Tuttnauer 25xx, 31xx, 28xx, 50xx Maintenance manual

OPERATION
&
MAINTENANCE
MANUAL
Laboratory Vertical Steam Sterilizers
models
2540, 3150, 3170, 3850, 3870, 5050, 5075
ELV
ELVC
ELVPRC
Cat. No. MAN205-0036000EN Rev AA
Tuttnauer Europe B.V., Hoeksteen 11 4815 PR P.O. Box 7191 4800 GD Breda, The Netherlands
Tel: 31 (0) 765423510, Fax: 31 (0) 765423540
Including Preparation for Fast Cooling System
Including Fast Cooling System
Standard Autoclave

TABLE OF CONTENTS
PARAGRAPH PAGE NO.
1 GENERAL......................................................................................................................4
1.1 Incoming Inspection...........................................................................................4
1.2 Warranty...............................................................................................................4
1.3 Warranty Statement ............................................................................................4
1.4 Ordering Information.........................................................................................5
2 SAFETY INSTRUCTIONS..........................................................................................6
3 TECHNICAL DATA.....................................................................................................8
3.1 Introduction..........................................................................................................8
3.2 Operating Conditions..........................................................................................9
3.3 Directives and Standards....................................................................................9
3.4 Environment Emission Information.................................................................9
3.5 Electrical Data:.................................................................................................10
3.6 Specifications....................................................................................................10
3.7 Loading Capacities...........................................................................................11
3.8 Utility..................................................................................................................11
3.9 Symbol Description ..........................................................................................12
3.10 Water Quality....................................................................................................17
3.11 Safety Features .................................................................................................18
3.12 Description of Operation.................................................................................19
4 STERILIZATION PROGRAMS..............................................................................20
4.1 Program 1 – Unwrapped Instruments (1 – Instruments)............................20
4.2 Program 2 (2- Instruments)............................................................................21
4.3 Program 3 (3 –Waste)......................................................................................22
4.4 Program 4 – (4 – Liquid)................................................................................. 23
4.5 Program 5 (5- Liquid)...................................................................................... 24
4.6 Program 6 (6-Liquid + Cool) – option on ELVC only.................................25
5 KEYBOARD (keys and display)...............................................................................26
5.1 Description and Functions of the Front Panel Keyboard ..........................27
5.2 Description of the Operational Messages .....................................................30
5.3 Displayed Error Messages and Safety Measures.........................................32
6 PRINTER ....................................................................................................................34
6.1 Printer Operation.............................................................................................34
6.2 DPU-20 Printer Handling ...............................................................................36
6.3 DPU 30 Printer Handling ...............................................................................37
7 PREPARATION BEFORE STERILIZATION......................................................38
7.1 Instruments .......................................................................................................38
7.2 Tubing................................................................................................................39
7.3 Liquids................................................................................................................39
7.4 Loading.............................................................................................................. 39
Page 1 of 58 Pages

8 OPERATION..............................................................................................................40
8.1 Operating Instructions.....................................................................................40
8.2 Moving the Autoclave......................................................................................41
8.3 Loading and Unloading the Device ...............................................................41
9 DOOR SAFETY SYSTEM ........................................................................................43
9.1 Solenoid locking device ................................................................................... 43
9.2 Door Safety System for models 5050, 5075...................................................43
9.3 Piston Lifting Device For 3850/3870/5050/5075 ELV ................................47
10 SERVICE AND MAINTENANCE..........................................................................48
10.1 Preventive Maintenance..................................................................................48
10.2 Replacing the Air Filter...................................................................................49
10.3 Replacing the Door Gasket ............................................................................. 50
10.4 Checking the Safety Valve...............................................................................50
10.5 Cleaning water outlet strainer........................................................................53
11 TROUBLESHOOTING.............................................................................................54
12 SPARE PARTS LIST.................................................................................................58
13 ACCESSORIES..........................................................................................................58
Page 2 of 58 Pages

TABLE OF CONTENT (Cont.)
DRAWINGS PAGE NO.
Overall Dimensions 2540 ELV...........................................................................................13
Overall Dimensions Drawing for the 3150 / 3170 ELV..................................................14
Overall Dimensions Drawing for the 3850 / 3870 ELV..................................................15
Overall Dimensions Drawing for the 5050/ 5075 ELV...................................................16
Front Panel Keyboard.........................................................................................................26
Baskets and Containers.......................................................................................................56
Page 3 of 58 Pages

1 GENERAL
1.1 Incoming Inspection
The autoclave should be unpacked and inspected for mechanical damage
upon receipt. Observe packing method and retain packing materials until
the unit has been inspected. Mechanical inspection involves checking for
signs of physical damage such as: scratched panel surfaces, broken knobs,
etc.
If damage is apparent, contact your dealer or point of purchase, so that
they may notify the manufacturer and file a claim with the appropriate
carrier.
All
Tuttnauer products are carefully inspected prior to shipment and all
reasonable precautions are taken in preparing them for shipment to assure
safe arrival at their destination.
1.2 Warranty
We certify that this instrument is guaranteed to be free from defects in
material and workmanship for one year against faulty components and
assembly with the exception of glassware, lamps and heaters.
