Page 1
version:1.0
GUANGDONG TRANSTEK MEDICAL ELECTRONICS CO., LTD
Zone A, 5/F., Investment Building, No. 12, Huizhan East Rd., Torch Development
District, Zhongshan, Guangdong, 528437, China
TEL:86-760-88282982 www.transtek.cn
Contains FCC ID: OU9AW8001-LS
User Manual
Glass Body Fat Analyzer
LS203-B
Please do read the user manual carefully and thoroughly so as to ensure
the safe usage of this product, and keep the manual well for further
reference in case you have problems.
Thank you very much for selecting the Transtek Glass Body Fat Analyzer
LS203-B.
Page 2

1
Table of Contents
Table of Contents
Safety Information
Your Scale and Its Environment
Efficient Use of Your Scale
Overview
Device Components
LCD Display
Initial Start-Up
General Instructions
Insert the Batteries
Install App and Pair-up
Start Measuring
Select Measurement Unit
Daily Measurement
Manage Your Health
Data Transmission
Troubleshooting
Error Prompt
When Measuring ...
When Data Transmitting...
Specifications
Maintenance
Warranty
FCC Regulations
Appendix
Table of Body Fat Level
Table of Body Water Level
Health Tips – About Body Fat
EMC Guidance
3
3
4
5
6
6
7
8
8
9
10
10-11
12
13
14
14
15
16
16
17
17-21
...............................................................................................
......................................................................................................
.................................................................................................................
.................................................................................................................
..........................................................................................................
.............................................................................................................................
....................................................................................................................
..................................................................................................................
..................................................................................................................
........................................................................................................
............................................................................................................................
...............................................................................................................................
......................................................................................................................................
................................................ ............................................................
......................................................................................................
.................................................................................................
.........................................................................................................................
..........................................................................................................
..............................................................................................................................
...................................................................................................................
........................................................................................................................
Contraindications
3
.
Indications For Use
2
Safety and Usage Information
2
...................................................................................................
................................................................................................................
......................................................................................................................
Page 3
2 3
Safety Information Safety Information
The warning signs and symbols are essential to ensure your correct and safe use of
this product and protect you and others from injury. Please kindly find the meanings of the
warning signs and symbols, which you may encounter in the label and user manual, as
follows:
Safety and Usage Information
Symbol for “THE OPERATION
GUIDE MUST BE READ”
Transtek’s Body Fat Analyzer offers you a seamless way to manage your health.
Please be aware that this device is designed for adults’ self-measuring and self-monitoring body
fat level. Any information provided by this device is in no way meant to treat, cure or prevent any
disease or illness from happening. This device should not be used by anyone who is acutely or
chronically ill, suffering from a disease or taking medications that affect your water levels. The
accuracy of readings for these patients has not been verified. Specific medical advice should be
obtained from a physician.
The device is equipped with automatic data transmission function. It may emit
electromagnetic energy so as to perform its intended function. Nearby portable and mobile RF
communications equipment can affect the performance of the device.
Kindly note that the use of accessories, transducers or cables other than those specified, with
the exception of transducers and cables sold by the manufacturer as replacement parts for
internal components, may result in increased EMISSIONS or decreased IMMUNITY of
the device.
Be aware that misuse of electrical equipments can cause electric shock, burns, fire and other
hazards.
Symbol for “MANUFACTURER”
Symbol for “COMPLIES WITH MDD
93/42/ECC REQUIREMENTS”
Symbol for “ENVIRONMENT
PROTECTION – Waste electrical
products should not be disposed of with
household waste. Please recycle where
facilities exist. Check with your local
authority or retailer for recycling advice”
Symbol for “TYPE BF APPLIED
PARTS”
Symbol for “SERIAL NUMBER”
Symbol for “DIRECT CURRENT”
Symbol for “INDOOR USE ONLY”
CAUTION
INDICATIONS FOR USE
The Transtek Body Fat Analyzer measures weight and uses bio-electrical impedance analysis
(BIA) technology to estimate body fat, total body water percentage, bone mass, and muscle
mass in generally healthy adults 18 years of age or older.
It is intended for use in the home / domestic setting only.
The Bluetooth Combination Mark
To ensure your safety as well as the service life of your scale, please avoid using
the scale under the following circumstances:
Concurrent use of this device and implantable medical electronic instruments, e.g.
