Page 1
USER MANUAL
Wrist blood pressure monitor
MEDEL SOFT
Model: TMB-1581-S
Page 2
EN
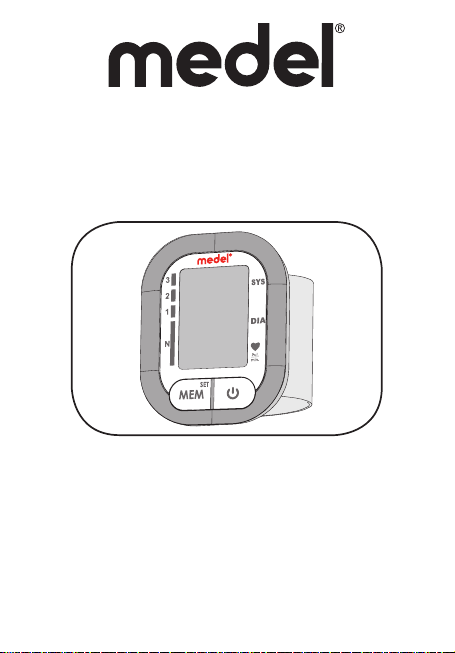
1
A
LCD DISPLAY
GRADE
D
MEM BUTTON
C
SET BUTTON
SYSTOLIC
E
DIASTOLIC
F
PULSE RATE
G
START/STOP BUTTON
B
2
+
-
-
+
4
A B
1
3
Page 3
EN
C
D
E
G
F
H
2
Page 4
EN
I
5
A B
C D
3
Page 5
6
A B
7
A B
C
EN
4
Page 6
EN
(DISPLAY SYMBOL (PIC. 3
SYMBOL DESCRIPTION EXPLANATION
SYS
DIA
INTRODUCTIONS
Dear customer,
ank you for choosing one of our products. Our name stands for high-quality, thoroughly tested products. Please read these instructions for
use carefully and keep them for later use, be sure to make them accessible
to other users and observe the information they contain.
With kind regards,
Your Medel team
INDICATIONS FOR USE
Medel So is a digital monitor intended for use in measuring blood pressure and heartbeat rate with a wrist circumference ranging from 13.5cm
to 21.5 cm ( about 5½˝-8½˝ ).
It is intended for adult indoor use only.
Systolic blood pressure High blood pressure
Dystolic blood pressure Low blood pressure
Pulse display Pulse in bests per minutes
Excessive Body Motion
Detector
Low battery Batteries are running low and
mmHg (1mmHg=0.133kPa)Measurement
Irregular heartbeat Detects an irregular heartbeat
Current time Month/Day/Year,Hour : Minute
Blood pressure level
indicator
Heartbeat Month/Day/Year,Hour : Minute
Motion may result in an inaccurate measurement
need to be replaced
unit the blood pressure
during measurement.
Indicate the blood pressure level
5
Page 7
CONTRAINDICATION
1.e device should not be used by any person who may be suspected
of,or is pregnant .
2.e device is not suitable for use on patients with implanted,electrical
devices, such as cardiac pacemakers, debrillators.
MEASUREMENT PRINCIPLE
is product uses the Oscillometric Measuring Method to detect blood
pressure. Before every measurement, the unit establishes a “zero point”
equivalent to the atmospheric pressure. en it starts inating the cu.
Meanwhile, the unit detects pressure oscillation generated by beat-to-beat
pulsatile, which is used to determine the systolic pressure and diastolic
pressure as well as pulse rate.
SAFETY INFORMATION
e signs below might be in the user manual, labeling or other component.ey are the requirement of standard and using.
Symbol for “THE OPERATION
GUIDE MUST BE READ”
Symbol for “COMPLIES WITH
MDD 93/42/EEC
REQUIREMENTS”
Symbol for “MANUFACTURER”
Symbol for “SERIAL NUMBER”
SN
Symbol for “RECYCLE”
Symbol for “MANUFACTURE
DATE”
The Green Dot is the license symbol of
a European network of industry-funded
systems for recycling the packaging
materials of consumer goods.
Symbol for “TYPE BF APPLIED
PARTS”
Symbol for “ENVIRONMENT
PROTECTION - Electrical waste
products should not be disposed of with
household waste. Please recycle where
facilities exist. Check with your local
authority or retailer for recycling advice”
Symbol for “DIRECT CURRENT”
Symbol for “Authorised Representative
EC REP
in the European Community
Caution: These notes must be
observed to prevent any damage
to the device.
ATTENTION!
is device is intended for adult use in homes only.
* e device is not suitable for use on neonatal patients, pregnant women,patients with implanted, electronical
devices, patients with pre-eclampsia, premature ventricular beats, atrial brillation, periph ral, arterial disease and
patients undergoing intravascular therapy or arterio-venous shunt or people who received a mastectomy. Please
consult your doctor prior to using the unit if you suer from illnesses.
* e device is not suitable for measuring the blood pressure of children. Ask your doctor before using it
on older children.
* e device is not intended for patient transport outside a healthcare facility.
* e device is not intended for public use.
* is device is intended for no-invasive measuring and monitoring of arterial blood pressure. It is not
intended for use on extremities other than the wrist or for functions other than obtaining a blood pressure
measurement.
* Do not confuse self-monitoring with self-diagnosis. is unit allows you to monitor your blood pressure.Do not
begin or end medical treatment without asking a physician for treatment advice.
* If you are taking medication,consult your physician to determine the most appropriate time to measure
your blood pressure. Never change a prescribed medication without consulting your physician.
EN
6
Page 8