The warranty does not include and does not replace routine treatment
and preventive maintenance to be performed according to instructions
in paragraph 10.1 (Preventive Maintenance).
Our obligation is limited to replacing the instrument or parts, after our
examination, if within one year after the date of shipment they prove to be
defective. This warranty does not apply to any instrument that has been
subjected to misuse, neglect, accident or improper installation or
application, nor shall it extend to products that have been repaired or
altered outside the factory without prior authorization from us.
The Autoclave should not be used in a manner not described in this
manual!
1.3 Warranty Statement
The warranty registration must be completed and returned to our service
departments; within fourteen (14) days of purchase or the warranty will be
void.
Our Technical Service Depts can be reached at:
Tuttnauer Europe b.v., Hoeksteen 11, 4815 PR Breda, Netherlands.
+31/76-5423510,
Fax: +31/76-5423540, E-mail: info@tuttnauer.nl
Rudolf Gunz & Co. PTY LTD:
Service Department, 26-34 Dunning Avenue, Ros, 2018, Sydney,
Australia.
Service Department, Locked bag 690, Beaconsfield, NSW 2014,
Australia.
+61-2-99356600 Fax: +61-2-99356650
Page 4 of 58 Pages

Note:
If there is any difficulty with this instrument, and the solution is not
covered in this manual, contact our representative or us first. Do not
attempt to service this instrument yourself. Describe the difficulty as
clearly as possible so we may be able to diagnose the problem and provide
a prompt solution.
If the autoclave is equipped with a printer, send along a copy of the last
printout for our inspection. If replacement parts are needed, stipulate the
model and serial number of the machine.
No products will be accepted for repair without proper authorization from
us. All transportation charges must be paid both ways by the owner. This
warranty will be void if the unit is not purchased from an authorized full
service
Tuttnauer dealer.
1.4 Ordering Information
Several items must be specified when ordering the unit from the dealer.
Exact model number (depending on desired chamber size).
Please specify the supply voltage available, 230/380V; 1PH or 3PH.
Temperature scale: Specify Centigrade or Fahrenheit.
Pressure scale: Specify kPa or psi.
1.4.1 Options
Printer option
Hanging temperature control probe for use with liquids, to
be placed directly inside a sample solution.
Cooling option - specify cooling method: with or without
compressed air. Air is used to balance the chamber
pressure during the cooling stage.
1.4.2 Accessories
Basket accessories: A set of two baskets is available for the unit.
The baskets are made of stainless steel wire and have a handle.
The basket allows the operator to load a large quantity of
materials into the chamber.
The pressure scale, printer option and cooling method can be set
up at any time by a technician.
Stainless steel containers: A stainless steel container, for waste
products, is available. This container has vent holes on its upper
part
Page 5 of 58 Pages

2 SAFETY INSTRUCTIONS
The autoclave has unique characteristics. Please read and understand the operation
instructions before first operation of the autoclave. The following issues may
require instructions guidance provided by the manufacturer: how to operate the
autoclave, the door safety mechanism, the dangers involved in circumventing
safety means, how to ensure that the door is closed, and how to select a correct
sterilization program.
Make sure that you know where the main power switch is, where the water cut-off
valve is and where the compressed air disconnection valves (if applicable) is
located.
Autoclave maintenance is crucial for the correct and efficient function of the
device. We enclose a log booklet that includes maintenance recommendations,
with every device.
The weekly spore test is part of the preventive maintenance plan, along with the
annual validation of the sterilization processes that ensures appropriate
temperature dispersion within the chamber.
Never use the autoclave to sterilize corrosive products, such as: acids, bases and
phenols, volatile compounds or solutions such ethanol, methanol or chloroform
nor radioactive substances.
1. Never start using a new autoclave before the safety, licensing and
authorization department has approved it for use.
2. All autoclave users must receive training in proper usage from an
experienced employee. Every new employee must undergo a training
period under an experienced employee.
3. A written procedure must be established for autoclave operation,
including: daily safety tests, seal inspection and door hinge inspection,
smooth action of the closing mechanism, chamber cleaning, prevention of
clogging and preservation from corrosion, what is permitted and what is
prohibited for sterilization and choosing a sterilization program.
4. Liquids may be sterilized only with the “liquids” program. The container
must be covered but not sealed. Sealed bottles may only be sterilized using
a special program. The bottle must be either Pyrex or a Borosilicate glass
bottle.
5. When sterilizing plastic materials, make sure that the item can withstand
sterilization temperature. Plastic that melts in the chamber is liable to
cause a great deal of damage.
6. Individual glass bottles may be placed within an appropriate container that
will be placed in a basket. Never place glass bottles on the floor of the
autoclave. Never fill more than 2/3 of the bottle volume.
7. On closing the autoclave's door, make sure it is properly locked before
activating.
8. Before withdrawing baskets, wear heat resistant gloves.
9. Before opening the door, verify that there is no pressure in the chamber
(chamber pressure gauge is located on the autoclave's front panel or door,
depends on model).
10. Open the door slowly to allow steam to escape and wait 5 minutes before
you remove the load. When sterilizing liquids, wait 10 minutes.
