TRUTH Vitality LUX RENEW Instruction Manual

Instruction Manual

TABLE OF CONTENTS
INTRODUCTION .................................................3
PRODUCT DESCRIPTION ...........................................3
SPECIFICATIONS.................................................4
GENERAL WARNINGS . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 5
General Precautions ..............................................5
General Instructions ..............................................5
General Preparation for Use.........................................5
LED (LIGHT EMITTING DIODE) ........................................6
Guide for Use for the LED Mode ......................................6
Contraindications for the LED Mode ...................................8
Warnings for the LED Mode .........................................8
Precautions for the LED Mode .......................................8
Risks for the LED Mode............................................8
Benefits for the LED Mode ..........................................8
Preparation for Use for the LED Mode ..................................9
INSTRUCTIONS, DIRECTIONS FOR USE, & OPERATING INSTRUCTIONS FOR THE LED MODE . 9
When to Seek Medical Assistance for the LED Mode ....................... 11
Alternative Procedures and Treatments for the LED Mode ................... 11
ULTRASONIC ..................................................12
Guide for Use for the Ultrasonic Mode.................................12
Contraindications for the Ultrasonic Mode ..............................12
Warnings for the Ultrasonic Mode ....................................12
Precautions for the Ultrasonic Mode ..................................12
Risks for the Ultrasonic Mode.......................................12
Benefits for the Ultrasonic Mode .....................................12
Preparation for Use for the Ultrasonic Mode.............................13
INSTRUCTIONS, DIRECTIONS FOR USE, & OPERATING INSTRUCTIONS FOR THE ULTRASONIC MODE
IMPORTANT INFORMATION.........................................15
INDICATIONS FOR USE ...........................................15
Glossary .....................................................15
After Use Information.............................................15
Troubleshooting ................................................15
..13
Home & Mobile Environment ........................................15
Travel with Device ..............................................16
Warranty Information.............................................16
2
INTRODUCTION
MODE
ON/OFF
CFMSHIFT
Thank you for purchasing the Truth Vitality Lux Renew LED and ultrasonic device. Please read these instructions before
use and keep them in a safe place for future reference.
The Lux Renew is designed for normal skin. Please consult your medical practitioner if you have any specifi c skincare
concerns or conditions.
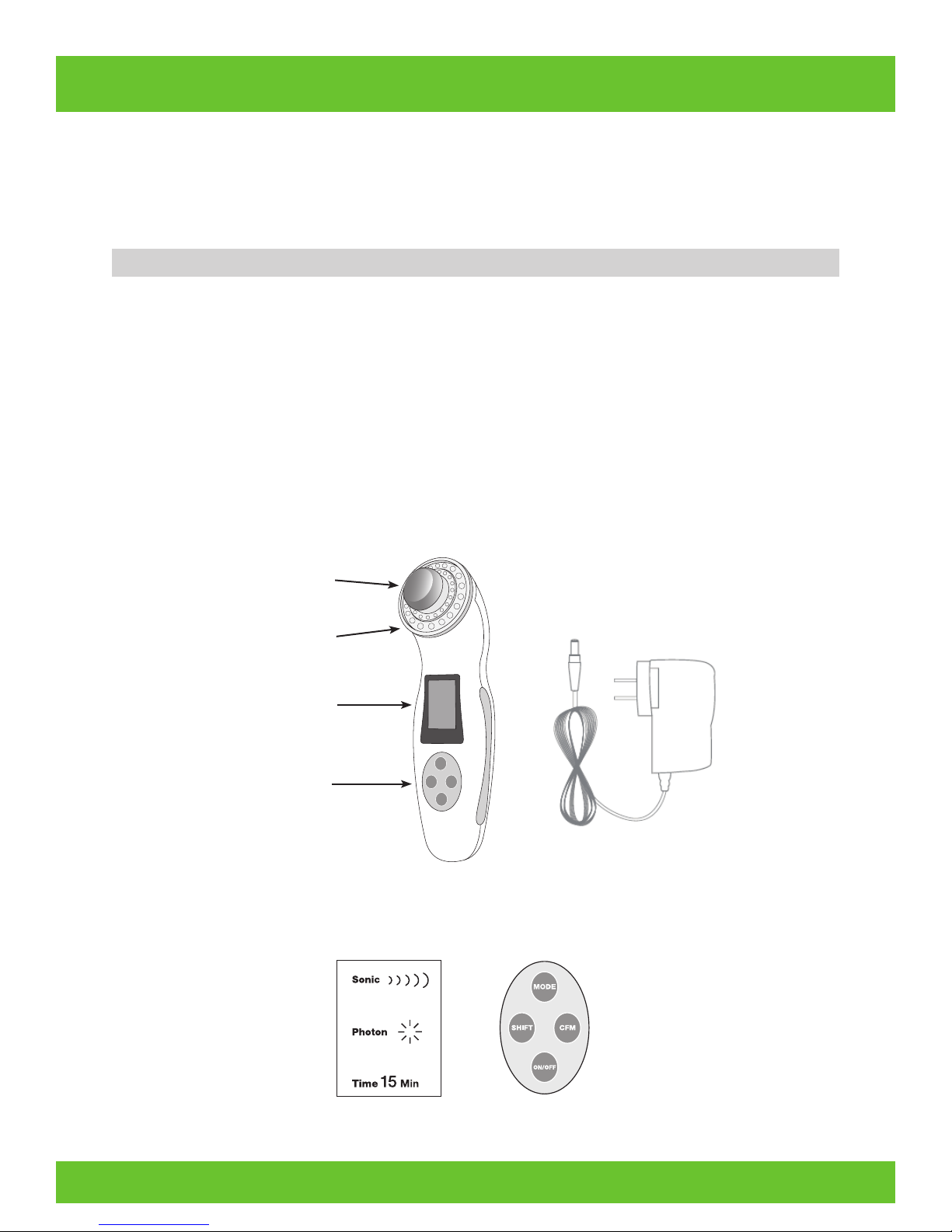
PRODUCT DESCRIPTION
The Lux Renew consists of two major components, the hand piece, which contains the functional elements of the device,
and the power adaptor. Protective goggles are provided as an accessory to the device.
The Lux Renew has two functional modes; LED and ultrasonic. Multiple types of treatments are available using the Lux
Renew. Each of the treatments is linked to a particular mode of the Lux Renew.
The LED mode has two colors available, red and blue. The ultrasonic mode uses ultrasound for anti-aging and to relieve
minor muscle aches and pains. The ultrasonic mode of Lux Renew has fi ve levels of intensity.
Treatment head
LED light
LCD display
Control panel
Power Adapter
LCD display Control panel
3
Type BF applied part.
SPECIFICATIONS
IPX0
Only use with Class II ungrounded power adaptor Kuantech KSAS7R51530040D5
Adaptor input power: AC 100-120V
Adaptor output power: 15V, 400mA
Ultrasonic frequency: 3 MHz ± 5%
Storage Conditions
• -25˚ C to 55˚ C
• 10% - 85% humidity, Non-condensing
• Altitude < 8000 ft
Transportation
• The unit should be protected against vibration and shock during transportation.
Operating Environment
• 10˚ C - 35˚ C
• 30% - 75% humidity, Non-condensing
• Altitude < 8000 ft.
The Lux Renew is considered IPX0 for ingress protection as defi ned by IEC 60601-1.
Attention, consult accompanying documents.
Consult instructions for use.
4

