Page 1
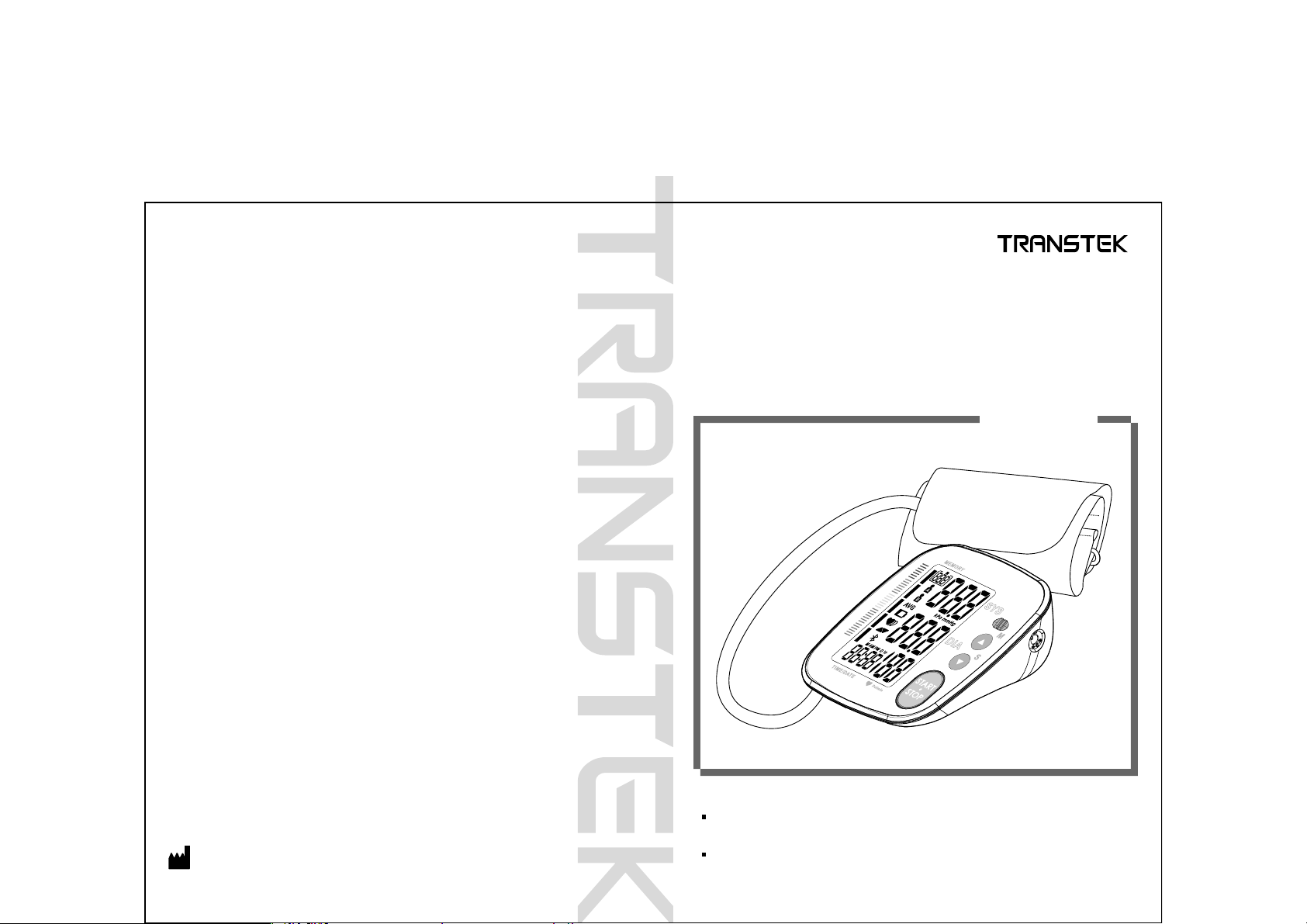
User Manual
Blood Pressure Monitor
TMB-1490-BS
Arm Type
Thank you very much for selecting TRANSTEK Blood Pressure Monitor
TMB-1490-BS.
Please do read the user manual carefully and thoroughtly so as to ensure
the safe usage of this product, and keep the manual well for further
reference in case you have problems.
version:1.0
FCC ID:OU9TMB1490BS
Guangdong Transtek Medical Electronics Co., Ltd.
Zone B, No.105 ,Dongli Road, Torch Development District,
Zhongshan,528437,Guangdong,China
Document No.: LS-INS-TMB-1490-BS-2
Version: A0
Page 2

INTRODUCTION..................................................................................................................2
General Description
Indications for Use
Safety Information
LCD Display Signal
Monitor Components
List
BEFORE YOU START..........................................................................................................8
The Choice of Power Supply
Installing and Replacing the Batteries
Measurement Principle
Setting Date, Time and Measurement Unit
MEASUREMENT................................................................................................................12
Tie the Cuff
Start the Measurement
DATA MANAGEMENT........................................................................................................15
Recall the Records
Delete the Records
INFORMATION FOR USER...............................................................................................17
Tips for measurement
Maintenances
ABOUT BLOOD PRESSURE.............................................................................................19
What are systolic pressure and diastolic pressure?
What is the standard blood pressure classification?
Irregular heartbeat detector
Why does my blood pressure fluctuate throughout the day?
Why do I get a different blood pressure at home compared to the hospital?
Is the result the same if measuring on the right arm?
TROUBLESHOOTING.......................................................................................................21
SPECIFICATIONS..............................................................................................................22
AUTHORIZED COMPONENT ...........................................................................................23
CONTACT INFORMATION.................................................................................................23
FCC STATEMENT...............................................................................................................24
COMPLIED STANDARDS LIST..........................................................................................25
EMC GUIDANCE................................................................................................................26
Table of Contents
CATALOGUE
Contraindications
Page 3
Thank you for selecting TRANSTEK arm type blood pressure monitor
(TMB-1490-BS). The monitor features blood pressure measurement, pulse
rate measurement and the result storage. The design provides you with
two years of reliable service.
Readings taken by the TMB-1490-BS are equivalent to those obtained by
a trained observer using the cuff and stethoscope auscultation method.
This manual contains important safety and care information, and
provides step by step instructions for using the product.
Read the manual thoroughly before using the product.
Features:
Maximum 250 records per each user
General Description
3rd technonoly: Measuring during inflation
(The updated technology in the world)
60.5 mm×92.5 mm Digital LCD display
INTRODUCTION INTRODUCTION
Indications for Use
The Transtek Blood Pressure Monitor is digital monitors intended for use in
measuring blood pressure and heartbeat rate with arm circumference ranging
IURPFPWRFPDERXWôÝòÝRUFPWRFPDERXWôÝòÝ
It is intended for adult indoor use only.
* Be careful to strangulation due to cables and hoses, particularly due to excessive length.
* At least 30 min required for ME equipment to warm from the minimum storage temperature between
uses until it is ready for intended use. At least 30 min required for ME equipment to cool from the
maximum storage temperature between uses until it is ready for intended use.
* This equipment needs to be installed and put into service in accordance with the information provided in
the ACCOMPANYING DOCUMENTS;
* Wireless communications equipment such as wireless home network devices, mobile phones, cordless
telephones and their base stations, walkie-talkies can affect this equipment and should be kept at least a
distance d away from the equipment. The distance d is calculated by the MANUFACTURER from the 80
MHz to 5.8 GHz column of Table 4 and Table 9 of IEC 60601-1-2:2014, as appropriate.
* Please use ACCESSORIES and detachable partes specified/ authorised by MANUFACTURE.
Otherwise, it may cause damage to the unit or danger to the user/patients.
* There is no luer lock connectors are used in the construction of tubing, there is a possibility that they
might be inadvertently connected to intravascular fluid systems, allowing air to be pumped into a blood
vessel.
* Please use the device under the environment which was provided in the user manual. Otherwise, the
performance and lifetime of the device will be impacted and reduced.
CAUTION
Safety Information
The signs below might be in the user manual, labeling or other component.
They are the requirement of standard and using.
Symbol for “THE OPERATION
GUIDE MUST BE READ”
Symbol for “MANUFACTURER”
Symbol for “SERIAL NUMBER”
Symbol for “TYPE BF APPLIED
PARTS”
Symbol for “DIRECT CURRENT”
Symbol for “ENVIRONMENT
PROTECTION - Electrical waste
products should not be disposed of
with household waste. Please recycle
where facilities exist. Check with your
local authority or retailer for recycling
advice”
Symbol for “MANUFACTURE
DATE”
For indoor use only
F1
7$9ĭ&&&
Symbol for “Class II Equipment”
Caution: These notes must be
observed to prevent any damage
to the device.
SN
The Green Dot is the license
symbol of a European network of
industry-funded systems for
recycling the packaging materials
of consumer goods.
Symbol for “Recycle”
1.The device is not suitable for use on may be pregnant women or pregnant women.
2.The device is not suitable for use on patients with implanted,electrical devices,
such as cardiac pacemakers, defibrillators.