Page 6 of 58 Pages

11. Once a month, ensure that the safety valves are functioning, and once
annually a certified tester must conduct pressure chamber safety tests.
12. Once annually, or more frequently, effective tests must be performed, i.e.,
calibration and validation.
13. Examine the condition of assemblies on a regular basis. Make sure there
are no leaks, breaks, blockages, whistles or strange noises.
14. It is required to conduct maintenance operations as instructed.
15. Immediately notify the person in charge of any deviation or risk for the
proper function of the device.
Page 7 of 58 Pages

3 TECHNICAL DATA
3.1 Introduction
Models 2540, 3150, 3170, 3850, 3870, 5050 and 5075, 5090 ELV are
vertical sterilizers designed especially for sterilization of instruments,
liquids and other materials in hospital laboratories, medical laboratories,
research institutes, food laboratories and pharmaceutical facilities.
A special feature of this sterilizer is the fast cooling operation, provided as
an option for the liquids sterilization cycle.
The sterilizer is fully automatic with a choice of six programs, eliminating
any need for operator intervention during a cycle. The sixth program is an
optional program including a cooling stage (program 7 is for leakage test
only). A computerized control unit enables precise control and monitoring
of physical parameters and clear documentation of the sterilization cycles.
The autoclave is equipped with a safety valve, which blows off at an
overpressure of over 25 psi, which is located on the rear of the autoclave.
The control system provides adequate protection, to ensure the safety of
personnel and reliable operation with a minimum of down time.
On all models (except 1730), a printer is an optional addition to the
autoclave. The printer prints the preset and actual parameters of the cycle
(temperature, time and pressure/vacuum).
The autoclave is equipped with a pressure gauge designed for reference
only. In case that there is any problem with electricity supply during a
sterilization cycle the pressure gauge will be used to verify that there is
pressure in the chamber.
This manual provides information for the following models:
ELV – this is the basic model of the vertical laboratory autoclave.
ELVC – this model includes a cooling system that enables cycles with
fast cooling.
ELVPRC – this model includes only the cooling coil as a preparation
for a cooling system but performs as ELV. A Tuttnauer technician can
upgrade this model to ELVC.
This manual is intended to give the user a general understanding of how
the autoclave works and indicates best ways to operate and maintain it in
order to obtain optimum results and a trouble free operation.
After reading this manual, operating the autoclave should be straight
forward. However, since the autoclave is built using high technology
sensitive components, no attempt should be made by the user or any other
unauthorized person to repair or re-calibrate it.
Only technical personnel having proper qualifications, holding technical
documentation and adequate test instrumentation, are authorized to
undertake repair or service.
Page 8 of 58 Pages

3.2 Operating Conditions
The autoclave is intended to work in ‘indoor’ conditions only.
Only autoclavable materials shall be used.
The ambient temperature shall not exceed 40ºC and a relative
humidity up to 85%.
The autoclave shall not be used in a manner not specified in this
manual!
Do not use the autoclave in the presence of dangerous gases.
The packed or unpacked device shall be stored in ‘indoor’ conditions.
Caution
Waste water should be brought into the public net in
accordance with the local rules or requirements i.e. only nonhazardous liquids shall be disposed in public sewage!
3.3 Directives and Standards
Every autoclave meets the provisions of the following Directives and is
constructed in compliance with the following Standards:
3.3.1 Technical Directives
1. Pressure Equipment Directive 97/23/EC.
2. Council Directive for voltage limits 73/23/EEC.
3. Electromagnetic Compatibility Requirements Directive
89/336/EC.
3.3.2 Technical Standards
1. ASME code (models 2540, 3150, 3850, 3870).
2. EN 13445 (models 5050, 5075).
3. IEC-61010-1 & IEC-61010-2-040 - Safety requirements for
medical device.
4. EN 61326 – EMC Requirements .
3.3.3 Quality standards
The manufacturing plant meets the following quality standards:
1. EN ISO 9001:2008– Quality System
2. ISO 13485:2003 – Quality systems – Medical devices.
3.4 Environment Emission Information
A. Peak sound level generated by the sterilizer is « 70 / dBA with a back
sound level of 60 dBA.