GENERAL WARNINGS
• If you are in the care of a physician, consult with your physician before using this device.
• Do not use if pregnant.
• Do not share the Lux Renew with another user due to the possibility of transferring bacteria/diseases.
• Do not use the Lux Renew for longer than 20 minutes continuous use.
• TO REDUCE THE RISK OF FIRE, ELECTRIC SHOCK OR PERSONAL INJURY:
Do not place or store the device where it can fall or be pulled into a tub or sink. Do not place in or drop into
water or other liquid.
Keep all electrical appliances away from water (including bath and shower).
If the device accidentally falls in water unplug immediately.
Turn off and unplug device before cleaning.
• Do not operate the unit if the cord or unit has been damaged. Electric shock could occur.
• Do not attempt to use alternate power supplies.
• Inspect this device prior to each use. Check for damage.
• Operate, transport and store the Lux Renew per the manner specified in this manual.
• No user serviceable parts. Do not attempt to disassemble the Lux Renew. This may cause the device to be
damaged, malfunction or may cause personal injury.
• Stop immediately if you experience any adverse effect or have a concern regarding use. Contact your healthcare provider.
• Do not operate this device in an environment where other devices, which intentionally radiate electromagnetic
energy in an unshielded manner, are being used. Portable or mobile Radio Frequency communication equipment
can affect medical electrical equipment.
GENERAL PRECAUTIONS
• This product should not be used in the presence of flammable anesthetics.
• Read all instructions before using your Lux Renew. Failure to do so may result in failure to understand safety
information or how the device is used.
• Inspect this device prior to each use. Check for damage. Damage includes cracks in the device, frayed wires,
buttons not present, or a broken display. Failure to do so may result in improper functioning of the device.
• Do not place device near any heat sources such as radiators, heat registers, stoves or other apparatus that produce heat.
• Keep the cord away from heated surfaces.
• Operate, transport and store the device per manner specified in this manual. Failure to do so may result in
damage to the unit.
GENERAL INSTRUCTIONS
• There are four buttons: ON/OFF; MODE (to select Sonic, Photon or Time); SHIFT (to choose intensity or
time); and CFM (CONFIRM) to start.
• To adjust the time of your session: Press MODE repeatedly until Time is selected (it will flash). Press SHIFT to
adjust Time from 1 – 19 minutes. Press CFM to start your session. The Lux Renew will turn off automatically
after the selected treatment time is completed.
GENERAL PREPARATION FOR USE
Plug the power adapter into bottom of your Lux Renew hand piece and then into a power outlet. The power jack should
fit snugly into the hand piece. Use only the power adaptor that comes with your Lux Renew. If you lose the power adaptor or it is damaged, contact us at customerservice@truthinaging.com for a new one.
Use on a clean face, without makeup. Men should shave before treatment, as hair can interfere with the treatment. The
facial area under a beard or mustache cannot be treated.
Use the Lux Renew in front of a mirror in a well lit room.
5
 Loading...
Loading...