Contraindications
Page 4

CAUTION
* This device is intended for adult use in homes only.
* The device is not suitable for use on neonatal patients, pregnant women,patients with implanted,
electronical devices, patients with pre-eclampsia, premature ventricular beats, atrial fibrillation, peripheral,
arterial disease and patients undergoing intravascular therapy or arterio-venous shunt or people who
received a mastectomy. Please consult your doctor prior to using the unit if you suffer from illnesses.
* The device is not suitable for measuring the blood pressure of children. Ask your doctor before using it on
older children.
* The device is not intended for patient transport outside a healthcare facility.
* The device is not intended for public use.
* This device is intended for no-invasive measuring and monitoring of arterial blood pressure.
It is not intended for use on extremities other than the arm or for functions other than obtaining a blood
pressure measurement.
* Do not confuse self-monitoring with self-diagnosis. This unit allows you to monitor your blood pressure.Do
not begin or end medical treatment without asking a physician for treatment advice.
* If you are taking medication,consult your physician to determine the most appropriate time to measure your
blood pressure. Never change a prescribed medication without consulting your physician.
* Do not take any therapeutic measures on the basis of a self measurement. Never alter the dose of a
medicine prescribed by a doctor. Consult your doctor if you have any question about your blood pressure.
* When the device was used to measure patients who have common arrhythmias such as atrial or ventricular
premature beats or atrial fibrillation, the best result may occur with deviation. Please consult your physician
about the result.
* Don't kink the connection tube during use, otherwise, the cuff pressure may continuously increase which
can prevent blood flow and result in harmful injury to the PATIENT.
* When using this device, please pay attention to the following situation which may interrupt blood flow and
influence blood circulation of the patient, thus cause harmful injury to the patient: connection tubing kinking
too frequent and consecutive multiple measurements; the application of the cuff and its pressurization on any
arm where intravascular access or therapy, or an arterio-venous (A-V) shunt, is present; inflating the cuff on
the side of a mastectomy.
* Warning: Do not apply the cuff over a wound;otherwise it can cause further injury.
*Do not inflate the cuff on the same limb which other monitoring ME equipment is applied around
simultaneously, because this could cause temporary loss of function of those simultaneously-used monitoring
ME equipment.
*On the rare occasion of a fault causing the cuff to remain fully inflated during measurement, open the cuff
immediately. Prolonged high pressure (cuff pressure > 300mmHg or constant pressure > 15mmHg for more
than 3 minutes) applied to the arm may lead to an ecchymosis.
*Please check that operation of the device does not result in prolonged impairment of patient blood
circulation.
* When measurement, please avoid compression or restriction of the connection tubing.
* The device cannot be used with HF surgical equipment at the same time.
* The ACCOMPANYING DOCUMENT shall disclose that the SPHYGMOMANOMETER was clinically
investigated according to the requirements of ISO 81060-2:2013.
* To verify the calibration of the AUTOMATED SPHYGMOMANOMETER, please contact the manufacturer.
* This device is contraindicated for any female who may be suspected of, or is pregnant. Besides providing
inaccurate readings, the effects of this device on the fetus are unknown.
* Too frequent and consecutive measurements could cause disturbances in blood circulation and injuries.
* This unit is not suitable for continuous monitoring during medical emergencies or operations.Otherwise, the
patient’s arm and fingers will become anaesthetic, swollen and even purple due to a lack of blood.
* When not in use, store the device with the adapter in a dry room and protect it against extreme moisture,
heat, lint, dust and direct sunlight. Never place any heavy objects on the storage case.
* This device may be used only for the purpose described in this booklet. The manufacturer cannot be held
liable for damage caused by incorrect application.
*This device comprises sensitive components and must be treated with caution. Observe the storage and
operating conditions described in this booklet.
CAUTION
* The equipment is not AP/APG equipment and not suitable for use in the presence of a flammable
anesthetic mixture with air of with oxygen or nitrous oxide.
* Warning: No servicing/maintenance while the ME equipment is in use.
* The patient is an intended operator.
* The patient can measure, transmit data and change batteries under normal circumstances and maintain
the device and its accessories according to the user manual.
* To avoid measurement errors, please avoid the condition of strong electromagnetic field radiated
interference signal or electrical fast transient/burst signal.
*The blood pressure monitor, its adaptor, and the cuff are suitable for use within the patient environment.
If you are allergic to polyester, nylon or plastic, please don't use this device.
* During use, the patient will be in contact with the cuff. The materials of the cuff have been tested and
found to comply with requirements of ISO 10993-5:2009 and ISO 10993-10:2010. It will not cause any
potential sensization or irritation reaction.
* Adaptor is specified as a part of ME EQUIPMENT.
* If you experience discomfort during a measurement, such as pain in the arm or other complaints, press
the START/STOP button to release the air immediately from the cuff. Loosen the cuff and remove it from
your arm.
* If the cuff pressure reaches 40 kPa (300 mmHg), the unit will automatically deflate. Should the cuff not
deflate when pressures reaches 40 kPa (300 mmHg), detach the cuff from the arm and press the
START/STOP button to stop inflation.
* Before use, make sure the device functions safely and is in proper working condition. Check the device,
do not use the device if it is damaged in any way. The continuous use of a damaged unit may cause
injury, improper results, or serious danger.
* Do not wash the cuff in a washing machine or dishwasher!
* The service life of the cuff may vary by the frequency of washing, skin condition, and storage state. The
typical service life is 10000 times.
* It is recommended that the performance should be checked every 2 years and after maintenance and
repair, by retesting at least the requirements in limits of the error of the cuff pressure indication and air
leakage (testing at least at 50mmHg and 200mmHg).
* Please dispose of ACCESSORIES, detachable parts, and the ME EQUIPMENT according to the local
guidelines.
* Manufacturer will make available on request circuit diagrams, component part lists, descriptions,
calibration instructions,etc., to assist to service personnel in parts repair.
* The plug/adapter plug pins insulates the device from the main supply. Do not position the device in a
position where it is difficult to disconnect from the supply mains to safely terminate operation of ME
equipment.
* The operator shall not touch output of batteries /adapter and the patient simultaneously.
* Cleaning :Dust environment may affect the performance of the unit. Please use the soft cloth to clean
the whole unit before and after use. Don’t use any abrasive or volatile cleaners.
* The device doesn’t need to be calibrated within two years of reliable service.
* If you have any problems with this device, such as setting up, maintaining or using, please contact the
SERVICE PERSONNEL of Transtek. Don’t open or repair the device by yourself in the event of
malfunctions. The device must only be serviced, repaired and opened by individuals at authorized
sales/service centers.
* Please report to Transtek if any unexpected operation or events occur.
* Keep the unit out of reach of infants, young children or pets to avoid inhalation or swallowing of small
parts. It is dangerous or even fatal.