B. Total heat transmitted by the sterilizer is < 100 W/h
Page 9 of 58 Pages
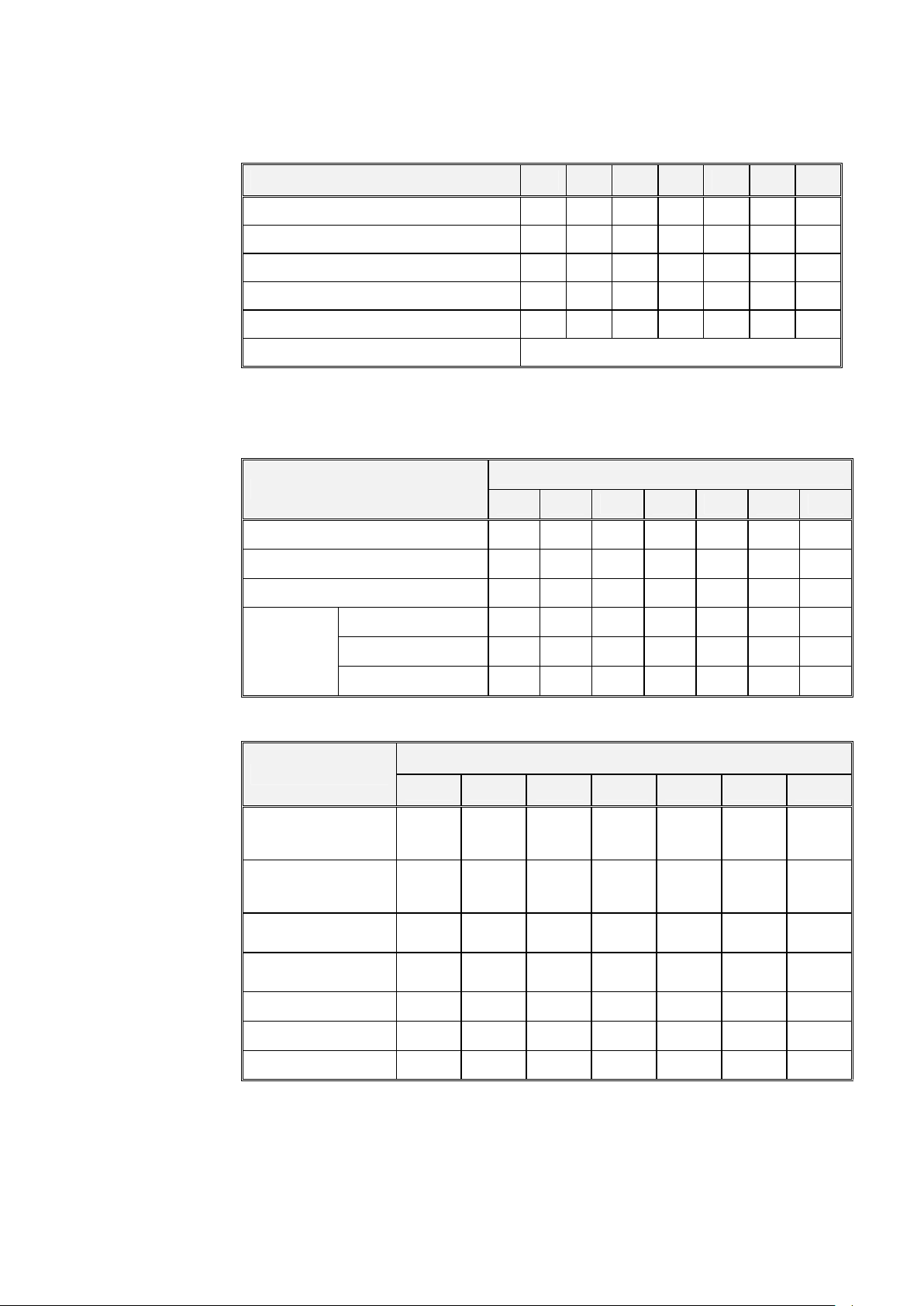
3.5 Electrical Data:
St. St.
St. St.
St. St.
St. St.
St. St.
St. St.
St. St.
St. St.
St. St.
Air supply pressure
Feed water pressure
To be regulated (Bar)
)
Chamber insulation
-
Fiber glass with reinforced material
2540 3150 3170 3850 3870 5050 5075
Ampere (A) at 1Ph/230V/50/60 Hz
Ampere (A) at 3Ph/380V/50/60 Hz
Ampere (A) at 3Ph/230V/50/60 Hz
Ampere (A) at 3Ph/208V/50/60 Hz
Watts (W) 2400 3300 3300 6000 6000 9000 9000
Protection against electrical shock Class I
Models 3850/70, 5050/75 are equipped with a switching box for 1 or 2 h to 3Ph
3.6 Specifications
3.6.1 Dimensions
DIMENSIONS
Chamber diameter in mm. 250 310 310 380 380 500 500
Chamber depth in mm. 400 550 730 480 680 520 750
Chamber volume (lit.) 23 40 59 65 85 110 160
Overall
dimensions
26 26 39 39
8.7 8.7 13.0 13.0
17 17 23 23
17 17 23 23
MODEL
2540 3150 3170 3850 3870 5050 5075
Height (mm) 665 780 960 760 960 770 1000
Width (mm) 500 580 580 650 650 700 700
Length (mm.) 335 420 420 500 500 880 880
3.6.2 Technical Data
PROPERTY
2540 3150 3170 3850 3870 5050 5075
Chamber material
Door material
to be regulated (Bar)
316 Ti
(1.4571)
ST.ST.
304L
3-8 3-8 3-8 3-8 3-8 3-8 3-8
2-3 2-3 2-3 2-3 2-3 2-3 2-3
Net weight (kg)
Shipping volume (m
Shipping weight (kg)
48 45 53 85 100 171 190
3
0.21 0.56 0.56 0.56 0.56 1.35 1.35
57 85 93 101 116 204 223
Outer Cabinet - Stainless Steel
316 Ti
ST.ST.
304L
316 Ti
ST.ST.
304L
MODEL
316 Ti
ST.ST.
304L
316 Ti
ST.ST.
