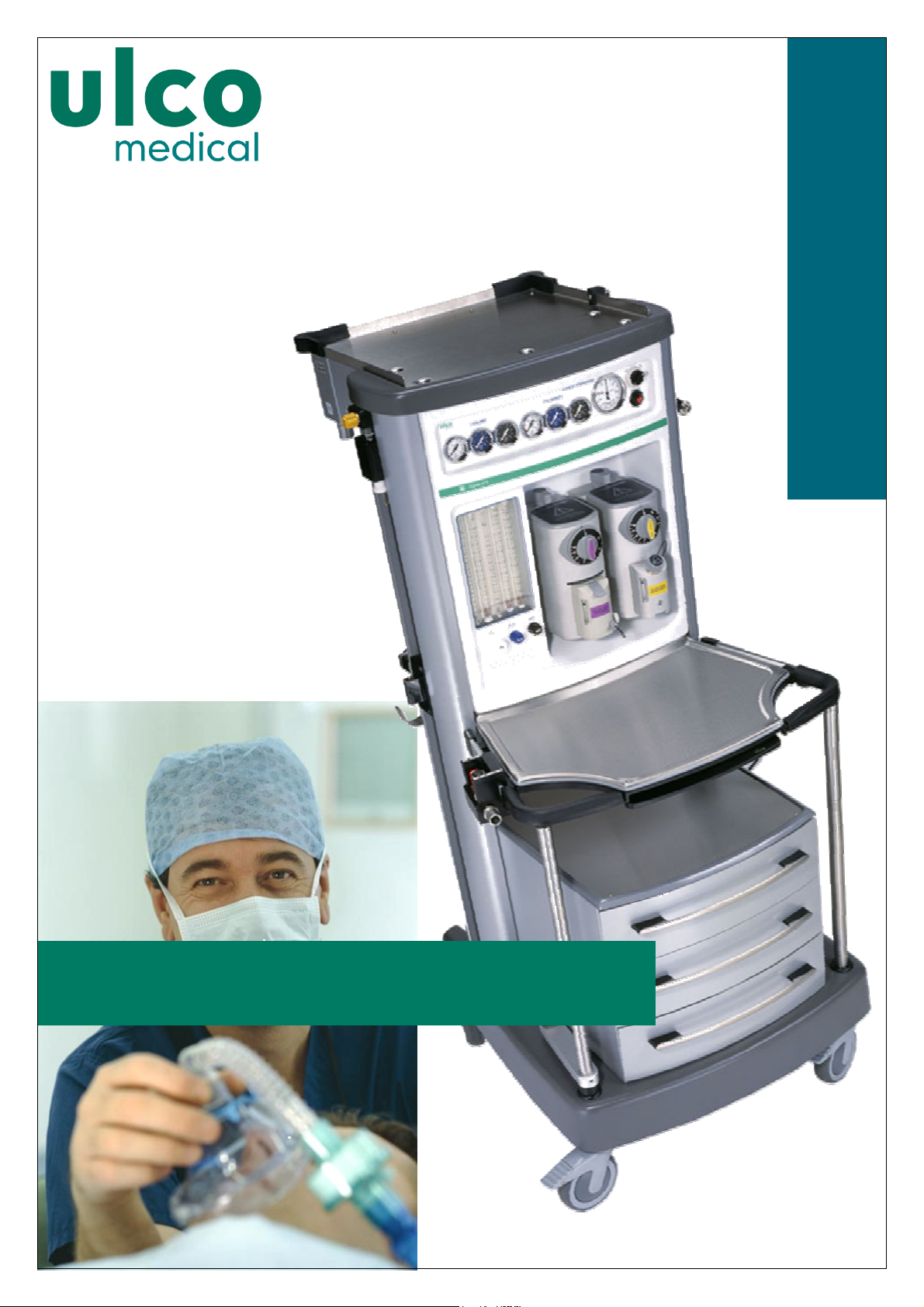
SIGNET 615 ANAESTHESIA WORKSTATION
SIGNET 615 ANAESTHESIA WORKSTATIONSIGNET 615 ANAESTHESIA WORKSTATION
SIGNET 615 ANAESTHESIA WORKSTATION
USER MANUAL
USER MANUAL
USER MANUALUSER MANUAL

Signet 615 User Manual
Copyright © 2002, 2005 by Ulco Medical
All rights reserved.
No part of this publication may be reproduced in any form, in an electronic retrieval system
or otherwise, without the written permission of the Ulco Medical.
All Ulco products are subject to a program of continuous development and the
manufacturer reserves the right to make alterations in design and equipment without prior
notice.
Document Number: SG6-UM-001
Version: 1.2
2