INTRODUCTION
INTRODUCTION
Page 5
Pul/min
MEM ORY
TIM E/DATE
S
M
STAR T
STO P
(1mmHg=0.133kPa)
(1kPa=7.5mmHg)
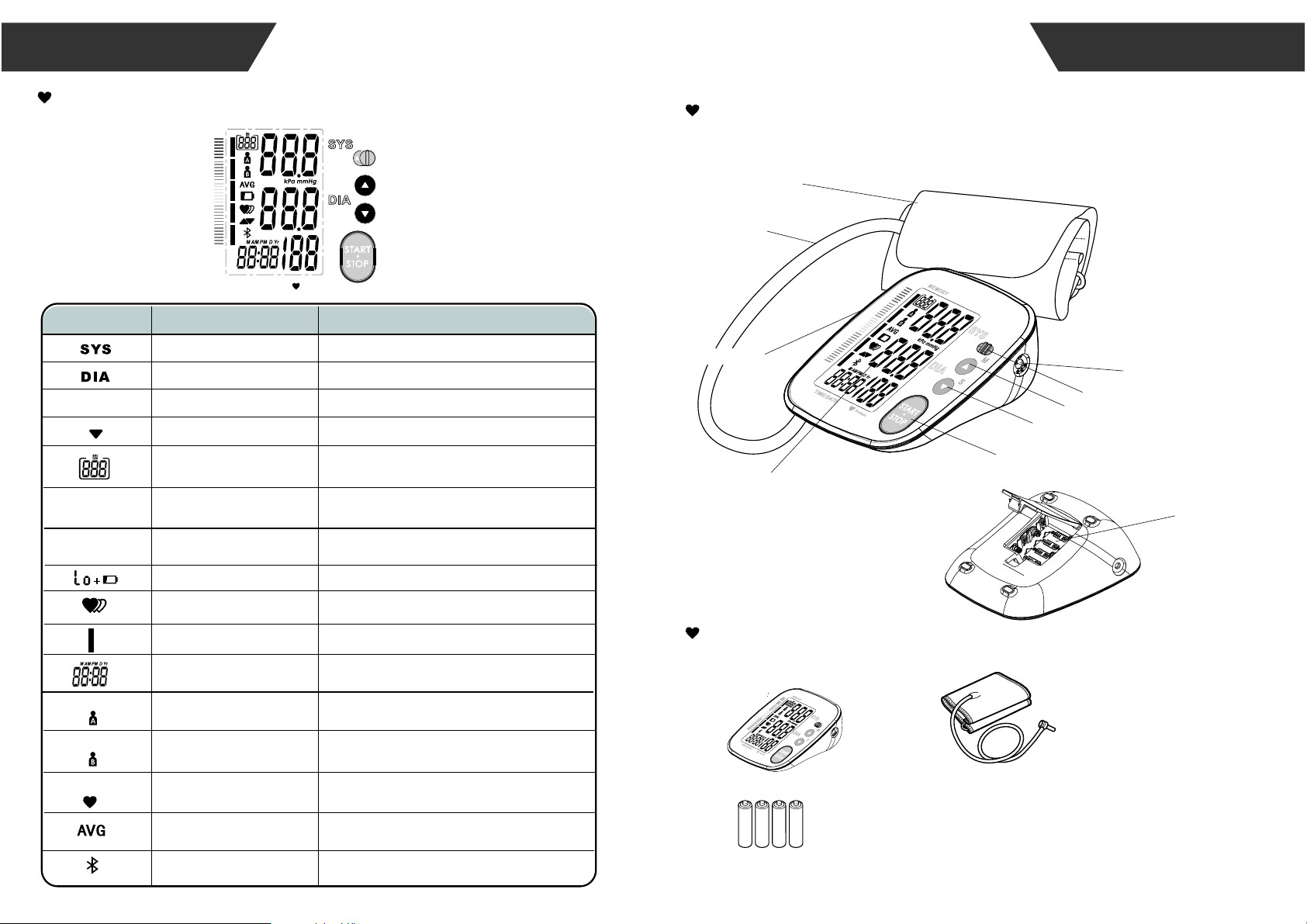
LCD display signal
SYMBOL DESCRIPTION
Systolic blood pressure High blood pressure
Diastolic blood pressure Low blood pressure
EXPLANATION
Memory
mmHg
kPa
Measurement Unit of the blood pressure
Measurement Unit of the blood pressure
Low battery
Batteries are low and need to be replaced
kPa
mmHg
Current Time
Month/Day/Year, Hour/Minute
Pul/min
User A
Start measurement,save and transmit the
measuring results for User A
User B
Start measurement,save and transmit the
measuring results for User B
Heartbeat
INTRODUCTION
Monitor Components
List
1.Blood Pressure Monitor
(TMB-1490-BS)
4.User manual
2.Cuff (Type BF applied part)
(22cm~32cm or 22cm~42cm)
Component list of
pressure measuring
system
1 Cuff
2 Air pipe
3 PCBA
4 Pump
5 Valve
3. 4×AAA batteries
BATTERY COMPARTMENT
CUFF
AIR HOSE
AIR CONNECTOR PLUG
LCD DISPLAY
MEM BUTTON
67$576723%87721
SET BUTTON
INTRODUCTION
USER SWITCH
The average value
The average value of the latest three
records
Bluetooth icon
The bluetooth icon blinks when the
bluetooth is working
5. AC Adaptor
(BLJ06L060100P-U)
Pulse in beats per minutePulse display
Deflation symbol The cuff is deflating.
Indicate it is in the memory mode and
which group of memory it is.
Blood pressure monitor is detecting an
irregular heartbeat during measurement.
Irregular heartbeat
Blood pressure
level indicator
Indicate the blood pressure level
Blood pressure monitor is detecting a
heartbeat during measurement.
DC POWER SOCKET
Page 6
Pul/min
MEM ORY
TIM E/DATE
S
M
STAR T
STO P
Pul/min
MEM ORY
TIM E/DATE
S
M
STAR T
STO P
In order to get the best effect and protect your monitor,please use the
the right batteries and special power adapter which complies with local
safety standard.
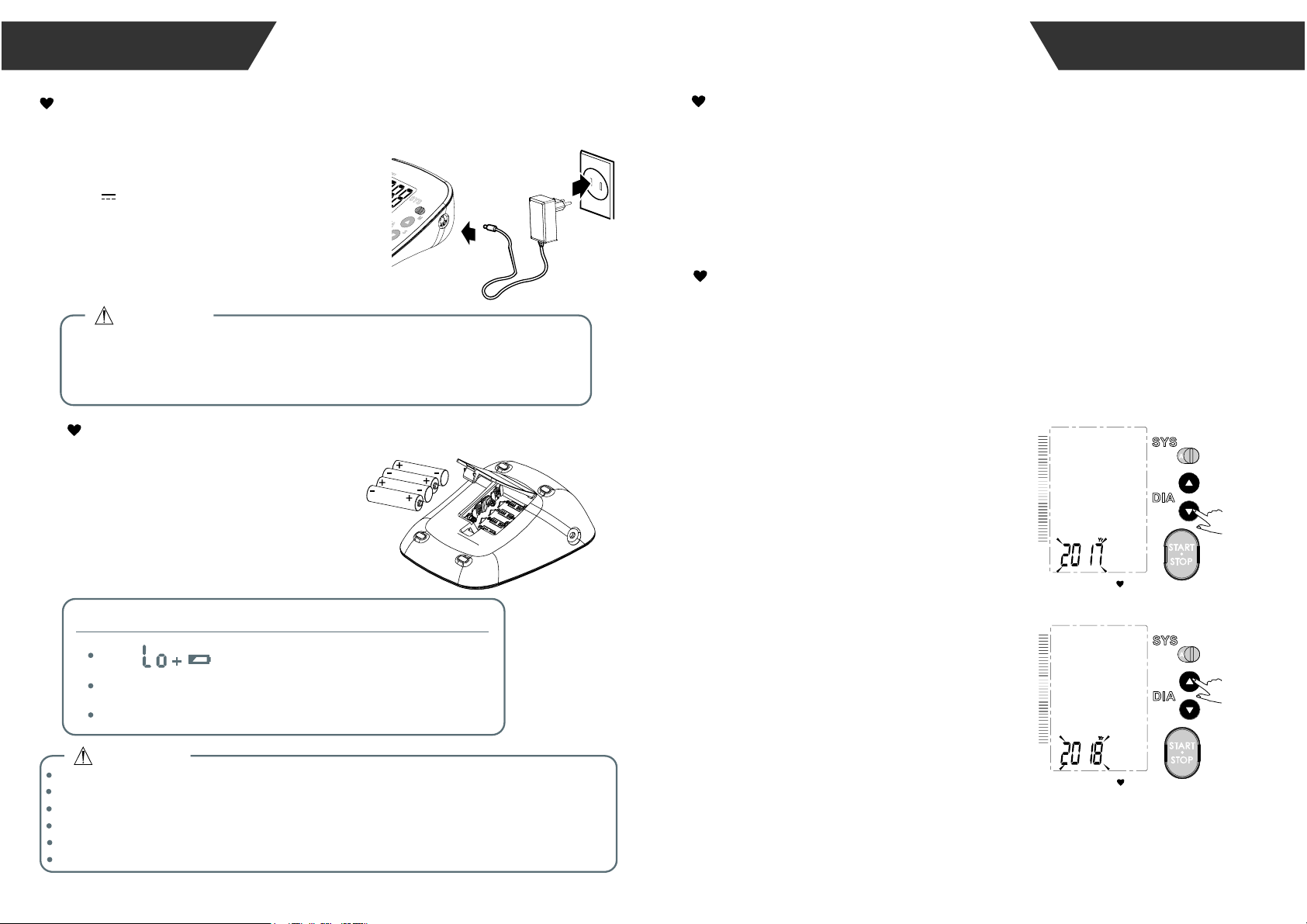
Open the battery cover.
Install the batteries by matching
the correct polarity, as shown.
Replace the battery cover.
Installing and Replacing the Batteries
CAUTION
CAUTION
Replace the batteries whenever the below happen
The shows
The display is dim.
The display does not light up
AC adaptor
The Choice of Power Supply
1.Battery powered mode:
6VDC 4×AAA batteries
2.AC adaptor powered mode:
(Please only use the recommended AC
adaptor model).
Please unplug the adaptor to depart from
the using utility power.
BEFORE YOU START
Measurement Principle
Setting Date, Time and Measurement Unit
It is important to set the clock before using your blood pressure
monitor, so that a time stamp can be assigned to each record that
is stored in the memory. (The setting range of the year :2017—2057
time format:12 H/24H)
This product uses the Oscillometric Measuring method to detect blood pressure.
Before every measurement, the unit establishes a “zero pressure” equivalent to the air
pressure. Then it starts inflating the arm cuff, meanwhile, the unit detects pressure
oscillations generated by beat-to-beat pulsatile, which is used to determine the systolic
and diastolic pressure, and also pulse rate.