EN
* Do not take any therapeutic measures on the basis of a self measurement. Never alter the dose of a medicine
prescribed by a doctor. Consult your doctor if you have any question about your blood pressure.
* When the device was used to measure patients who have common arrhythmias such as atrial or ventricular
premature beats or atrial brillation, the best result may occur with deviation. Please consult your physician about
the result.
* Warning: Do not apply the cu over a wound;otherwise it can cause further injury.
*Do not inate the cu on the same limb which other monitoring ME equipment is applied around simultaneously,
because this could cause temporary loss of function of those simultaneously-used monitoring ME equipment.
*On the rare occasion of a fault causing the cu to remain fully inated during measurement, open the cu
immediately. Prolonged high pressure (cu pressure > 300mmHg or constant pressure >15mmHg for
more than 3 minutes) applied to the wrist may lead to an ecchymosis.
*Please check that operation of the device does not result in prolonged impairment of patient blood
circulation.
* When measurement, please avoid compression or restriction of the connection tubing.
* e device cannot be used with HF surgical equipment at the same time.
* e ACCOMPANYING DOCUMENT shall disclose that the SPHYGMOMANOMETER was clinically
investigated according to the requirements of ISO 81060-2:2013.
* To verify the calibration of the AUTOMATED SPHYGMOMANOMETER, please contact the manufacturer.
* is device is contraindicated for any female who may be suspected of, or is pregnant. Besides providing
inaccurate readings, the eects of this device on the fetus are unknown.
* Too frequent and consecutive measurements could cause disturbances in blood circulation and injuries.
* is unit is not suitable for continuous monitoring during medical emergencies or operations.Otherwise,
the patient’s wrist and ngers will become anaesthetic, swollen and even purple due to a lack of blood.
* When not in use, store the device in a dry room and protect it against extreme moisture, heat, lint, dust
and direct sunlight. Never place any heavy objects on the storage case.
* is device may be used only for the purpose described in this booklet. e manufacturer cannot be held
liable for damage caused by incorrect application.
*is device comprises sensitive components and must be treated with caution. Observe the storage and
operating conditions described in this booklet.
* e maximum temperature that the applied part can be achieved is 42.5°C while the environmental
temperature is 40°C.
* e equipment is not AP/APG equipment and not suitable for use in the presence of a ammable
anesthetic mixture with air of with oxygen or nitrous oxide.
* Warning: No servicing/maintenance while the ME equipment is in use.
* e patient is an intended operator.
* e patient can measure data and change batteries under normal circumstances and maintain the device
and its accessories according to the user manual.
* To avoid measurement errors, please avoid the condition of strong electromagnetic eld radiated
interference signal or electrical fast transient/burst signal.
* e blood pressure monitor and the cu are suitable for use within the patient environment. If you are
allergic to polyester, nylon or plastic, please don’t use this device.
* During use, the patient will be in contact with the cu. e materials of the cu have been tested and
found to comply with requirements of ISO 10993-5:2009 and ISO 10993-10:2010. It will not cause any
potential sensization or irritation reaction.
* If you experience discomfort during a measurement, such as pain in the wrist or other complaints, press
the START/STOP button to release the air immediately from the cu. Loosen the cu and remove it from
your wrist.
* If the cu pressure reaches 40 kPa (300 mmHg), the unit will automatically deate. Should the cu not
deate when pressures reaches 40 kPa (300 mmHg), detach the cu from the wrist and press the
START/STOP button to stop ination.
* Before use, make sure the device functions safely and is in proper working condition. Check the device,
do not use the device if it is damaged in any way. e continuous use of a damaged unit may cause injury,
improper results, or serious danger.
* Do not wash the cu in a washing machine or dishwasher!
* e service life of the cu may vary by the frequency of washing, skin condition, and storage state. e
typical service life is 10000 times.
* It is recommended that the performance should be checked every 2 years and aer maintenance and
repair, by retesting at least the requirements in limits of the error of the cu pressure indication and air
leakage (testing at least at 50mmHg and 200mmHg).
* Manufacturer will make available on request circuit diagrams, component part lists, descriptions,
calibration instructions,etc., to assist to service personnel in parts repair.
* e operator shall not touch output of batteries and the patient simultaneously.
Cleaning :Dust environment may aect the performance of the unit. Please use the so cloth to clean the
whole unit before and aer use. Don’t use any abrasive or volatile cleaners.
* e device doesn’t need to be calibrated within two years of reliable service.
* If you have any problems with this device, such as setting up, maintaining or using, please contact the
SERVICE PERSONNEL of Transtek. Don’t open or repair the device by yourself in the event of
.
7
Page 9