304L
316 Ti
316 Ti
316 Ti
316 Ti
Page 10 of 58 Pages
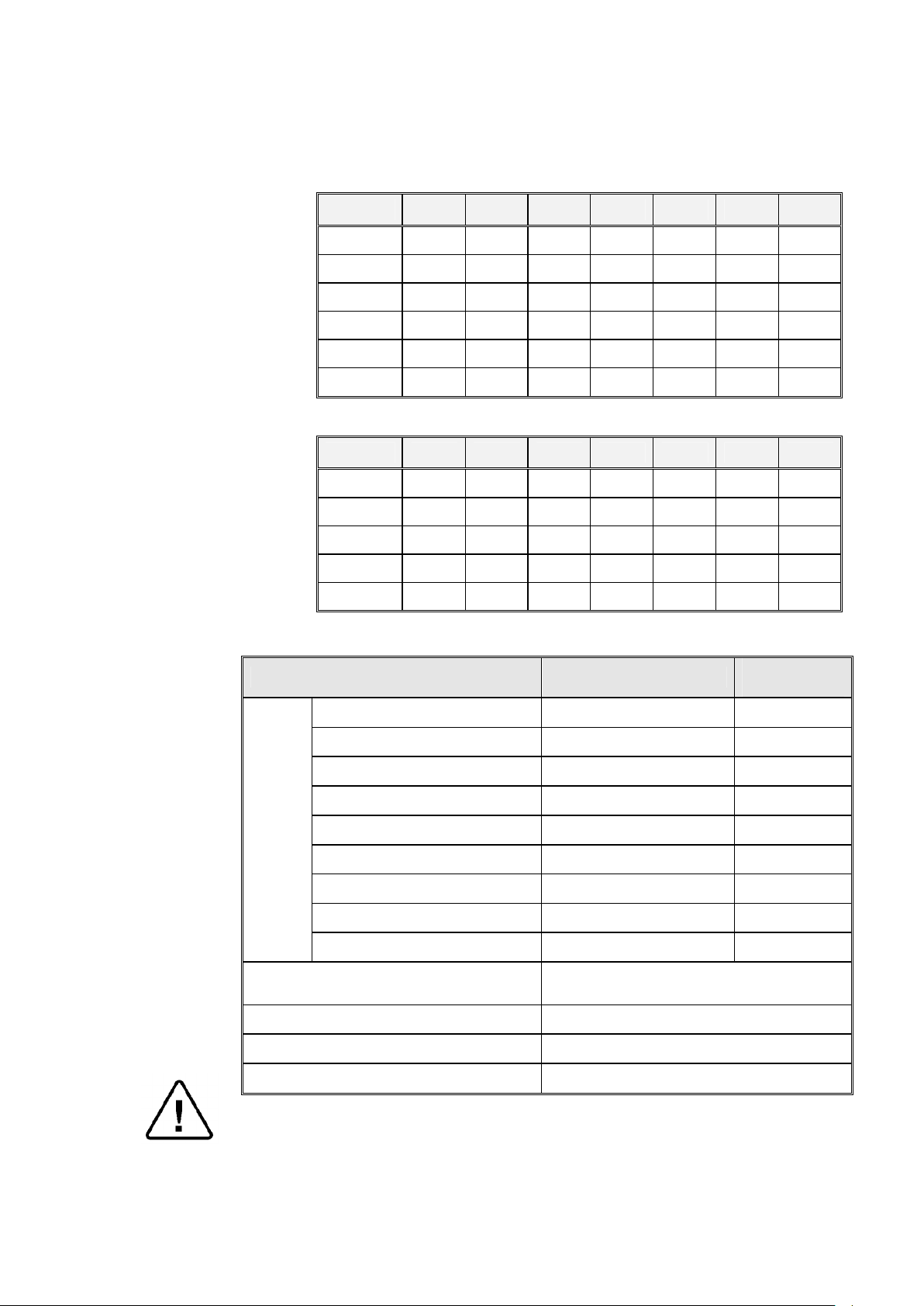
3.7 Loading Capacities
Recommended
Compressed Air (ELVC models
3.7.1 Erlenmeyer Flasks
SIZE 2540 3150 3170 3850 3870 5050 5075
250 ml 2 x 4 3 x 7 3 x 7 2 x 12 3 x 12 2 x 21 3 x 21
500 ml 1 x 2 2 x 4 3 x 4 2 x 8 3 x 8 2 x 14 3 x 14
1000 ml 1 2 x 2 3 x 2 1 x 5 2 x 5 2 x 8 3 x 8
2000 ml 1 1 2 x 1 1x2 2 x 2 1x5 2 x 5
3000 ml 1 1 2 x 1 1 2 x 1 1x4 2 x 4
5000 ml — 1 1 1 1 1x2 1x2
3.7.2 Medium Flasks (Schott)
SIZE 2540 3150 3170 3850 3870 5050 5075
250 ml. 2 x 7 3 x 11 3 x 11 2 x 19 3 x 19 2 x 32 3 x 32
500 ml 1 x 4 2 x 7 3 x 7 2 x 12 3 x 12 2 x 21 3 x 21
1000 ml 1x3 1 x 5 2 x 5 1 x 8 2 x 8 2 x 15 3 x 15
2000 ml 1 31x2 2 x 2 1x4 2 x 4 1x8 2 x 8
3.8 Utility
Power
Supply
only)
Tap water
5000 ml 1 1 1 1 1 1x4 2 x 4
Utility Power
2540
3150/70
3850/70
3850/70
3850/70 with switching box
3850/70, 5050/75
5050/75
5050/75
1 or 2 Ph, 230V/50/60Hz 25A
3 Ph, 208V/50/60Hz 25A
3 Ph, 230V/50/60Hz 25A
1 or 2 Ph, 230V/50/60Hz 30A
3 Ph, 400V/50/60Hz 20A
3 Ph, 208V/50/60Hz 30A
3 Ph, 230V/50/60Hz 30A
5050/75 with switching box
1Ph, 230V/50/60Hz 20A
Circuit Breaker
45A
1/2" 3-4 Bar
1/2", 2-3 Bar
Mineral free water
Drain
Withstanding temp. of 80°C
1/2", 2-4 Bar
Attention:
The electrical net must be protected with a current leakage safety relay.