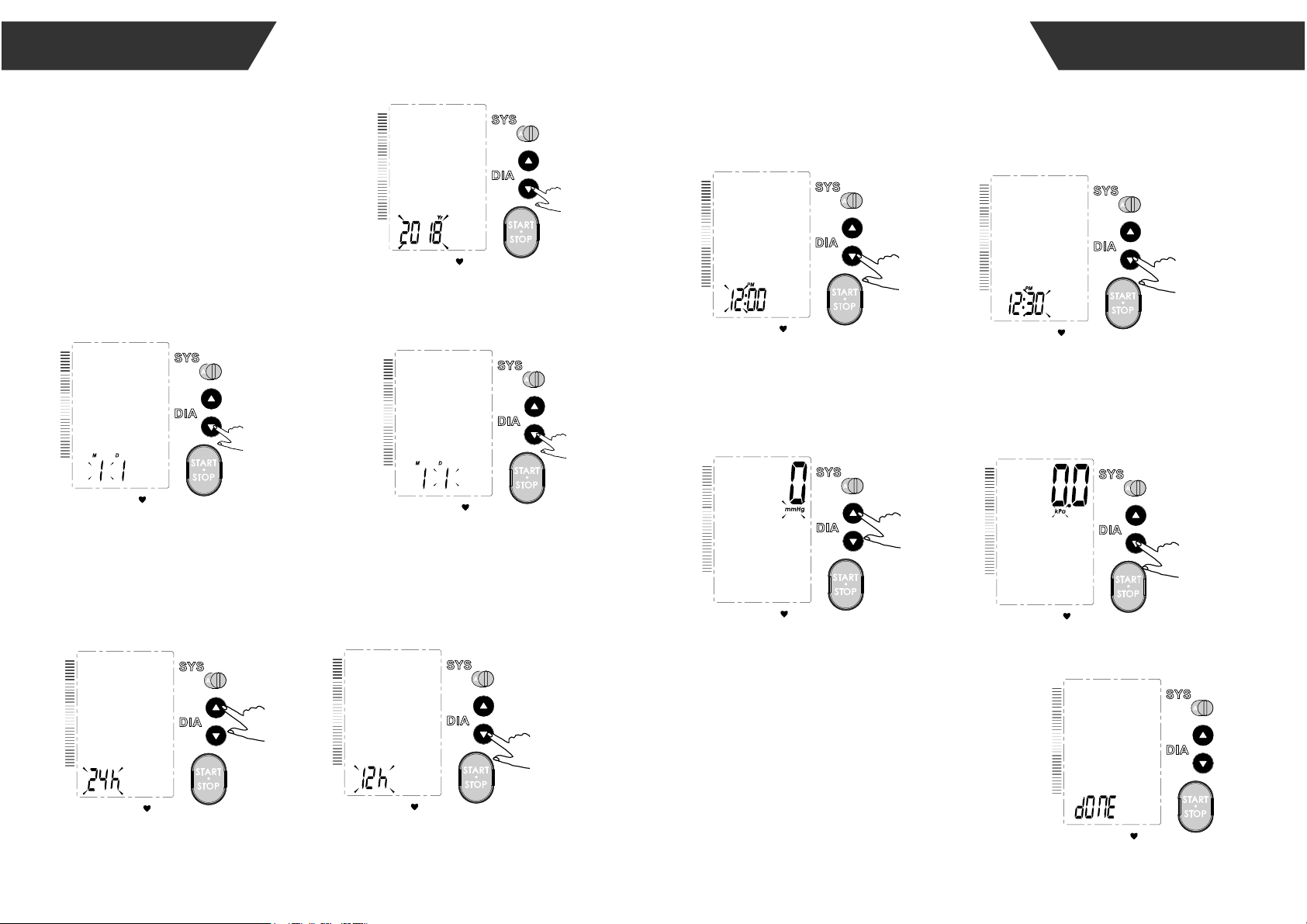
1.When the monitor is off,
press “SET”button,it will
display the time.Then
press and hold “ SET ”
button to enter the
mode for year setting.
2.Press the “MEM” to
change the [YEAR].
Each press will increase
the numeral by one in a
cycling manner.
BEFORE YOU START
6V 1A
Do not use new and used batteries together.
Do not use different types of batteries together.
Do not dispose the batteries in fire. Batteries may explode or leak.
Remove batteries if the device is not likely to be used for some time.
Worn batteries are harmful to the environment. Do not dispose with daily garbage.
Remove the old batteries from the device following your local recycling guidelines.
Page 7
Pul/min
MEM ORY
TIM E/DATE
S
M
STAR T
STO P
Pul/min
MEM ORY
TIM E/DATE
S
M
STAR T
STO P
Pul/min
MEM ORY
TIM E/DATE
S
M
STAR T
STO P
Pul/min
MEM ORY
TIM E/DATE
S
M
STAR T
STO P
Pul/min
MEM ORY
TIM E/DATE
S
M
STAR T
STO P
Pul/min
MEM ORY
TIM E/DATE
S
M
STAR T
STO P
BEFORE YOU START
BEFORE YOU START
3.When you get the right
year, press “SET” to set
down and turn to next
step.
4.Repeat steps 2 and 3 to set
the [MONTH] and [DAY].
5.Repeat steps 2 and 3 to set the [TIME FORMAT]
between 12h and 24h.
6.Repeat steps 2 and 3 to set the [HOUR]
and [MINUTE].
8.After the unit is set,the LCD will display
“dOnE”first,then display all the settings you
have done and then it will turn off.
7.Repeat steps 2 and 3 to set the [UNIT].
Pul/min
MEM ORY
TIM E/DATE
S
M
STAR T
STO P
Pul/min
MEM ORY
TIM E/DATE
S
M
STAR T
STO P
Pul/min
MEM ORY
TIM E/DATE
S
M
STAR T
STO P
Pul/min
MEM ORY
TIM E/DATE
S
M
STAR T
STO P
Page 8
Pul/min
MEM ORY
TIM E/DATE
S
M
STAR T
STO P
MEASUREMENT
Tie the cuff
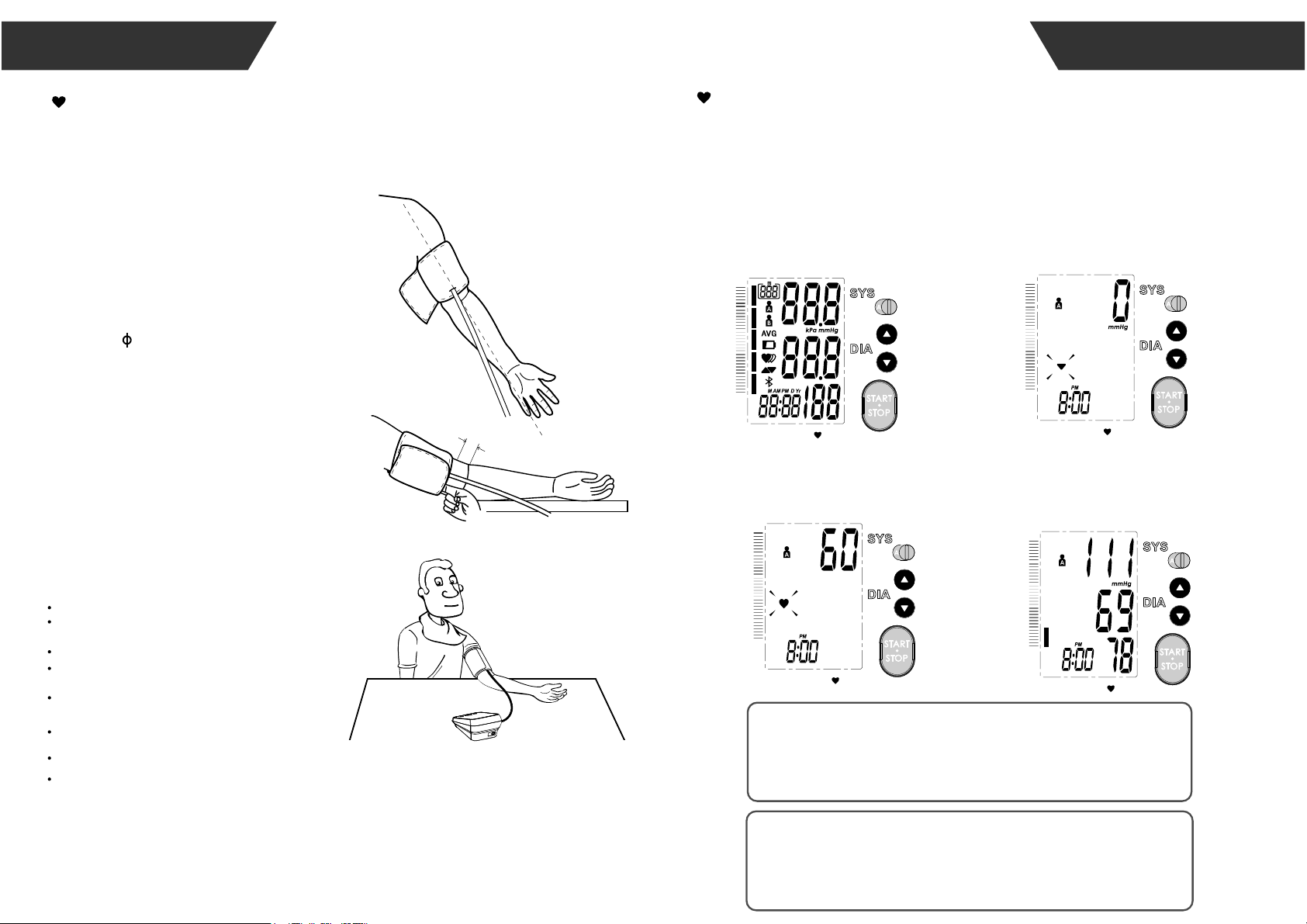
1.Before you start the measurement, switch the User button to select the user
between User A and User B. Switch to right to select User A, switch to left to
VHOHFW8VHU%:KHQWKHPRQLWRULVRIISUHVVWKH³67$576723EXWWRQWRWXUQ
on the monitor, and it will finish the whole measurement,save and transmit the
measurement data for the desired user. (Take User A for example.)