malfunctions. e device must only be serviced, repaired and opened by individuals at authorized
sales/service centers.
* Please report to Transtek if any unexpected operation or events occur.
* Keep the unit out of reach of infants, young children or pets to avoid inhalation or swallowing of small
parts. It is dangerous or even fatal.
* Be careful to strangulation due to cables and hoses, particularly due to excessive length.
* At least 30 min required for ME equipment to warm from the minimum storage temperature between
uses until it is ready for intended use. At least 30 min required for ME equipment to cool from the
maximum storage temperature between uses until it is ready for intended use.
* is equipment needs to be installed and put into service in accordance with the information provided in
the ACCOMPANYING DOCUMENTS;
* Wireless communications equipment such as wireless home network devices, mobile phones, cordless
telephones and their base stations, walkie-talkies can aect this equipment and should be kept at least a
distance d away from the equipment. e distance d is caculated by the MANUFACTURER from the
80MHz to 5.8 GHz column of Table 4 and Table 9 of IEC 60601-1-2:2014, as appropriate.
* Please use ACCESSORIES and detachable partes specied/ authorised by MANUFACTURE.
Otherwise, it may cause damage to the unit or danger to the user/patients.
* ere is no luer lock connectors are used in the construction of tubing, there is a possibility that they
might be inadvertently connected to intravascular uid systems, allowing air to be pumped into a blood
vessel.
* Please use the device under the environment which was provided in the user manual. Otherwise, the
performance and lifetime of the device will be impacted and reduced.
2. BLOOD PRESSURE MONITOR COMPONENT (PIC. 1)
A.
LCD Display
B.
Start / Stop button
C.
Memory button / Set button (MEM)
D.
Hypertension grade indicator
E.
Systolic blood pressure
F.
Diastolic blood pressure
G.
Pulse rate
COMPONENT LIST OF PRESSURE MEASURING SYSTYEM:
1. PCBA;
2. Air Pipe;
3. Pump;
4. Valve;
5. Cu.
LIST OF ACCESSORIES:
1. Wrist blood pressure monitor
2. Storage case
3. 2 x AAA batteries
4. User manual
EN
8
Page 10

EN
3. BEFORE YOU START
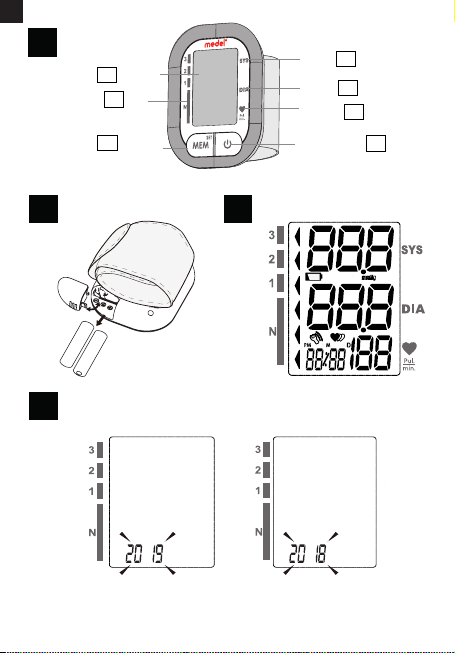
3.1 INSTALLING AND REPLACING THE BATTERIES (PIC. 2)
Slide o the battery cover.
Install the batteries by matching the correct polarity, as show pic. 2.
Always use the correct battery type (2 x AAa batteries).
Replace the battery cover.
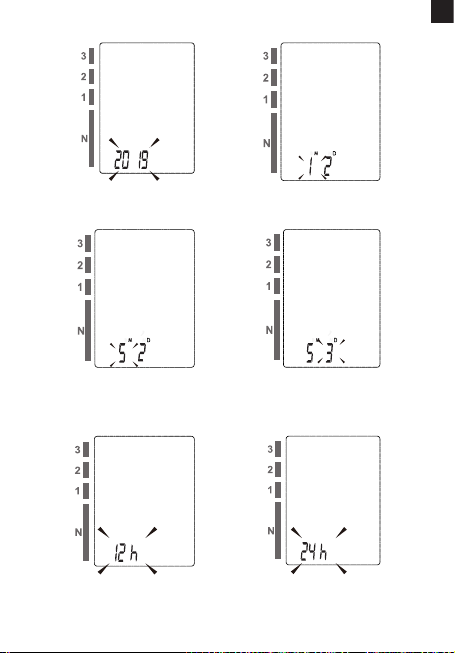
3.2 SETTING DATE, TIME AND MEASUREMENT UNIT (PIC. 4)
It is important to set the clock before using your blood pressur monitor, so that a time stamp can be assigned to each record that is stored in
the memory. (year: 2018 - 2058, time format: 12H/24H)
1. When the monitor is o, hold pressing “MEM” button for about 3
seconds to enter into setting mode. e blinking numeral represents [YEAR] (pic. 4.A)
2. Press the “MEM” button to change the [YEAR]. Each press will
increase the numeral by one in a cycling manner (pic. 4.B).
3. When you get the right year, press “START/STOP” button to
conrm your selection and it will turn to the next step (pic. 4.C)
(pic.4.D).
4. Repeat step 2 and 3 to conrm [MONTH] and [DAY]
5. (pic. 4.E) (pic. 4.F)
6. Repeat step 2 and 3 to conrm the time formate [12H] and [24H]
(pic. 4.G) (pic. 4.H)
7. Repeat step 2 and 3 to conrm [HOUR] and [MINUTE] (pic. 4.I)
Replace the batteries whenever the below happen
The shows
The display is dim.
The display does not light up
CAUTION
Do not use new and used batteries together.
Do not use different types of batteries together.
Do not dispose the batteries in fire. Batteries may explode or leak.
Remove batteries if the device is not likely to be used for some time.
Worn batteries are harmful to the environment. Do not dispose with daily garbage.
Remove the old batteries from the device following your local recycling guidelines.
9
Page 11