Cardiac Pacemaker
Concurrent use of this device and wearable medical electronic instruments, e.g.
electrocardiograph
Concurrent use of this device and other medical electronic instruments for life support,
e.g. mechanical heart
Slippery floor such as tile floor
Jumping onto the platform immediately after bath or with wet hands
Near a cell phone or microwave oven
Avoid storage in the following locations:
Where there is water
Where the device may be exposed to extreme temperatures, humidity, moisture, direct
sunlight, dust, or salt air
Where there is risk of shock or drop
Where you store chemicals or full of corrosive gases
Where in reach of the infants or children
Your Scale and Its Environment
Efficient Use of Your Scale
To ensure the accuracy of measurement, please follow below instructions when
you start measurement.
Place the scale on a flat, hard surface. Soft surface such as carpet will affect the
performance of the scale.
Step onto the platform with bare feet. Stand still and keep full contact with the electrodes
until the measurement is complete.
Start measurement at least two hours after Getting up or Dinning.
Avoid measurement immediately after strenuous exercise, sauna or bath, drinking, and
dinning.
Always start measurement in the same time slot and on the same scale located on the
same flat, hard surface.
CONTRAINDICATIONS
1. This device is contraindicated for any female subject who may be suspected of, or is pregnant.
Besides provided inaccurate readings, the affects of this device on the fetus are unknown.
2. This device is contraindicated for any person who is connected to a wearable or implantable
electronic device or instrument such as a pacemaker or defibrillator.
The patient is an intended operator. The patient can perform all the operations in the
manual, such as measurement, data transmitting, changing batteries.
Page 4

5
4
Overview
Overview
List
1. Glass Body Fat Analyzer LS203-B
2. Four AAA-size Alkaline Batteries (1.5V each)
3. User Manual
Device Components
LCD Display
Electrode
Battery Compartment
UNIT and activation Button
Electrode
Stone
Pound
Bioelectrical Impedance Analysis
Kilogram
Data transmitting
Pending to transmit to wireless
wellness system
Low Battery
LCD Display
Page 5
7
6
Initial Start-Up Initial Start-Up
Transtek Glass Body Fat Analyzer LS203-B applies BIA (Bio-impedance Analysis)
technology. A small amount of weak current flows through the human body so as to detect