Adjust the zero. LCD display
Inflating and measuring. Display and save the
measurement result.
Start the Measurement
MEASUREMENT
2~3cm
1.
4.
Hold your arm with your palm facing up and
tie the cuff on your upper arm, then position
the tube off-center toward the inner side of
arm in line with the little finger. Or position the
artery mark over the main artery (on the
inside of your arm). Note: Locate the main
artery by pressing with 2 fingers
approximately 2 cm above the bend of your
elbow on the inside of your left arm. Identify
where the pulse can be felt the strongest.
This is your main artery.
The cuff should be snug but not too tight.
You should be able to insert one finger
between the cuff and your arm.
Remove all jewelry, such as watches and
bracelets from your left arm.
Note: If your doctor has diagnosed you with
poor circulation in your left arm, use your right
arm.
Roll or push up your sleeve to expose the
skin. Make sure your sleeve is not too tight.
2.
3.
5.
Sit comfortably with your tested arm resting on a
flat surface.
Place your elbow on a table so that
the cuff is at the same level as your heart. Turn
your palm upwards. Sit upright in a chair, and take
5-6 deep breaths.
Rest for 5 minutes before first measuring.
Wait at least 3 minutes between measurements.
This allows your blood circulation to recover.
The patient must relax as much as possible and do
not move and talk during the measurement procedure.
For a meaningful comparison, try to measure under
similar conditions. For example, take daily
measurements at approximately the same time, on the
same arm, or as directed by a physician.
6.
Take the measurement in a silent room.
The cuff should maintain at the same level as the
right atrium of the heart.
Please sit comfortably. Do not cross your legs and
keep your feet flat on the ground.
Keep your back against the backrest of the chair.
Helpful tips for Patients, especially for Patients with
Hypertension:
Pul/min
MEM ORY
TIM E/DATE
S
M
STAR T
STO P
Pul/min
MEM ORY
TIM E/DATE
S
M
STAR T
STO P
Pul/min
MEM ORY
TIM E/DATE
S
M
STAR T
STO P
Bluetooth Module No.: LS51802
RF Frequency Range: 2402 MHz to 2480 MHz
Output Power Range: 0 dBm
Supply Voltage: 2V-3.6 V
Transmitting Distance: 10 meters
List of compatible devices:
For iOS devices:
The operating system must be iOS 8 or more, such as iPhone
4S, iPhone 5/5C/5S, iPhone 6/6 Plus and so on.
For Android devices:
The operating system must be 4.3 or more.
Page 9

Pul/min
MEM ORY
TIM E/DATE
S
M
STAR T
STO P
Pul/min
MEM ORY
TIM E/DATE
S
M
STAR T
STO P
Pul/min
MEM ORY
TIM E/DATE
S
M
STAR T
STO P
MEASUREMENT
STAR T
STOP
43UHVVWKH³67$576723´WRSRZHU
off.
Tips: Maximum 250 records are both for User A and User B.
Recall the Records
2. Press the “MEM” or “SET” to get the record you
want.
The most recent record (1) is shown first. Each new measurement is
assigned to the first (1) record. All other records are pushed back one
digit (e.g., 2 becomes 3, and so on), and the last record (60) is
dropped from the list.
The current No. is No 2. The corresponding
time is P.M. 10:08.
The corresponding
date is January 6th.
CAUTION
The date and time
of the record
will be shown
alternately.
DATA MANAGEMENT
3. If you want to look over another user’s data, switch the User button to select
the desired user. Then you can look over its historical records.
1. When the monitor is off,
please press the “MEM”,it will display the
latest record first when the records are less than
three groups. When there are three or more than
three groups ,it will display the average value of
the latest three records first.
2.This device will proceed
to data transmission
after measurement.
The Bluetooth symbol
blinks on the LCD
indicates data is
transmitting.
3.If the data is successfully
transmitted, the Bluetooth
symbol doesn’t blink any more,
and then it will turn off within 5
seconds.
If the data transmission fails,
it will turn off within 3 minutes.
Pul/min
MEM ORY
TIM E/DATE
S
M
STAR T
STO P
Pul/min
MEM ORY
TIM E/DATE
S
M
STAR T
STO P
CAUTION
1.
2.
Interference may occur in the vicinity of equipment marked with the following
symbol . And TMB-1490-BS may interfering vicinity electrical equipment.
Sensitive people, including pregnant women pre-eclamptic and those who
implanted medical electronic instruments, should avoid using the unit
whenever possible.
Keep the monitor at least 20 centimeters away from the human body
(especially the head) when the data transmission is proceeding after
measurement.
To enable the data transmission function, this product should be paired to
Bluetooth end at 2.4 GHz.
How to mitigate possible interference?
The range between the device and BT end should be reasonably close, from
1 meter to 10 meters. Please ensure no obstacles between the device and
BT end so as to obtain quality connection and to lower the RF output range.
To avoid interference, other electronic devices (particularly those with
wireless transmission / Transmitter) should be kept at least 1 meter away
from the monitor.
Page 10

Pul/min
MEM ORY
TIM E/DATE
S
M
STAR T
STO P
Pul/min
MEM ORY
TIM E/DATE
S
M
STAR T
STO P
Pul/min
MEM ORY
TIM E/DATE
S
M
STAR T
STO P
DATA MANAGEMENT
If you did not get the correct measurement, you can delete all
results for the selected user by following steps below.
Delete the Records
1.Hold pressing “SET”
for 3 seconds when the
monitor is in the memory
recall mode ,the
flash display “dEL ALL” will
show.
2.Press “SET” to
confirm deleting and
the monitor will turn
off.
3.If you don’t want to delete the
records, press “START/STOP”
to escape.
4. If there is no record,
press “MEM” button,
the right display will
show.
STAR T
STOP
INFORMATION FOR USER
Tips for Measurement
Within 1 hour
after dinner or drinking
Within 20 minutes
after taking a bath
In a very cold environment
Immediate measurement
after tea, coffee, smoking
When talking or moving your fingers
When you want to discharge urine
Measurements may be inaccurate if taken in the following
circumstances.
Page 11
INFORMATION FOR USER
Maintenance
In order to get the best performance, please follow the
instructions below.
Put in a dry place and
avoid the sunshine
Avoid intense shaking
and collisions
Using wet cloths to
remove dirt
Avoid touching water,
clean it with a dry cloth in case.
Avoid dusty and unstable
temperature environment
Do not attempt to clean the reusable
cuff with water and never immerse
the cuff in water.
ABOUT BLOOD PRESSURE
What are systolic pressure and diastolic pressure?
press
artery
vein
blood discharging
Systolic
relax
blood entering
Diastolic
When ventricles contract and pump blood out of the
heart, the blood pressure reaches its maximum value
in the cycle, which is called systolic pressure. When
the ventricles relax, the blood pressure reaches its
minimum value in the cycle, which is called diastolic
pressure.
Irregular Heartbeat Detector
CAUTION
The appearance of the IHB icon indicates that a pulse irregularity consistent with an irregular
heart-beat was detected during measurement. Usually this is NOT a cause for concern.
However, if the symbol appears often, we recommend you seek medical advice. Please note
that the device does not replace a cardiac examination, but serves to detect pulse irregularities
at an early stage.
An irregular heartbeat is detected when a heartbeat rhythm varies while the unit is measuring
the systolic and diastolic blood pressure.During each measurement, the monitor records all the
pulse intervals and calculate the average ; if there are two or more pulse intervals ,the difference
between each interval and the average is more than the average value of ±25% , or there are four
or more pulse intervals ,the difference between each interval and the average is more than the
average value of ±15%,the irregular heartbeat symbol appears on the display when the
measurement results are appear.
What is the standard blood pressure classification?
The chart on the right is the
standard blood pressure classification published by American
Heart Association (AHA).
Please consult a physician if your measuring result falls outside the range.
Please note that only a physician can tell whether your blood pressure value
has reached a dangerous point.