The electrical network must comply with local rules or regulations.
Page 11 of 58 Pages
3.9 Symbol Description
On-Off
Caution! Consult accompanying documents
Caution! Hot surface.
Caution! Hot steam.
Protective earth (Ground)
Page 12 of 58 Pages

OVERALL DIMENSIONS 2540 ELV
Page 13 of 58 Pages
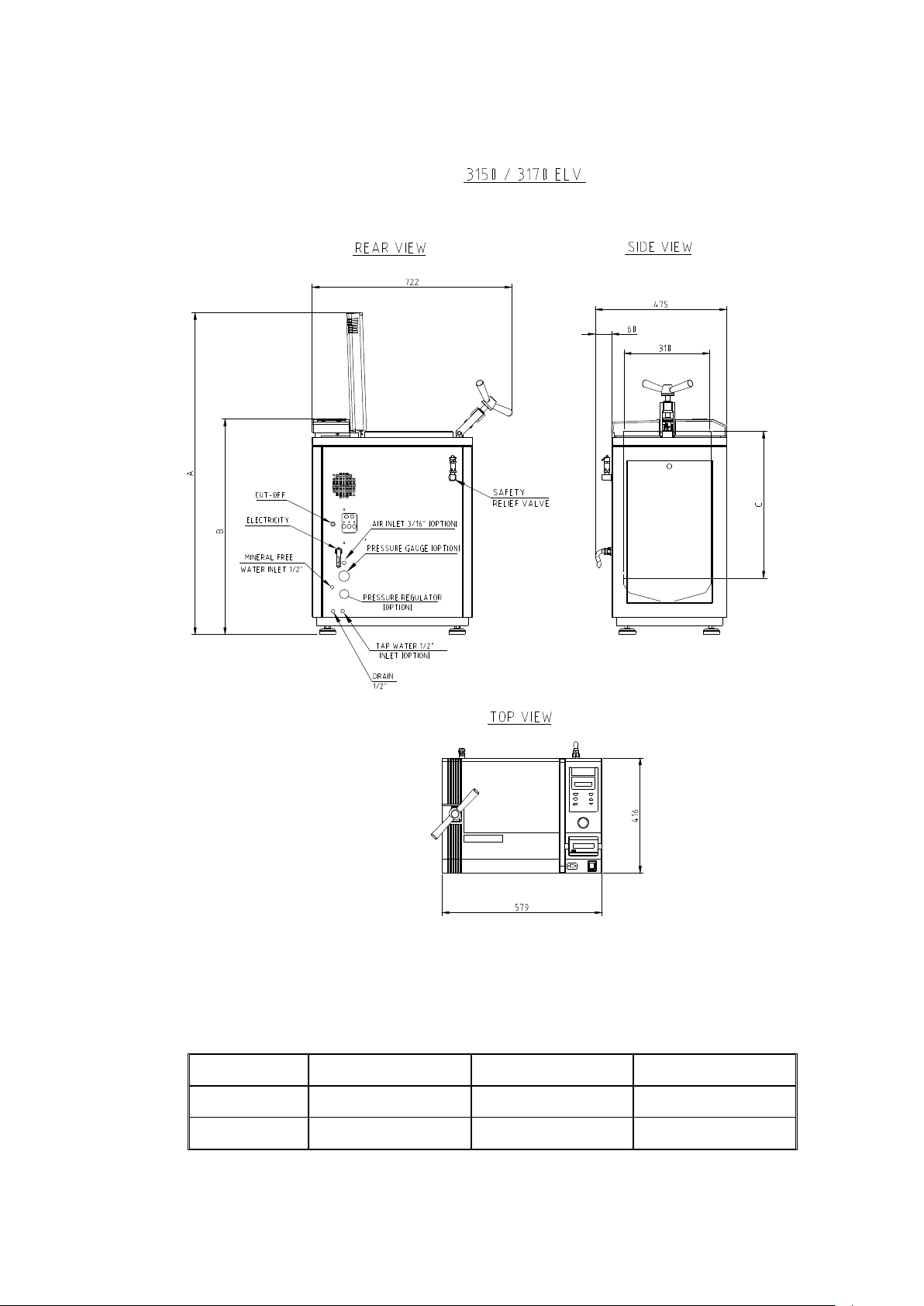
OVERALL DIMENSIONS DRAWING FOR THE 3150 / 3170 ELV
Note:
The dimensions A, B and C are different for the models 3150 and 3170, as
indicated below
TYPE A B C
3150
3170
1165 780 530
1345 960 710
Page 14 of 58 Pages
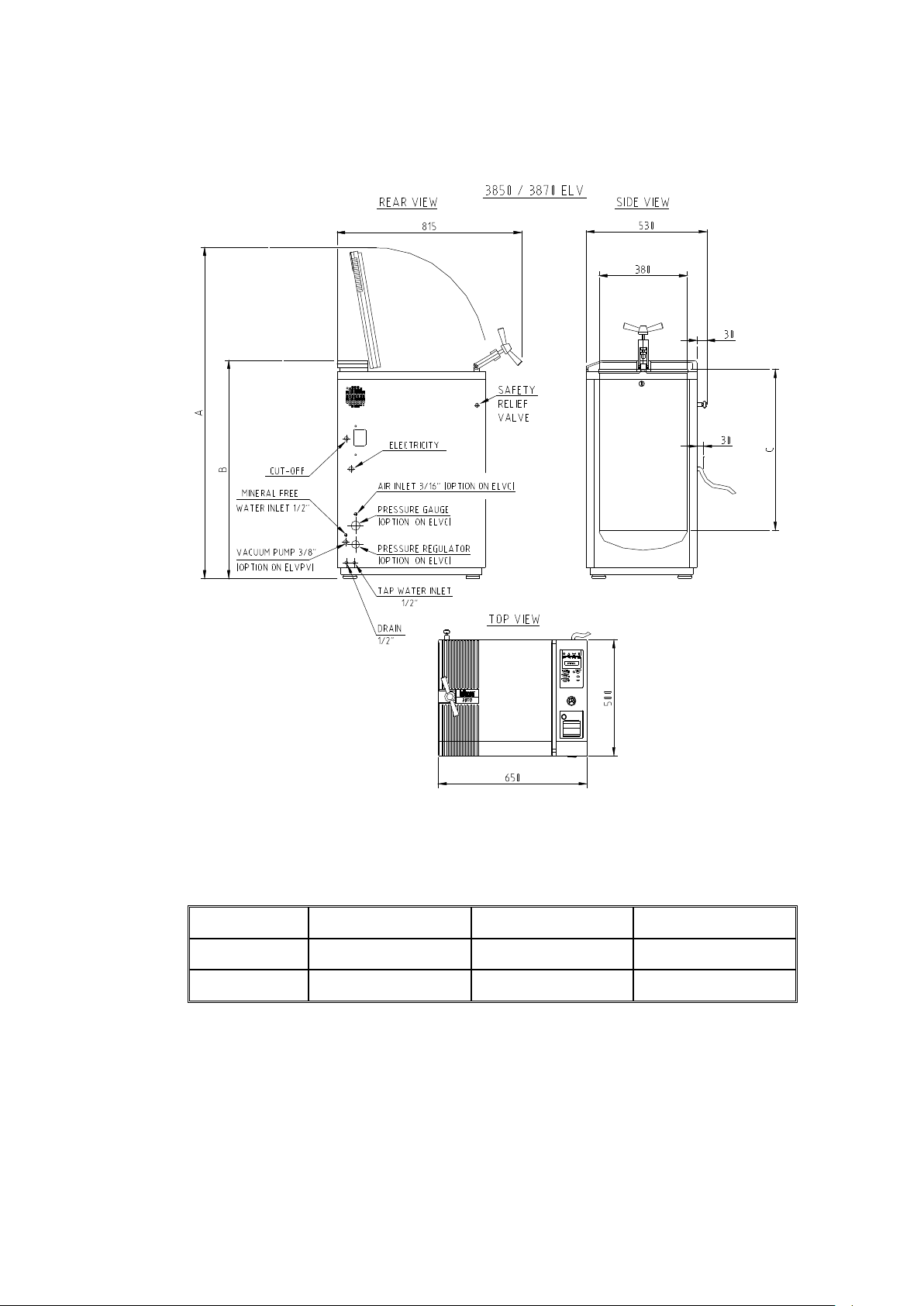
OVERALL DIMENSIONS DRAWING FOR THE 3850 / 3870 ELV
Note:
The dimensions A, B and C are different for the models 3850 and 3870, as
indicated below
TYPE A B C
3850
3870
1220 745 500
1400 925 680
Page 15 of 58 Pages