the bio-impedance and estimate body fat. The electrical current is small and may not be felt.
This BIA technology is cheap, safe, non-invasive, toxic-free and harmless. It also
possesses the characteristics of simple operation and abundant information.
The current mentioned above is less than 1mA. However, please be aware that anyone
with an wearable or implantable medical electronic instrument, such as a pacemaker, must
avoid using this device.
The intended use of this device is for adult’s indoor use only.
General Instructions
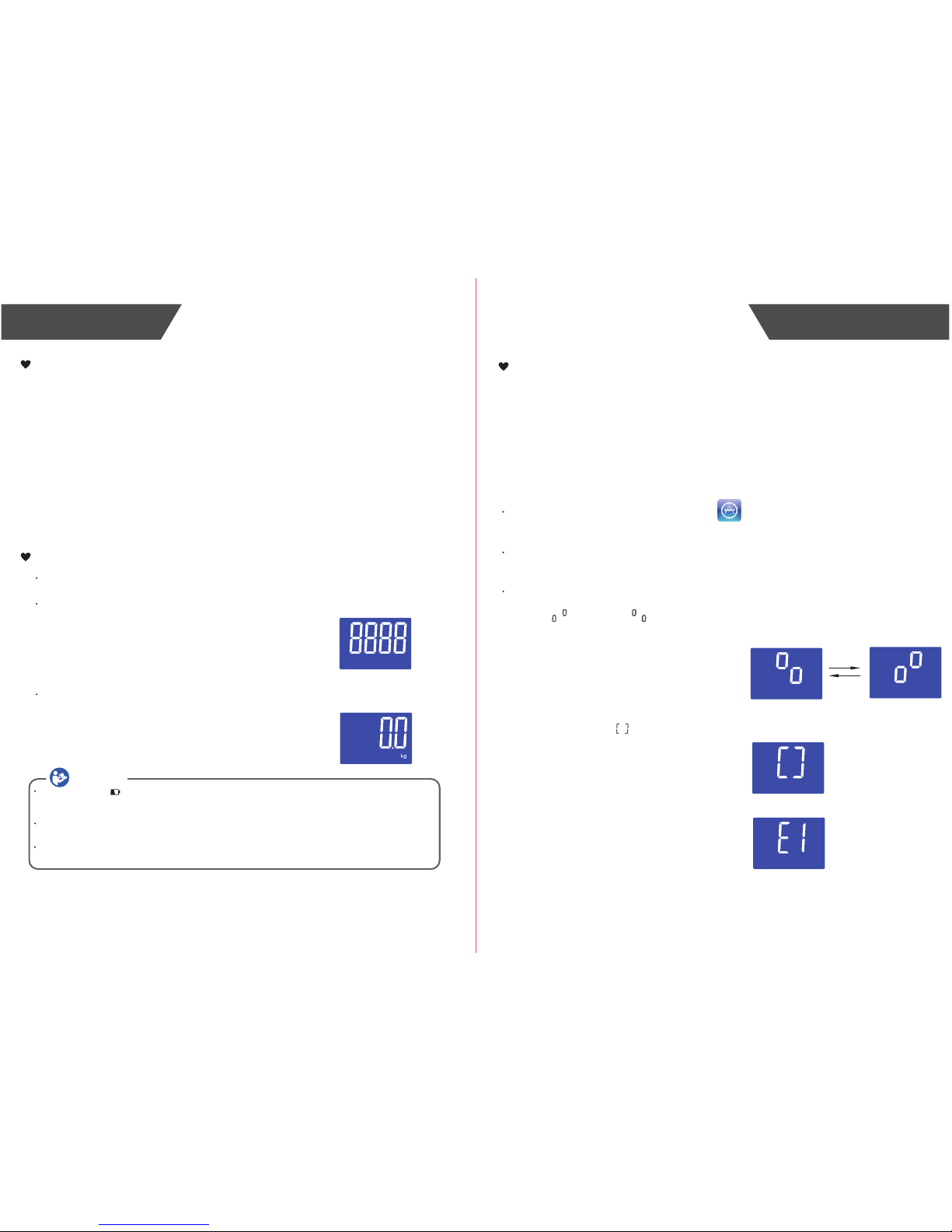
Insert the Batteries
Open the battery door in the back of the scale.
Insert the batteries (4 x 1.5V AAA) into the battery
compartment according to the polarity indications
marked inside the compartment.
* The digits “8888” will be shown on the LCD.
Close the battery door and wait until the digits
“0.0kg” are shown on the LCD.
CAUTION
When the symbol appears, the device will power off in four seconds. Then you shall replace with a
new set of batteries. Please replace all four batteries in the same time. Do NOT mix the old batteries with
the new one.
Worn batteries are hazardous waste. Do NOT dispose of them together with the household garbage.
Please refer to the local ordinances and recycling instructions regarding disposal of the worn batteries.
If SUCCEED, symbol will be shown on the LCD.
If FAIL, symbol “E1” will be shown on the LCD.
Install App and Pair-Up
The App is now available in App Store
You may search and install the app in your iPhone.
With the advanced Bluetooth 4.0 technology applied, the mobile or portable equipments,
which are equipped with Bluetooth function in line with BLE Technical Specifications as
well as BLP Protocol established by global organization Bluetooth SIG, are capable to
receive your personal health data.
Just simply install the specially-designed app and pair up your scale with
your mobile or portable equipments. Then you may enjoy the comprehensive health
solution provided by Transtek.
Turn on Bluetooth and App. Make sure both are ON when pair-up is
proceeding.
Press and hold “UNIT” button in the back of the scale to start pair-up.
Symbol and symbol will be shown on the
LCD alternatively, indicating pair-up is proceeding.
To remove primary batteries when me equipment is not likely to be used for some time.
Not touch the patient and battery output simultaneously.