CAUTION
Blood Pressure Category
Normal
Elevated
High Blood Pressure
(Hypertension) Stage 1
High Blood Pressure
(Hypertension) Stage 2
Hypertensive Crisis
(Consult your doctor immediately)
Systolic
mmHg (upper#)
Diastolic
mmHg (lower#)
less than 120
120-129
130-139
140 or higher
Higher than 180
and
or
or
and/or
less than 80
80-89
90 or higher
Higher than 120
This chart reflects blood pressure categories defined by American Heart Association.
and less than 80
Page 12

ABOUT BLOOD PRESSURE
Why does my blood pressure
fluctuate throughout the
day?
Is the result the same
if measuring on the
right arm?
Why do I get a different
blood pressure at home
compared to the hospital?
1. Individual blood pressure varies
multiple times everyday. It is also affected
by the way you tie your cuff and your
measurement position, so please take the
measurement under the same conditions.
2.If the person takes medicine, the
pressure will vary more.
3.Wait at least 3 minutes for another
measurement.
The blood pressure is different even
throughout the day due to weather,
emotion, exercise etc, Also, there is the
“white coat” effect, which means blood
pressure usually increases in clinical
settings.
It is ok for both arms, but there
will be some different results for
different people. We suggest you
measure the same arm every time.
What you need to pay
attention to when you measure
your blood pressure at home:
If the cuff is tied properly.
If the cuff is too tight or too loose.
If the cuff is tied on the upper arm.
If you feel anxious.
Taking 2-3 deep breaths before
beginning will be better for measuring.
Advice: Relax yourself for 4-5
minutes until you calm down.
TROUBLESHOOTING
This section includes a list of error messages and frequently
asked questions for problems you may encounter with your blood
pressure monitor. If the products not operating as you think it
should, check here before arranging for servicing.
PROBLEM SYMPTOM CHECK THIS REMEDY
No power
Low
batteries
Error
message
Display will not
light up.
Batteries are exhausted.
Replace with new batteries
Insert the batteries
correctly
Replace with new batteries
Batteries are inserted
incorrectly.
Display is dim or
show
Batteries are low.
E 01 shows
E 03 shows
E 04 shows
EExx,shows on
the display.
A calibration error
occurred.
(XX can
be some digital symbol,
such as 01, 02,etc., if
this similar situation
appear, all belong to
calibration error.)
Retake the measurement.
If the problem persists,
contact the retailer or our
customer service
department for further
assistance.Refer to the
warranty for contact
information and return
instructions.
The treatment of the
measurement failed.
AC adaptor is inserted
incorrectly.
Insert the AC adaptor
tightly
E 02 shows
The cuff is too tight
or too loose.
Refasten the cuff and then
measure again.
The monitor
detected motion
while measuring.
Movement can affect the
measurement.Relax for a
moment and then
measure again.
Relax for a moment and
then measure again.
The measurement
process does not
detect the pulse
signal.
Loosen the clothing on
the arm and then
measure again.
Warning
message
Relax for a moment.
Refasten the cuff and then
measure again. If the
problem persists, contact
your physician.
“out ” shows
Out of measurement
range
Page 13

Contact Information
For more information about our products, please visit www.transtek.cn.you can get customer
service, usual problems and customer download, transtek will serve you anytime.
Manufactured by:
Company:
Address:
Authorized Component
1please use the TRANSTEK
authorized adapter.
AUTHORIZED COMPONENT
Guangdong Transtek Medical Electronics Co., Ltd.
Zone B, No.105 ,Dongli Road, Torch Development District,
Zhongshan,528437,Guangdong,China
Guangdong Transtek Medical Electronics Co., Ltd.
Adapter
Model:
BLJ06L060100P-U
Input:AC 100-240V
50/60Hz 0.2A Max
Output:6V 1000mA
SPECIFICATIONS
6VDC 4×AAA batteries
Approx.250g(Excluding the batteries and cuff)
A01
Digital LCD V.A.60mm × 92mm
Approx.140mm×130mm×49.7mm
4×AAA batteries,user manual,AC adapter
6V 1A
Battery powered mode:
AC adaptor powered mode:
(Please only use the recommended AC
adaptor model).
About 22cm~32cm or 22cm~42cm
Type BF applied part
WARNING: No modification of this equipment is allowed.
Power supply
Display mode
Measurement mode
Oscillographic testing mode
Measurement range
Measurement perimeter
of the upper arm
Weight
External dimensions
Attachment
Mode of operation
Continuous operation
Degree of protection
Protection against
ingress of water
Accuracy
Normal working condition
Storage & transportation
condition
Software Version
Pressure:
5℃-40℃within±3mmHg(0.4kPa)
Pulse value:±5%
Rated cuff pressure:
0mmHg~299mmHg(0kPa ~ 39.9kPa)
Measurement pressure:
SYS: 60mmHg~230mmHg (8.0kPa~30.7kPa)
DIA: 40mmHg~130mmHg (5.3kPa~17.3kPa)
Pulse value: (40-199)beat/minute
IP21 It means the device could protected against
solid foreign objects of 12.5mm and greater, and
protect against vertically falling water drops.
Device Classification
Battery Powered Mode:
Internally Powered ME Equipment
AC Adaptor Powered Mode: Class II ME Equipment
A temperature range of :+5°C to +40°C
A relative humidity range of 15% to 90%,
non-condensing, but not requiring a water vapour
partial pressure greater than 50 hPa
An atmospheric pressure range of :
700 hPa to 1060 hPa
Temperature:-20°C to +60°C
$UHODWLYHKXPLGLW\UDQJHRIQRQFRQGHQVLQJ
at a water vapour pressure up to 50hPa
Page 14

COMPLIED STANDARDS LIST
Complied Standards List
EN ISO 14971:2012 / ISO 14971:2007 Medical devices -
Application of risk management to medical devices
EN 1041:2008 Information supplied by the manufacturer of medical
devices
EN 60601-1:2006/ IEC 60601-1:2005+A1:2012 Medical electrical
equipment - Part 1: General requirements for basic safety and
essential performance
EN 60601-1-11:2015/ IEC 60601-1-11:2015 Medical electrical
equipment - Part 1-11: General requirements for basic safety and
essential performance - Collateral standard: Requirements for medical
electrical equipment and medical electrical systems used in the home
healthcare environment
EN 60601-1-2:2015/ IEC 60601-1-2:2014 Medical electrical
equipment - Part 1-2: General requirements for basic safety and
essential performance - Collateral standard: Electromagnetic
disturbances - Requirements and tests
EN ISO 81060-1:2012 Non-invasive sphygmomanometers - Part 1:
Requirements and test methods for non-automated measurement type
EN 1060-3:1997+A2:2009 Non-invasive sphygmomanometers Part 3: Supplementary requirements for electro-mechanical blood
pressure measuring systems
EN 1060-4:2004 Non-invasive sphygmomanometers - Part 4: Test
procedures to determine the overall system accuracy of automated
non-invasive sphygmomanometers
EN 60601-1-6:2010/IEC 60601-1-6:2010+A1:2013 Medical
electrical equipment - Part 1-6: General requirements for basic safety
and essential performance - Collateral standard: Usability
EN 62366-1:2015/
IEC 62366-1:2015 Medical devices - Part 1:
Application of usability engineering to medical devices
EN 62304:2006/AC: 2008 / IEC 62304:2006 Medical device
software - Software life-cycle processes
Risk management
Labeling
User manual
General Requirements
for Safety
Electromagnetic
compatibility
Performance
requirements
Clinical investigation
Usability
Software life-cycle
processes
Bio-compatibility
ISO 10993-1:2009 Biological evaluation of medical devices- Part
1: Evaluation and testing within a risk management process
ISO 10993-5:2009 Biological evaluation of medical devices -
Part 5: Tests for in vitro cytotoxicity
ISO 10993-10:2010 Biological evaluation of medical devices -
Part 10: Tests for irritation and skin sensitization
EN ISO 15223-1:2016 / ISO 15223-1:2016 Medical devices.
Symbols to be used with medical device labels, labelling and
information to be supplied. Part 1 : General requirements
IEC 80601-2-30:2013 Medical electrical equipment- Part 2-30:
Particular requirements for the basic safety and essential
performance of automated non-invasive sphygmomanometers
ISO 81060-2:2013 Non-invasive sphygmomanometers - Part 2:
Clinical validation of automated measurement type
This device complies with Part 15 of the FCC Rules. Operation is subject
to the following two conditions: (1) this device may not cause harmful
interference, and (2) this device must accept any interference received,
including interference that may cause undesired operation.
Caution: The user is cautioned that changes or modifications not
expressly approved by the party responsible for compliance could void
the user's authority to operate the equipment.
NOTE: This equipment has been tested and found to comply with the
limits for a Class B digital device, pursuant to Part 15 of the FCC Rules.
These limits are designed to provide reasonable protection against
harmful interference in a residential installation. This equipment generates,
uses and can radiate radio frequency energy and, if not installed and used in
accordance with the instructions, may cause harmful interference to
radio communications. However, there is no guarantee that interference will
not occur in a particular installation.