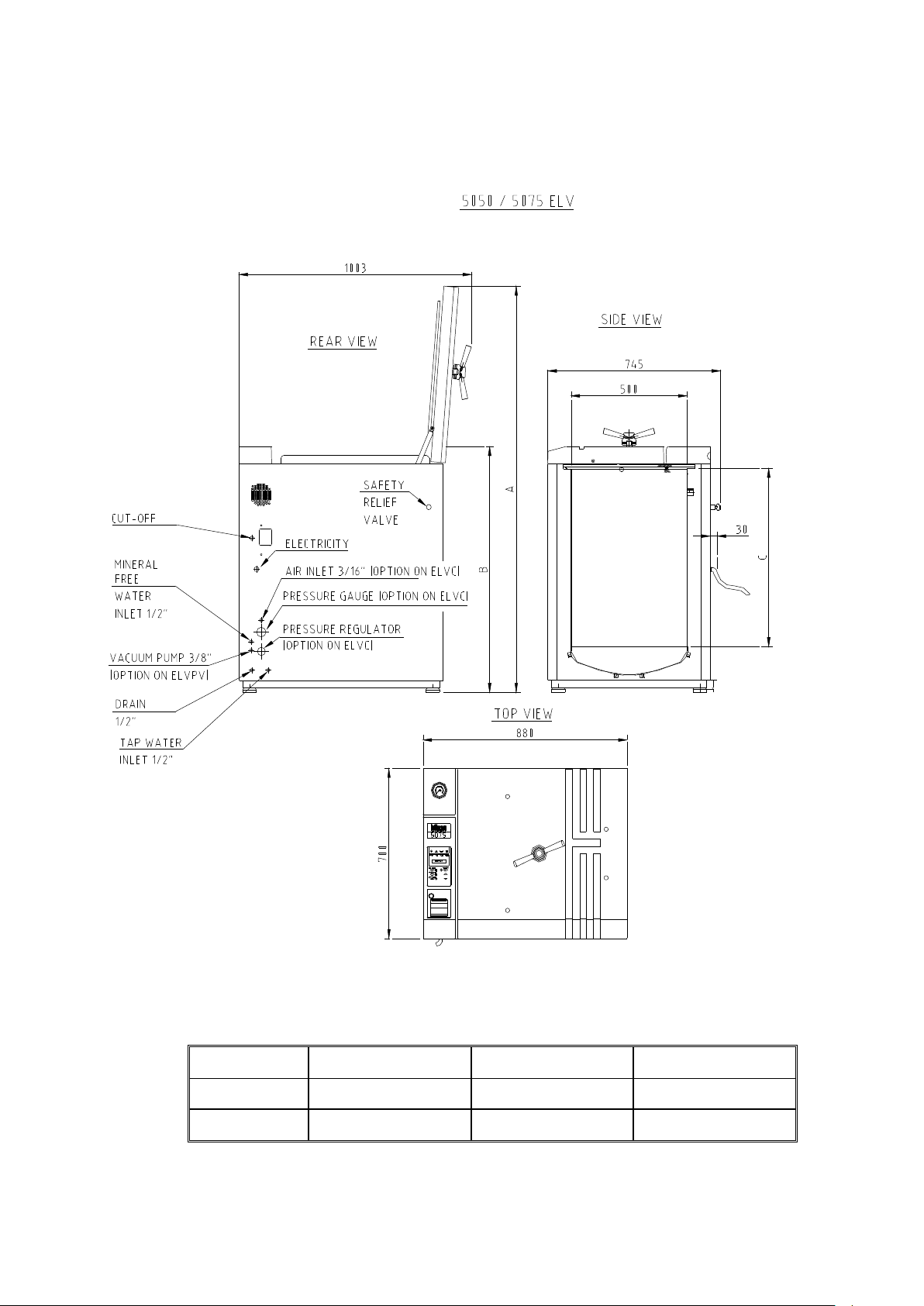
OVERALL DIMENSIONS DRAWING FOR THE 5050/ 5075 ELV
Note:
The dimensions A, B and C are different for the models 5050 and 5075, as
indicated below
TYPE A B C
5050
5075
1440 780 503
1670 1010 733
Page 16 of 58 Pages
3.10 Water Quality
Physical Characteristics and Maximum acceptable
Condensate
–
allowable
Rest of metals except iron,
Colourless clean without
Hardness (Σ Ions of alkaline
3.10.1 Water for Steam Generation
The distilled or mineral – free water supplied to the autoclave
should have the physical characteristics and maximum
acceptable level of contaminants indicated in the table below:
contaminants levels in steam for sterlizers
(According to EN 13060:2004).
Element
Silicium oxide. SiO
2
≤0.1 mg/kg
content
Iron ≤0.1 mg/kg
Cadmium ≤0.005 mg/kg
Lead ≤ 0.05 mg/kg
cadmium, lead
≤0.1 mg/kg
Chloride (Cl) ≤0.1 mg/kg
Phosphate (P2O5) ≤0.1 mg/kg
Conductivity (at 20°C) ≤3 μs/cm
pH value (degree of acidity) 5 to 7
Appearance
earth)
sediment
≤0.02 mmol/l
Compliance with the above data should be tested in accordance
with acknowledged analytical methods, by an authorized
laboratory.
Attention:
month. The use of water for autoclaves that does not comply
with the table above may have severe impact on the working
life of the sterilizer and can invalidate the manufacturer’s
guarantee.
3.10.2 Drain Cooling
The feed water supplied to the drain cooling must meet the
following requirements:
Hardness: 0.7 - 0.2 mmol/l.
Water temperature shall not exceed 15°C.
We recommend testing the water quality once a
Page 17 of 58 Pages
 Loading...
Loading...