3.3 TIE THE CUFF
1. Remove all accessories (watch, bracelet,etc) from your wrist. If your physician
has diagnosed you with poor circulation in your wrist, use the other one.
2. Roll or push up your sleeve to expose the skin.
3. Apply the cu to your wrist with your palm facing up.
4. Position the edge of the cu about 1cm~1.5cm from wrist joints.
5. Fasten the wrist cu around your wrist, leaving no extra room between the cu
and your skin. If the cu is too loose, the measurement will not be accurate.
6. Sit comfortably with your tested wrist resting on a at surface. Place your elbow on a table so that the cu is at the same level as your heart. Turn your palm
upwards. Sit upright in a chair, and take 5-6 deep breaths.
7. Patients with Hypertension: e middle of the cu should be at the level of the
right atrium of the heart; Before starting measurement, please sit comfortably
with legs uncrossed, feet at on the oor, back and wrist supported. Rest for 5
minutes before measuring.
Wait at least 3 minutes between measurements. is allows your blood circulation to
recover. Take the measurement in a silent room. e patient must relax as much as
possible and do not move and talk during the measurement procedure.
e cu should maintain at the same level as the right
atrium of the heart. Do not cross your legs and keep your
feet on the ground. Keep your back against the backrest of
the chair. For a meaningful comparison, try to measure
under similar conditions. For example, take daily measurements at approximately the same time, on the same
wrist, or as directed by a physician.
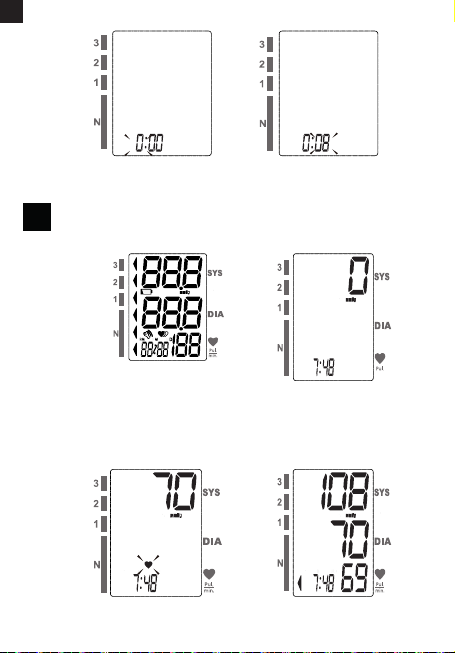
3.4. START THE MEASUREMENT
1. When the monitor is o, press “START/STOP” button to turn on
the monitor, and it will nish the whole measurement (pic. 5.A)
(pic. 5.B).
2. Press “START/STOP” button to power o, otherwise it will turn o
within 1 minute (pic. 5.C) (pic. 5.D).
DATA MANAGEMENT
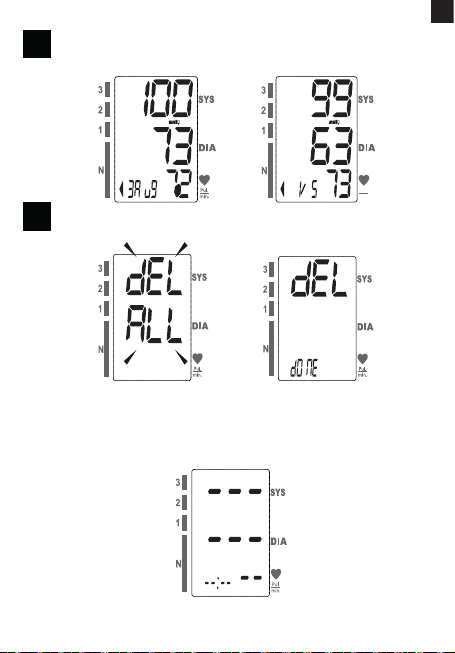
4.1 RECALL THE RECORDS
1. When the monitor is o, press “MEM” button to show the average
value of the latest three measurement records.If the records are
less than 3 groups,it will display the latest record instead (pic. 6. A).
2. Press “MEM” button again, it will display the latest measurement
result, date and time. Press “MEM” button again, it will display the
EN
10
Page 12