Table of Contents
Table of Contents
Table of ContentsTable of Contents
1
Introduction ....................................................................................... 6
1.1 Warranty Statement .......................................................................................6
1.2 About this manual ..........................................................................................6
1.3 Intended Use..................................................................................................6
1.4 Device Classification......................................................................................7
1.5 Background....................................................................................................7
1.6 Major features ................................................................................................7
1.6.1 Overall strengths .................................................................................7
1.6.2 Typical configuration ...........................................................................7
1.7 Safety Precautions.........................................................................................8
1.7.1 Powering the unit up for the first time ..................................................8
Signet 615 User Manual
1.7.2 Follow the Instructions for Use ............................................................8
1.7.3 Liability for proper function or damage ................................................8
1.7.4 Maintenance and Repairs ...................................................................8
1.7.5 Use of anaesthetic agents...................................................................8
1.7.6 Power Connection...............................................................................8
1.7.7 Monitoring and alarms.........................................................................9
2
Major components of the machine................................................. 10
2.1 Gas controls.................................................................................................10
2.2 Flowmeters ..................................................................................................10
2.3 Patient Block ................................................................................................11
2.4 Vaporisers....................................................................................................11
2.5 Ventilator......................................................................................................12
2.6 Soda-lime absorber......................................................................................12
2.7 The anti-hypoxic device ...............................................................................12
2.8 Gas manifold................................................................................................12
2.9 Main regulators ............................................................................................13
2.10 Second stage regulators...........................................................................13
2.11 Pressure gauges ......................................................................................13
2.12 Scavenging...............................................................................................13
2.13 Auxiliary oxygen outlet..............................................................................13
2.14 Pipeline hose assemblies .........................................................................13
3

2.15 Patient circuitry.........................................................................................14
2.15.1 The circle circuit...............................................................................14
2.16 Oxygen failure warning device .................................................................14
2.17 Auxiliary Power Outlets ............................................................................14
2.18 Other accessories ....................................................................................15
2.18.1 Accessory power .............................................................................15
3
Preparing for use............................................................................. 16
3.1 Protocol for checking the Anaesthetic Machine ...........................................16
3.2 Operational checklist procedure – pre testing ..............................................19
3.2.1 Visual examination ............................................................................19
3.2.2 General preparation – pre check.......................................................20
3.2.3 Cylinder Fitting ..................................................................................20
3.2.4 Component integrity ..........................................................................20
Signet 615 User Manual
3.2.5 General .............................................................................................20
4
Operation ......................................................................................... 21
4.1 Medical Gas Mixing......................................................................................21
4.1.1 Accuracy and the Effects of Backpressure........................................21
4.2 Circle Systems .............................................................................................21
4.3 Troubleshooting ...........................................................................................21
4.3.1 Leaks.................................................................................................21
5
Care and Cleaning ........................................................................... 23
5.1 Cleaning.......................................................................................................23
5.2 Disinfecting ..................................................................................................23
5.3 Steam Autoclaving .......................................................................................23
5.4 Filters ...........................................................................................................23
6
Servicing .......................................................................................... 24
6.1 Service intervals and service components for Signet ‘615’ ..........................24
7
Specifications .................................................................................. 25
7.1 Physical........................................................................................................25
7.2 Electromagnetic Compatibility......................................................................25
7.2.1 Electromagnetic Emissions ...............................................................25
7.2.2 Electromagnetic Immunity .................................................................25
4
7.2.3 Recommended Separation Distance.................................................26
7.2.4 Recommended Separation Distances from Portable and Mobile RF
Communication Equipment ...........................................................................27
7.3 Symbols .......................................................................................................27
7.4 Standard items.............................................................................................28
7.5 Optional Items..............................................................................................28
8
Terms and conditions ..................................................................... 29
Signet 615 User Manual
5