If this equipment does cause harmful interference to radio or television
reception, which can be determined by turning the equipment off and on,
the user is encouraged to try to correct the interference by one or more
of the following measures:
-- Reorient or relocate the receiving antenna.
-- Increase the separation between the equipment and receiver.
-- Connect the equipment into an outlet on a circuit different from that to
which the receiver is connected.
-- Consult the dealer or an experienced radio/TV technician for help.
FCC Radiation Exposure Statement:
This equipment complies with FCC radiation exposure limits set forth for
an uncontrolled environment.
This transmitter must not be co-located or operating in conjunction with
any other antenna or transmitter.
FCC Statement
FCC ID:OU9TMB1490BS
FCC STATEMENT
Page 15
EMC GUIDANCE
EMC GUIDANCE
EMC Guidance
1)This product needs special precautions regarding EMC and needs to be
installed and put into service according to the EMC information provided,
and this unit can be affected by portable and mobile RF communications
equipment.
2)* Do not use a mobile phone or other devices that emit electromagnetic
fields, near the unit. This may result in incorrect operation of the unit.
3)Caution: This unit has been thoroughly tested and inspected to assure
proper performance and operation!
4)* Caution: This machine should not be used adjacent to or stacked with
other equipment and that if adjacent or stacked use is necessary, this
machine should be observed to verify normal operation in the configuration in
which it will be used.
Table 1
Guidance and manufacturer’s declaration – electromagnetic emissions
RF emissions
CISPR 11
Group 1
Class B
Class A
Complies
Compliance
Harmonic emissions
IEC 61000-3-2
Voltage fluctuations/
flicker emissions
IEC 61000-3-3
RF emissions
CISPR 11
Emissions test Electromagnetic environment - guidance
The device is intended for use in the electromagnetic environment specified below. The customer or the
user of the device should assure that it is used in such an environment.
The device uses RF energy only for its internal
function. Therefore, its RF emissions are very low
and are not likely to cause any interference in nearby
electronic equipment.
The device is suitable for use in all establishments,
other than domestic and those directly connected to
the public low-voltage power supply network that
supplies buildings used for domestic purposes.
Guidance and manufacturer’s declaration – electromagnetic immunity
Immunity test
±8 kV contact
±15 kV air
±8 kV contact
±15 kV air
±2 kV
power supply lines:
line(s) to line(s): ±1 kV
line(s) to earth: ±2 kV
0%UT; 0.5 cycle
At 0°, 45°, 90°, 135°,
180°,225°,270° and 315°
0%U
T
; 1 cycle
and
70%U
T
; 25/30 cycles
Single phase: at 0°
0% U
T
; 300 cycle
30 A/m
50Hz/60Hz
NOTE UT is the a.c. mains voltage prior to application of the test level.
Table 2
Compliance level
Electrostatic
discharge (ESD)
IEC 61000-4-2
Electromagnetic
environment - guidance
30 A/m
50Hz/60Hz
Power frequency
(50Hz/60Hz)
magnetic field
IEC 61000-4-8
Voltage dips,
short interruptions
and voltage
variations on
power supply
input lines
IEC 61000-4-11
Electrical fast
transient/burst
IEC 61000-4-4
Surge
IEC61000-4-5
input/output lines:
±1 kV
The device is intended for use in the electromagnetic environment specified below. The customer or the
user of the device should assure that it is used in such an environment.
IEC 60601 test level
Floors should be wood, concrete
or ceramic tile. If floors are
covered with synthetic material,
the relative humidity should be at
least 30%.
±2 kV
power supply lines:
100 kHz repetition
frequency
line(s) to line(s): ±1 kV
100 kHz repetition
frequency
Mains power quality should be
that of a typical commercial or
hospital environment.
Mains power quality should be
that of a typical commercial or
hospital environment.
Mains power quality should be
that of a typical commercial or
hospital environment.
Power frequency magnetic fields
should be at levels characteristic
of a typical location in a typical
commercial or hospital
environment.
0% U
T
; 0.5 cycle
At 0°, 45°, 90°, 135°,
180°,225°,270° and
315°
0% U
T
; 1 cycle
and
70% U
T
; 25/30 cycles
Single phase: at 0°
0% U
T
;300 cycle
Page 16
EMC GUIDANCE
Table 3
Guidance and manufacturer’s declaration – electromagnetic immunity
Immunity test
Compliance
level
IEC 60601
Test level
Conducted RF
IEC 61000-4-6
Radiated RF
IEC 61000-4-3
10V/m, 80% Am
at 1kHz
150 kHz to
80 MHz:
3 Vrms
6Vrms (in ISM
and amateur
radio bands)
80% Am at 1kHz
Electromagnetic environment - guidance
NOTE 1 At 80 MHz and 800 MHz, the higher frequency range applies.
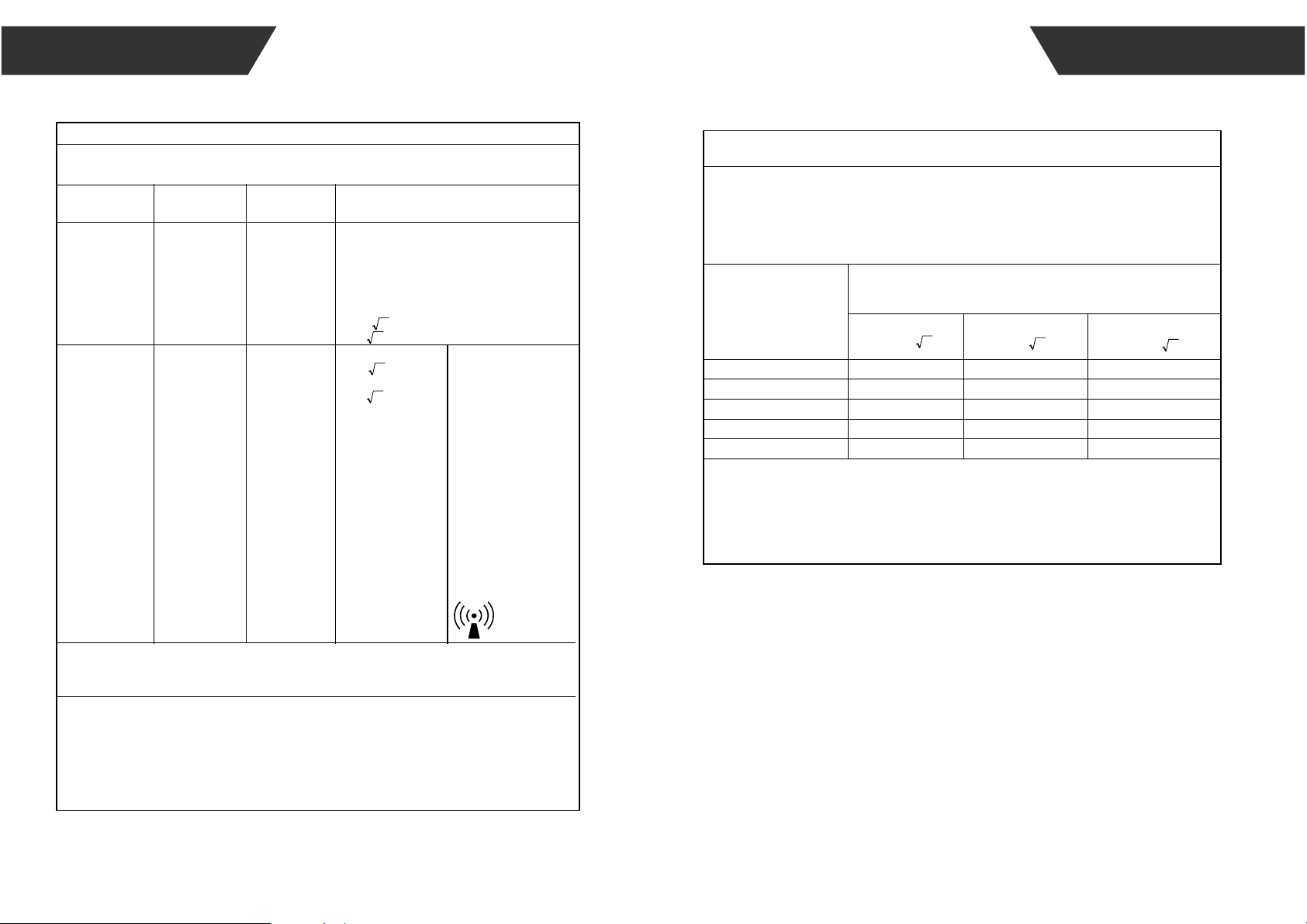
NOTE 2 These guidelines may not apply in all situations. Electromagnetic propagation is affected by
absorption and reflection from structures, objects and people.
a
Field strengths from fixed transmitters, such as base stations for radio (cellular / cordless)
telephones and land mobile radios, amateur radio, AM and FM radio broadcast and TV broadcast
cannot be predicted theoretically with accuracy. To assess the electromagnetic environment due to
fixed RF transmitters, an electromagnetic site survey should be considered. If the measured field
strength in the location in which the device is used exceeds the applicable RF compliance level
above, the device should be observed to verify normal operation. If abnormal performance is
observed, additional measures may be necessary, such as re-orienting or relocating the device.