EN
next record,and so on. During the process of displaying the results,
if there is no operation, the blood pressure monitor will turn o
in one minute. Or you can press “START/STOP” button to turn it
o. (pic. 6.B).
It means the total
records is 5, the
current No. is No. 1.
CAUTION
The most recent record )1( is shown first. Each new m easurement
is assigned t o the first )1( record. All other records a re pushed back
one digit ) e.g., 2 becomes 3, and so on(, and the last record ) 60(
dropped from the list.
4.2 DELETE THE RECORDS
If you did not get the correct measurement, you can delete all results by
followings steps below.
1. Hold pressing “MEM” button for 3 seconds when the monitor is
in the memory recall mode, the “dEL All” will ash on the display
(pic. 7.A).
2. Press “MEM” to conrm deleting , the LCD displays “ dEL donE”
and the monitor will turn o (pic. 7.B).
Note: To exit out of delete mode without deleting any records, press START/
STOP button before pressing “MEM” to conrm any delete commands.
The corresponding
date is May 14 .
th
The corresponding
time is 8:16.
3. If there is no record, the right display will show (pic. 7.C).
5. INFORMATION FOR USER
5.1 MAINTENANCE
To obtain the best performance, please follow the instructions below.
• Put in a dry place and avoid the sunshine
• Avoid immersing it in the water. Clean it with a dry cloth in case.
• Avoid shaking and collision.
• Avoid dusty environment and unstable temperature surrounding.
• Use the slightly damp cloth to remove the dirt.
• Avoid washing the cu.
11
Page 13

6. ABOUT BLOOD PRESSURE
6.1 WHAT ARE SYSTOLIC PRESSURE AND DIASTOLIC PRESSURE?
When ventricles contract and pump blood out of
the heart, blood pressure reaches its maximum
value, the highest pressure in the cycle is known as
systolic pressure.
When the heart relaxes between heartbeats, the lowest blood pressure is
diastolic pressure.
6.2 WHAT IS THE STANDARD BLOOD PRESSURE CLASSIFICATION?
e blood pressure
classication published by World Health Organization
(WHO) and International Society of
Hypertension (ISH)
in 1999 is as follows:
Level
Blood
pressure
(mmHg)
Optimal Normal Mild Moderate SevereNormal
<120
SYS
<80
DIA
120~129
80~84
Hight-
130~139
85~89
140~159
90~99
Systolic
blood discharging
artery
press
160~179
100~109
Diastolic
blood entering
vein
relax
>
180
110
>
EN
12
Page 14

EN
6.3 IRREGULAR HEARTBEAT DETECTOR
An irregular heartbeat is detected when a heartbeat rhythm varies while
the device is measuring systolic pressure and diastolic pressure. During
each measurement, blood pressure monitor will keep a record of all the
pulse intervals and calculate the average value of them. If there are two or
more pulse intervals , the dierence between each interval and the average
is more than the average value of ±25% , or there are four or more pulse
intervals ,the dierence between each interval and the average is more
than the average value of ±15%, then the irregular heartbeat symbol will
appear on the display with the measurement result.
6.4 WHY DOES MY BLOOD PRESSURE FLUCTUATE THROUGHOUT THE DAY?
1. Individual blood pressure varies multiple times everyday. It is also
aected by the way you tie your cu and your measurement position, so please take the measurement under the same conditions.
2. If the person takes medicine, the pressure will vary more.
3. Wait at least 3 minutes for another measurement.
7. ERROR MESSAGE MALFUNCTION
PROBLEM SYMPTOM CHECK THIS REMEDY
No power
Low
batteries
Error
message
Warning
message
13
Batteries are exhausted.
Display is dim or
Batteries are inserted
will not light up.
incorrectly.
Show on
Batteries are low.
the display
E1 shows
E 2 shows The cuff is very tight
E 3 shows
E 10 or
E 11 shows
E 20 shows
E 21 shows
EExx,shows on
the display.
“out ” shows
The cuff is not secure.
The pressure of the
cuff is excess.
The monitor detected motion,
talking,or the pulse is too poor
while measuring.
The measurement process
does not detect the pulse
signal.
Measure incorrectly.
A calibration error
occurred.
Out of measurement
range
Replace with new batteries
Insert the batteries correctly
Replace with new batteries
Refasten the cuff and then
Refasten the cuff and then measure again.
Relax for a moment and then measure again.
movement can affect the measurement.
Relax for a moment and then measure
again.
Loosen the clothing on the arm and then
measure again.
Relax for a moment and then measure again.
Retake the measurement.If the problem
persists,contact the retailer or our customer
service department for further assistance.
Refer to the warranty for contact information
and return instructions.
Relax for a moment. Refasten the cuff and
thenmeasure again. If the problem persists,
contact your physician.
measure again.
Page 15