Signet 615 User Manual
1111 Introduction
Introduction
IntroductionIntroduction
1.1
1.1 Warranty Statement
Warranty Statement
1.11.1
Warranty StatementWarranty Statement
All Ulco products are guaranteed to be free of defects of workmanship or material for a period of one year from
the date of delivery. The following are exceptions to this warranty:
1. Defects caused by misuse, mishandling, tampering, or by modifications not authorised by Ulco or its
appointed agents are not covered.
2. Rubber and plastic components and materials are warranted to be free of defects at the time of
delivery.
Any product, which proves to be defective in workmanship or material will be replaced, credited or repaired with
Ulco holding the option. Ulco is not responsible for deterioration, wear or abuse. In any case, Ulco will not be
liable beyond the original selling price.
Goods are subject to the terms of applicable warranty. Defective products will be accepted for return at Ulco’s
discretion, and only during the warranty period. Application of this warranty is subject to the following conditions:
1. Ulco or its authorised agents must be promptly notified, in writing upon detection of the defective
material or equipment.
2. Defective material or equipment must be returned, shipping prepaid, to Ulco or its authorised agent.
3. Examination by Ulco or its authorised agent must confirm that the defect is in fact covered by the terms
of this warranty.
4. Notification in writing, of defective material or equipment must be receive by Ulco or its agent no later
than two (2) weeks following expiration of this warranty.
In order to assume complete protection under this warranty, the warranty registration card, and or periodic
manufacturer’s service record (if applicable) must be returned to Ulco within 2 weeks of receipt of the equipment.
The above is the sole warranty provided by Ulco. No other warranty expressed or implied is intended.
Representatives or agents of Ulco are not authorised to modify or amend the terms and conditions of this
warranty.
1.2
1.2 About this manual
About this manual
1.21.2
About this manualAbout this manual
This manual provides information for the preparation, assembly and care of the Signet 615 anaesthesia machine,
together with suitable equipment from the Ulco range. Although this equipment has been carefully designed for
simplicity of assembly and use, it is recommended that the contents of this manual be studied before attempting
preparation or care of the equipment. Explanatory diagrams are provided in order to help the reader understand
the concepts described.
This user manual should be read in conjunction with the user manual(s) for the vaporisers, ventilator and
absorbers.
1.3
1.3 Intended Use
Intended Use
1.31.3
Intended UseIntended Use
The Signet 615 Anaesthesia Machine is an apparatus for providing continuous gas inhalation anaesthesia for
human adults and children (above 5kg body weight).
The apparatus is intended for used solely in an operating or induction room.
The apparatus is intended to be use with hospital gas supply pressures within the range 300-500 kPa.
The apparatus is intended to be use under the continuous control of a person suitably trained and clinically
qualified in its use.
6

Signet 615 User Manual
To comply with the Anaesthetic workstation station standard IEC 60601-2-13, the Signet 615 must be used with
adequate monitoring and alarms. The stated monitors and alarms can be found in Section 1.7.7 of this manual.
1.4
1.4 Device Classification
Device Classification
1.41.4
Device ClassificationDevice Classification
The Signet 615 Anaesthesia Machine is classified as follows:
• Class I equipment for the purposes of electrical safety
• Type CF applied part
• Continuous Operation
1.5
1.5 Background
Background
1.51.5
BackgroundBackground
Anaesthesia machines such as the Signet 615 are engineered to very high standards of design and finish and all
units are able to accommodate the most comprehensive specifications required in modern anaesthesia. Ulco
machines are manufactured from non-magnetic materials such as stainless steel, anodised aluminium and
chromed brass or acetal, with moulded covers made from Kydex.
The construction of the frames provides a stable and unobstructed unit for the mounting of a wide range of
anaesthesia equipment accessories. The Signet 615 can be easily upgraded from its basic configuration with
optional accessories and attachments including a full range of patient monitors to provide a comprehensive
anaesthesia workstation.
If equipment that has not been specifically designed or supplied by Ulco is to be attached to the Ulco machine, it is
recommended that customers consult Ulco as to the suitability of the equipment and necessary modifications to
the apparatus.
Ulco and its agents provide a comprehensive regular maintenance service and it is recommended that advantage
be taken for the safe and reliable upkeep of this equipment. Refer to the accompanying service manual for
details on how to maintain your Ulco machine. There is a service contract included with the equipment – please fill
it in and return it to Ulco.
Customers requiring further service or advice with operating problems should contact Ulco or one of their
accredited agents.
1.6
1.6 Major features
Major features
1.61.6
Major featuresMajor features
All working surfaces are designed to be easy to clean and front wheels are lockable to prevent
unwanted movement
A monitor shelf is available as an option.
An aluminium accessory rail is fitted to the sides of the working area for easy attachment of many
options
The absorber post can be mounted either on the left or right hand sides or both, allowing the absorber
to be swapped from side to side
Internally mounted mains outlets used to power options
1.6.1
1.6.1 Overall strengths
1.6.11.6.1
1.6.2
1.6.2 Typical configuration
1.6.21.6.2
Overall strengths
Overall strengthsOverall strengths
sturdy, strong, durable and easy to understand construction
high quality and quantity of patient safety features
pure flexibility of design allowing tailoring of options to exact requirements
Typical configuration
Typical configurationTypical configuration
Signet 615 - 3 gas 5 Tube machine fitted with anti-hypoxic device as standard
3 drawers standard
4 internally mounted mains power outlets (IEC type)
7