Over the frequency range 150 kHz to 80 MHz, field strengths should be less than 3V/m.
b
The device is intended for use in the electromagnetic environment specified below. The customer or the
user of the device should assure that it is used in such an environment.
150 kHz to
80 MHz:
3 Vrms
6Vrms (in ISM
and amateur
radio bands)
80% Am at 1kHz
Portable and mobile RF communications
equipment should be used no closer to any part
of the device, including cables, than the
recommended separation distance calculated
from the equation appropriate for the frequency
of the transmitter.
Recommended separation distances:
d=0.35 ;
d=1.2
10V/m, 80% Am
at 1kHz
80 MHz to 800 MHz:
d=1.2
800 MHz to 2.7 GHz:
d=2.3
where, P is the maximum
output power rating of the
transmitter in watts (W)
according to the
transmitter manufacturer,
d is the recommended
separation distance in
meters (m). Field
strengths from fixed RF
transmitters, as
determined by an
electromagnetic site
survey, should be less
than the compliance level
in each frequency range.
Interference may occur in
the vicinity of equipment
marked with the following
symbol:
P
P
P
P
150 kHz to 80 MHz 80 MHz to 800 MHz 800 MHz to 2.7 GHz
Table 4
Recommended separation distances between portable and mobile RF communications
equipment and the device.
Rated maximum output
power of transmitter
(W)
Separation distance according to frequency of transmitter (m)
0.01
0.1
1
10
100
0.12 0.12
0.38
1.2
3.8
12
0.23
0.73
2.3
7.3
23
=d
=d 1.2
2.3=d
0.37
1.2
3.8
12
3.5
The device is intended for use in an electromagnetic environment in which radiated RF disturbances are
controlled. The customer or the user of the device can help prevent electromagnetic interference by
maintaining a minimum distance between portable and mobile RF communications equipment
(transmittters) and the device as recommended below, according to the maximum output power of the
communications equipment.
For transmitters rated at a maximum output power not listed above, the recommended separation
distance d in metres (m) can be estimated using the equation applicable to the frequency of the
transmitter, where P is the maximum output power rating of the transmitter in watts (W) according to the
transmitter manufacturer.
NOTE 1 At 80 MHz and 800 MHz, the separation distance for the higher frequency range applies.
NOTE 2 These guidelines may not apply in all situations. Electromagnetic propagation is affected by
absorption and reflection from structures, objects and people.
P
P
P
EMC GUIDANCE
Page 17
EMC GUIDANCE
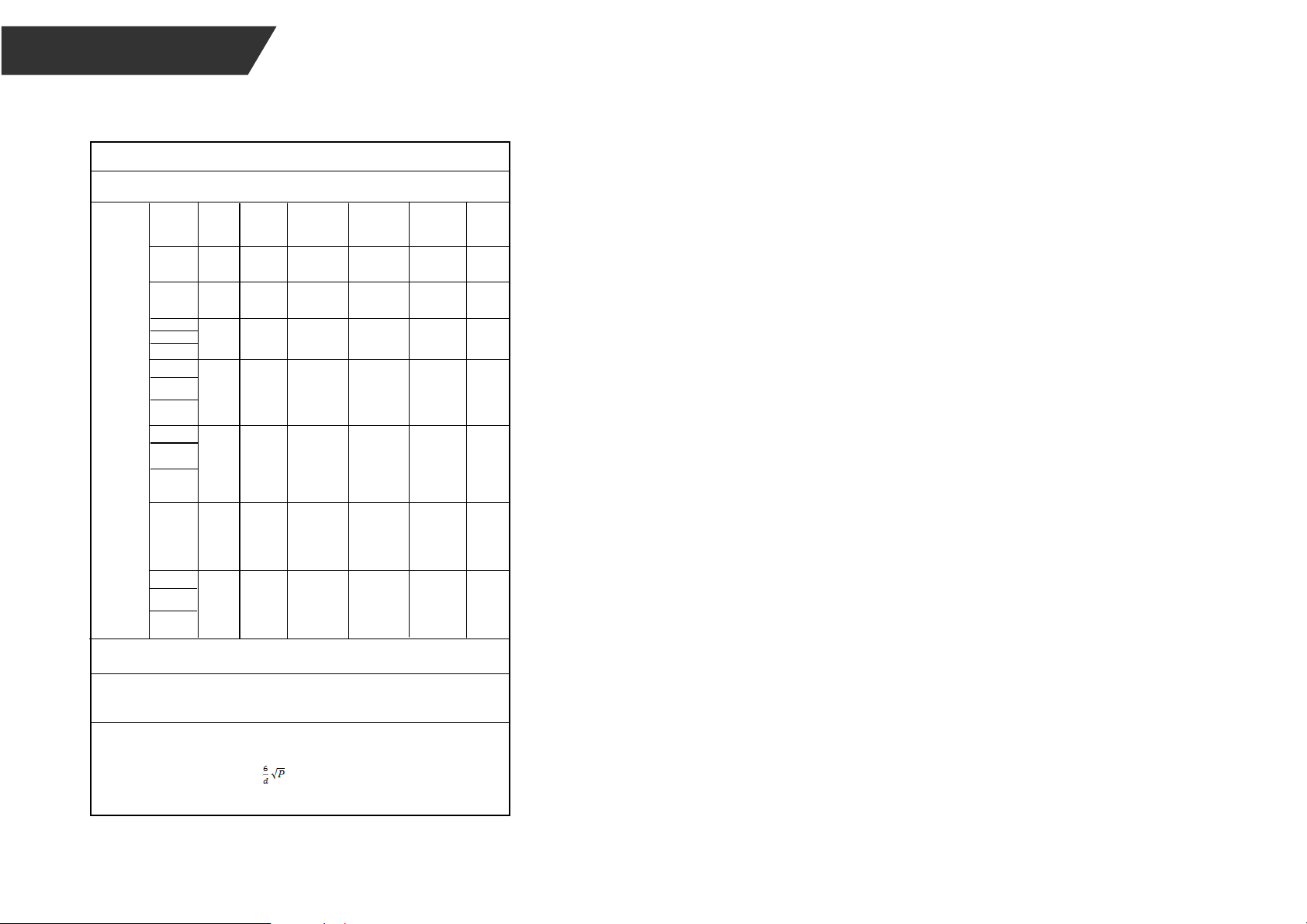
Table 5
Guidance and manufacturer’s declaration - electromagnetic i mmunity
Test
Frequency
(MHz)
385
0.3 27
The device is intended for use in the electromagnetic environment specified below. The customer or the
user of the device, should assure that it is used in such an environment.
NOTE If necessary to achieve the IMMUNITY TEST LEVEL, the distance between the transmitting antenna
and the ME EQUIPMENT or ME SYSTEM may be reduced to 1 m. The 1 m test distance is permitted by
IEC 61000-4-3.
Radiated RF
IEC61000-4-3
(Test
specifications
for
ENCLOSURE
PORT
IMMUNITY to
RF wireless
communicatio
ns equipment)
Band a)
(MHz)
Service a) Modulation b) Modulation b)
(W)
Distance (m)
IMMUNITY
TEST
LEVEL
(V/m)
380-390 TETRA
400
Pulse
modulation b)
18Hz
1.8
450 380-390
GMRS 460,
FRS 460
FM c) ± 5kHz
deviation 1kHz
sine
2 0.3 28
710 704-787
745
780
LTE Band
13,
17
Pulse
modulation b)
217Hz
0.2 0.3 9
810
870
930
800-960
GSM
800/900,
TETRA 800,
iDEN 820,
CDMA 850,
LTE Band 5
Pulse
modulation b)
18Hz
2
0.3 28
1720
1845
1970
17001990
GSM 1800;
CDMA 1900;
GSM 1900;
DECT;
LTE Band 1,
3,
4,25; UMTS
Pulse
modulation b)
217Hz
2
0.3 28
2
0.3 282450 2400-
2570
Bluetooth,
WLAN,
802.11
b/g/n, RFID
2450, LTE
Band 7
Pulse
modulation b)
217 Hz
5240
5240
5785
51005800
WLAN
802.11
a/n
Pulse
modulation b)
217 Hz
0.2
0.3 9
a) For some services, only the uplink frequencies are included.
b) The carrier shall be modulated using a 50% duty cycle square wave signal.
c) As an alternative to FM modulation, 50% pulse modulation at 18 Hz may be used because while it does
not represent actual modulation, it would be worst case.
The MANUFACTURER should consider reducing the minimum separation distance, based on RISK
MANAGEMENT, and using higher IMMUNITY TEST LEVELS that are appropriate for the reduced
minimum separation distance. Minimum separation distances for higher IMMUNITY TEST LEVELS shall be
calculated using the following equation:
E=
Where P is the maximum power in W, d is the minimum separation distance in m, and E is the IMMUNITY
TEST LEVEL in V/m.
 Loading...
Loading...