8. SPECIFICATION
EN
Power supply
Display mode
Measurement mode
Measurement range
Accuracy
Working condition
Storage & transportation
condition
Measurement perimeter
of the upper arm
Weight
External dimensions
Attachment
Battery powered mode:
Digital LCD V.A.32mmx45mm
Oscillographic testing mode
Rated cuff pressure:
0mmHg~299mmHg)0kPa ~ 39.9kPa(
Measurement pressure:
SYS: 60mmHg~230mmHg )8.0kPa~30.7kPa(
DIA: 40mmHg~130mmHg )5.3kPa~17.3kPa(
Pulse value: )40-199(beat/minute
Pressure:5°C-40°C within±3mmHg)0.4kPa(
Pulse value:±5%
A temperature range of :+5°C to +40°C
A relative humidity range of 15% to 90%, non-condensing,
but not requiring a water vapour partial pressure greater
than 50 hPa
An atmospheric pressure range of : 700 hPa to 1060 hPa
About 13.5cm-21.5cm
Approx.104g)Excluding the batteries(
Approx.85mm×67mm×23mm)Excluding the cuff(
2*AAA batter ies,user manual,PP case
Mode of operation
Degree of protection Type BF applied part
Device Classification
Continuous operation
Internally Powered ME Equipment
2*AAA batteries
IP Classification
Software Version
A01
WARNING: No modication of this equipment is allowed.
14
Page 16

EN
9.WARRANTY CONDITIONS
• The device is guaranteed for 5 years from the date of original
purchase against any defect in materials or workmanship.
• The warranty consists in the replacement and/or repair, free
of charge, of originally defective components.
• The warranty does not cover the accessories supplied and the
parts subject to normal wear and tear. The device may only be
repaired by authorised technical service centres.
• The appliance must be sent to MEDEL CUSTOMER SERVICE
for repairs.
• The transport costs shall be borne by the user.
• Any repair out of warranty shall be borne by the user. The
warranty lapses if the device has been tampered with, if the
defect was caused by improper use or in case the damage is
not due to the manufacturer (accidental fall, incorrect transport
etc.).
• The warranty does not involve any direct or indirect damages
of any kind to people or property during the period of inefficiency of the product.
• The warranty is valid from the date of purchase certified by
the receipt or invoice.
15
Page 17

10. REFERENCE TO STANDARD
10.1 COMPLIED LIST
Risk management
Labeling
User manual
General Requirements
for Safety
Electromagnetic
compatibility
Performance
requirements
Clinical investigation
Usability
Software life-cycle
processes
Bio-compatibility
EN ISO 14971:2012 / ISO 14971:2007 Medical devices -
Application of risk management to medical devices
EN ISO 15223-1:2016 / ISO 15223-1:2016 Medical devices.
Symbols to be used with medical device labels, labelling and
information to be supplied. Part 1 : General requirements
EN 1041:2008 Information supplied by the manufacturer of
medical devices
EN 60601-1:2006+A1:2013/ IEC 60601-1:2005+A1:2012 Medical
electrical equipment - Part 1: General requirements for basic safety
and essential performance
EN 60601-1-11:2015/ IEC 60601-1-11:2015 Medical electrical
equipment - Part 1-11: General requirements for basic safety and
essential performance - Collateral standard: Requirements for
medical electrical equipment and medical electrical systems used in
the home healthcare environment
EN 60601-1-2:2015/ IEC 60601-1-2:2014 Medical electrical
equipment - Part 1-2: General requirements for basic safety and
essential performance - Collateral standard: Electromagnetic
disturbances - Requirements and tests
EN ISO 81060-1:2012 Non-invasive sphygmomanometers - Part
1: Requirements and test methods for non-automated measurement
type
EN 1060-3:1997+A2:2009 Non-invasive sphygmomanometers Part 3: Supplementary requirements for electro-mechanical blood
pressure measuring systems
IEC 80601-2-30:2009+A1:2013 Medical electrical equipmentPart 2-30: Particular requirements for the basic safety and
essential performance of automated non-invasive
sphygmomanometers
EN 1060-4:2004 Non-invasive sphygmomanometers - Part 4: Test
procedures to determine the overall system accuracy of automated
non-invasive sphygmomanometers
ISO 81060-2:2013 Non-invasive sphygmomanometers - Part 2:
Clinical validation of automated measurement type
EN 60601-1-6:2010+A1:2015/IEC 60601-1-6:2010+A1:2013
Medical electrical equipment - Part 1-6: General requirements for
basic safety and essential performance - Collateral standard:
Usability
IEC 62366-1:2015 Medical devices - Part 1: Application of
usability engineering to medical devices
EN 62304:2006/AC: 2008 / IEC 62304: 2006+A1:2015 Medical
device software - Software life-cycle processes
ISO 10993-1:2009 Biological evaluation of medical devicesPart 1: Evaluation and testing within a risk management process
ISO 10993-5:2009 Biological evaluation of medical devices -
Part 5: Tests for in vitro cytotoxicity
ISO 10993-10:2010 Biological evaluation of medical devices -
Part 10: Tests for irritation and skin sensitization
EN
16
Page 18