Signet 615 User Manual
1 external mains outlet for use with powered vaporisers (IEC type)
EV500 ventilator (OPTIONAL)
2kg absorber (OPTIONAL
Vaporiser(s) (OPTIONAL)
Auxiliary flowmeter (OPTIONAL)
Writing table
Universal arm (OPTIONAL)
Second absorber mounting post (OPTIONAL)
Auxiliary power outlet APB70 (OPTIONAL)
1.7
1.7 Safety Precautions
Safety Precautions
1.71.7
Safety PrecautionsSafety Precautions
1.7.1
1.7.1 Powering the unit up
1.7.11.7.1
The unit must be connected to an AC mains supply of 240 V only. When power is supplied, the power available
lamp at the inlet will light. All Ulco-supplied options such as patient monitors or ventilators will be powered when
the main front panel power switch is turned to the ON position. The electro-luminescent backlight of the medical
gas flow meters will only be turned on when the driving gas supply is connected to the air or oxygen ports at the
rear of the machine.
1.7.2
1.7.2 Follow the Instruc
1.7.21.7.2
In order to use the equipment, these instructions must be fully read and understood and strictly followed. Any
equipment mentioned is only to be used for the intended purpose.
1.7.3
1.7.3 Liability for proper function or damage
1.7.31.7.3
This equipment is an adjunct to patient safety and it must in no way replace the normal monitoring by skilled
personnel. The manufacturers accept no responsibility for incidents arising from either incorrect use or
malfunction of this equipment.
If the equipment is serviced or repaired by persons not employed or authorised by Ulco, or if the equipment is not
used for the purposes for which it is intended, then the liability for the proper function of the equipment is
transferred to the owner/operator.
Where, the Signet 615 is assembled with other components of an anaesthetic system, such as ventilators or
breathing systems, and Ulco did not supply these components, then the personnel performing the assembly are
responsible for providing a checklist for the anaesthetic system.
1.7.4
1.7.4 Maintenance and Repairs
1.7.41.7.4
An authorised Ulco Technical Service Representative must perform maintenance of this equipment. Ulco products
in need of factory repairs must be sent to the nearest local agent or direct to Ulco.
Ulco recommends that anaesthesia products/equipment be serviced at intervals stated in Section 6.1 of this
manual. Periodic Manufacturer’s Service Contracts are available for products manufactured and or sold by Ulco.
These agreements are available from Ulco Technical Services.
1.7.5
1.7.5 Use of anaesthetic agents
1.7.51.7.5
Explosive anaesthetic agents, such as ether or cyclopropane, must not be used due to the risk of fire. Ulco
accepts no liability if the wrong anaesthetic agent is used.
1.7.6
1.7.6 Power Connection
1.7.61.7.6
Ulco equipment is to be used only in rooms with mains power supply installations complying with national safety
standards.
Powering the unit up for the first time
Powering the unit up Powering the unit up
Follow the Instructions for Use
Follow the InstrucFollow the Instruc
Liability for proper function or damage
Liability for proper function or damageLiability for proper function or damage
Maintenance and Repairs
Maintenance and RepairsMaintenance and Repairs
Use of anaesthetic agents
Use of anaesthetic agentsUse of anaesthetic agents
Power Connection
Power ConnectionPower Connection
for the first time
for the first timefor the first time
tions for Use
tions for Usetions for Use
8

Signet 615 User Manual
Electrical connections for other equipment not listed here should only be made following consultations with the
respective manufacturers or other expert.
For full details of the electrical environment in which the Signet may be used see Section 7.2 of this document.
1.7.7
1.7.7 Monitoring and alarms
1.7.71.7.7
If the unit alarms, check the patient. Establish that the patient is being ventilated correctly.
This unit must be used in conjunction with equipment providing the following alarms and monitoring capabilities:
These will help ensure patient safety, make precise ventilation possible and so achieve the best possible
ventilator parameters for the patient.
Monitoring and alarms
Monitoring and alarmsMonitoring and alarms
• Inspiratory oxygen concentration
• Anaesthetic agent concentration
• Airway pressure
• CO2 concentration
• Exhaled volume
• Breathing system integrity
• Continuing pressure
9
 Loading...
Loading...