EN
12. EMC Guidance
1)is product needs special precautions regarding EMC and needs to
be installed and put into service according to the EMC information provided,
and this unit can be aected by portable and mobile RF communications
equipment.
2)* Do not use a mobile phone or other devices that emit electromagnetic
elds, near the unit. is may result in incorrect operation of the unit.
3)Caution: is unit has been thoroughly tested and inspected to assure
proper performance and operation!
4)* Caution: is machine should not be used adjacent to or stacked with
other equipment and that if adjacent or stacked use is necessary, this machine should be observed to verify normal operation in the conguration
in which it will be used.
Table 1
Guidance and manufacturer’s declaration – electromagnetic emissions
The device is intended for use in the electromagne tic environment specified below. The customer or the
user of the device should assure that it is used in such an environment.
Emissions test Electromagnetic environment - guidance
RF emissions
CISPR 11
RF emissions
CISPR 11
Harmonic emissions
IEC 61000-3-2
Voltage fluctuations/
flicker emissions
IEC 61000-3-3
Compliance
Group 1
Class B
Class A
Complies
The device uses RF energy only for its internal
function. Therefore, its RF emissions are very low
and are not likely to cause any interference in nearby
electronic equipment.
The device is suitable for use in all establishments,
other than domestic and those directly connected to
the public low-voltage power supply network that
supplies buildings used for domestic purposes.
17
Page 19

Table 2
Guidance and manufacturer’s declaration – electromagnetic immunity
The device is intended for use in the electromagnetic environment specified below. The customer or the
user of the device should assure that it is used in such an environment.
Immunity test
Electrostatic
discharge )ESD(
IEC 61000-4-2
Electrical fast
transient/burst
IEC 61000-4-4
Surge
IEC61000-4-5
Voltage dips,
short interruptions
and voltage
variations on
power supply
input lines
IEC 61000-4-11
IEC 60601 test level
±8 kV contact
±15 kV air
power supply lines:
±2 kV
input/output lines:
±1 kV
line)s( to line)s(: ±1 kV
line)s( to earth: ±2 kV
100 kHz repetition
frequency
0%UT; 0.5 cycle
At 0°, 45°, 90°, 135°,
180°,225°,270° and 315°
0%U
and
70%U
Single phase: at 0°
0% U
T ; 1 cycle
T ; 25/30 cycles
T ; 300 cycle
Compliance level
±8 kV contact
±15 kV air
power supply lines:
±2 kV
line)s( to line)s(: ±1 kV
100 kHz repetition
frequency
0% U
T ; 0.5 cycle
At 0°, 45°, 90°, 135°,
180°,225°,270° and
315°
0% U
T ; 1 cycle
and
70% U
T ; 25/30 cycles
Single phase: at 0°
0% U
T ;300 cycle
Electromagnetic
environment - guidance
Floors should be wood, concrete
or ceramic tile. If floors are
covered with synthetic material,
the relative humidity should be at
least 30%.
Mains power quality should be
that of a typical commercial or
hospital environment.
Mains power quality should be
that of a typical commercial or
hospital environment.
Mains power quality should be
that of a typical commercial or
hospital environment.
EN
Power frequency
)50Hz/60Hz(
magnetic field
IEC 61000-4-8
NOTE UT is the a.c. mains voltage prior to application of the test level.
30 A/m
50Hz/60Hz
30 A/m
50Hz/60Hz
Power frequency magnetic fields
should be at levels characteristic
of a typical location in a typical
commercial or hospital
environment.
18
Page 20

EN
Table 3
Guidance and manufacturer’s declaration – electromagnetic immunity
The device is intended for use in the electromagnetic environment specified below. The customer or the
user of the device should assure that it is used in such an environment.
Immunity test
Conducted RF
IEC 61000-4-6
Radiated RF
IEC 61000-4-3
IEC 60601
Test level
150 kHz to
80 MHz:
3 Vrms
6Vrms )in ISM
and amateur
radio bands(
80% Am at 1kHz
10V/m, 80% Am
at 1kHz
Compliance
level
150 kHz to
80 MHz:
3 Vrms
6Vrms )in ISM
and amateur
radio bands(
80% Am at 1kHz
10V/m, 80% Am
at 1kHz
Electromagnetic environment - guidance
Portable and mobile RF communications
equipment should be used no closer to any part
of the device, including cables, than the
recommended separation distance calculated
from the equation appropriate for the frequency
of the transmitter.
Recommended separation distances:
d=0.35 ;
P
d=1.2
P
P
P
where,
output power rating of the
transmitter in watts )W(
according to the
transmitter manufacturer,
d is the recommended
separation distance in
meters )m(. Field
strengths from fixed RF
transmitters, as
determined by an
electromagnetic site
survey, should be less
than the compliance level
in each frequency range.
Interference may occur in
the vicinity of equipment
marked with the following
symbol:
80 MHz to 800 MHz:
d=1.2
800 MHz to 2.7 GHz:
d=2.3
P is the maximum
a
b
NOTE 1 A t 80 MHz and 800 MHz, the higher frequency range applies.
NOTE 2 T hese guidelines may not apply in all situations. Electromagnetic propagation is affected by
absorption and reflection from structures, objects and people.
a
Field strengths from fixed transmitters, such as base stations for radio )cellular / cordless(
telephones and land mobile radios, amateur radio, AM and FM radio broadcast and TV broadcast
cannot be predicted theoretically with accuracy. To assess the electromagnetic environment due to
fixed RF transmitters, an electromagnetic site survey should be considered. If the measured field
strength in the location in which the device is used exceeds the applicable RF compliance level
above, the device should be observed to verify normal operation. If abnormal performance is
observed, additional measures may be necessary, such as re-orienting or relocating the device.
b
Over the frequency range 150 kHz to 80 MHz, field strengths should be less than 3V/m .
19
Page 21

Table 4
Recommended separation distances between portable and mobile RF communications
equipment and the device.
The device is intended for use in an electromagnetic environment in which radiated RF disturbances are
controlled. The customer or the user of the device can help prevent electromagnetic interference by
maintaining a minimum distance between portable and mobile RF communications equipment
)transmittters( and the device as recommended below, according to the maximum output power of the
communications equipment.
EN
Rated maximum output
power of transmitter
)W(
0.01
0.1
1
10
100
For transmitters rated at a maximum output power not listed above, the recommended separation
distance
d in metres )m( can be estimated using the equation applicable to the frequency of the
transmitter, where P is the maximum output power rating of the transmitter in watts )W( according to the
transmitter manufacturer.
NOTE 1 At 80 MHz and 800 MHz, the separation distance for the higher frequency range applies.
NOTE 2 These guidelines may not apply in all situations. Electromagnetic propagation is affected by
absorption and reflection from structures, objects and people.
Separation distance according to frequency of transmitter )m(
150 kHz to 80 MHz 8 0 MHz to 800 MHz 800 MHz to 2.7 GHz
=d
3.5
P
0.12 0.12
0.38
1.2
3.8
12
=d 1.2
0.38
1.2
3.8
12
P
0.23
0.73
2.3
7.3
23
2.3=d
P
20
Page 22

EN
Table 5
Guidance and manufacturer’s declaration - electromagnetic immunity
The device is intended for use in the electromagnetic environment specified below. The customer or the
user of the device, should assure that it is used in such an environment.
Radiated RF
IEC61000-4-3
)Test
specifications
for
ENCLOSURE
PORT
IMMUNITY to
RF wireless
communications
equipment(
NOTE If necessary to achieve the IMMUNITY TEST LEVEL, the distance between the transmitting antenna
and the ME EQUIPMENT or ME SYSTEM may be reduced to 1 m. The 1 m test distance is permitted by
IEC 61000-4-3.
a( For some services, only the uplink frequencies are included.
b( The carrier shall be modulated using a 50% duty cycle square wave signal.
c( As an alternative to FM modulation, 50% pulse modulation at 18 Hz may be used because while it does
not represent actual modulation, it would be worst case.
The MANUFACTURER should consider reducing the minimum separation distance, based on RISK
MANAGEMENT, and using higher IMMUNITY TEST LEVELS that are appropriate for the reduced
minimum separation distance. Minimum separation distances for higher IMMUNITY TEST LEVELS shall be
calculated using the following equation:
E=
Where P is the maximum power in W, d is the minimum separation distance in m, and E is the IMMUNITY
TEST LEVEL in V/m.
Test
Band a(
Service a( Modulation b( Modulation b(
)MHz(
Frequency
)MHz(
385
450 430-470
710 704-787
745
780
810
870
930
1720
1845
1970
5240
5500
5785
380-390 TETRA
800-960
17001990
2570
51005800
400
GMRS 460,
FRS 460
LTE Band
13,
17
GSM
800/900,
TETRA 800,
iDEN 820,
CDMA 850,
LTE Band 5
GSM 1800;
CDMA 1900;
GSM 1900;
DECT;
LTE Band 1,
3,
4,25; UMTS
Bluetooth,
WLAN,
802.11
b/g/n, RFID
2450, LTE
Band 7
WLAN
802.11
a/n
Pulse
modulation b(
18Hz
FM c( ± 5kHz
deviation 1kHz
sine
Pulse
modulation b(
217Hz
Pulse
modulation b(
18Hz
Pulse
modulation b(
217Hz
Pulse
modulation b(
217 Hz
Pulse
modulation b(
217 Hz
Distance )m(
)W(
1.8
20 .3 28
0.20 .3 9
2
2
2
0.2
21
IMMUNITY
TEST
LEVEL
)V/m(
0.32 7
0.32 8
0.32 8
0.32 82450 2400-
0.39
Page 23

Page 24

Manufacturer:
Guangdong Transtek Medical Electronics Co.,
Ltd. Zone A, No.105 ,Dongli Road, Torch Development District, Zhongshan,528437 Guangdong,China
MDSS - Medical Device Safety Service GmbH
EC REP
Schigraben 41, 30175 Hannover- Germany
Distributed by:
Medel International Srl
Via Villapizzone 26 - 20156 Milano / Italy
 Loading...
Loading...