Page 6
8
9
Start Measuring Manage Your Health
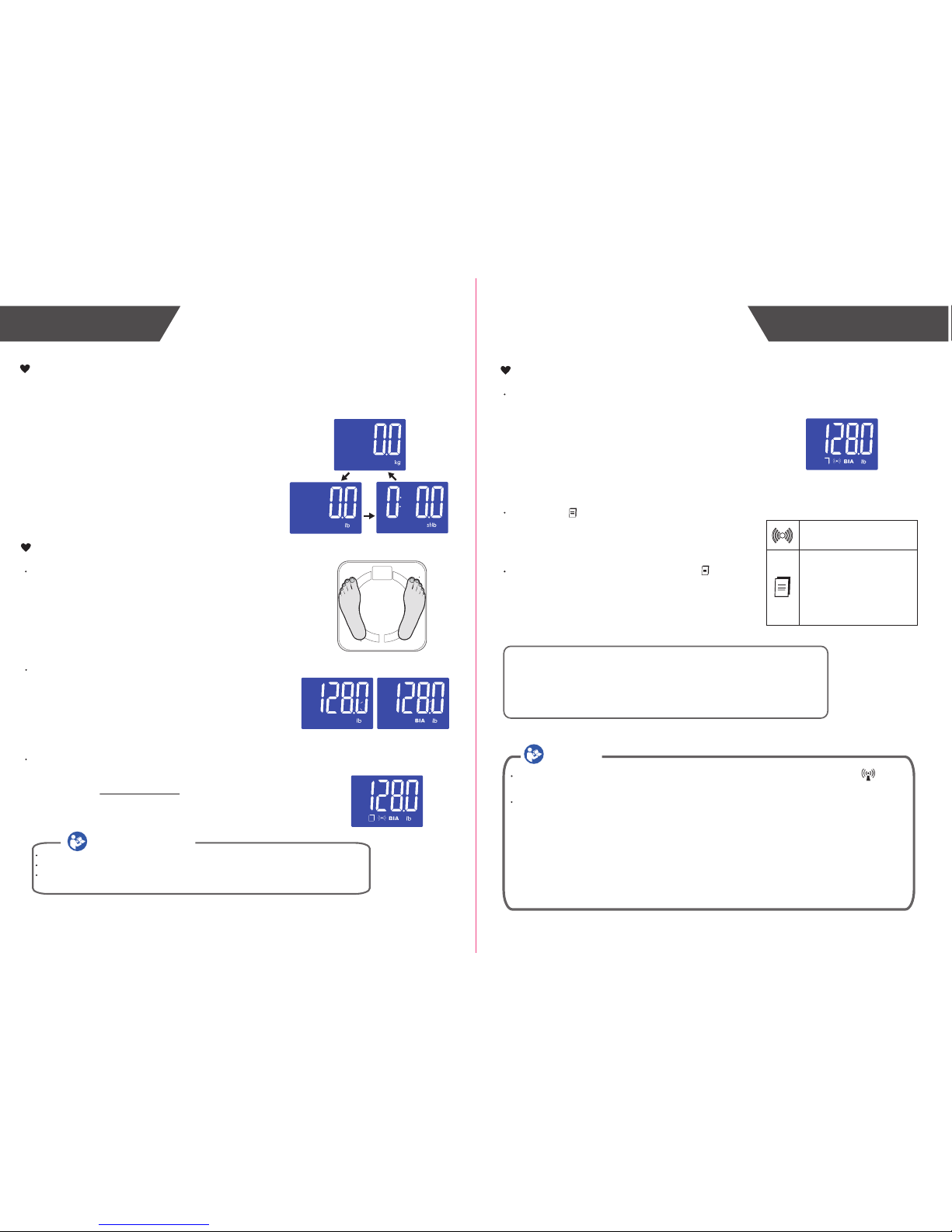
With batteries correctly installed, press “UNIT” button in the back of the scale to select
measurement unit. The default measurement unit is “kg”. You may press “UNIT”
button to choose among kilogram, stone and pound.
Select Measurement Unit
With original SENSE ON patent technology, the scale
will automatically switch on as you step on the platform
barefooted.
Stand still and keep full contact with the electrodes until
the LCD stops blinking “BIA”.
When your scale is successfully paired with your iPhone and the Bluetooth is ON,
it will process data transmission automatically.
(Please refer to Data Transmission for more details.)
Daily Measurement
With the scale successfully pair-up with your
iPhone, the measurement data will be automatically
transmitted to your mobile via Bluetooth.
The symbol will disappear after successful data
transmission, and you may check your personal
health data stored in your iPhone.
If the data transmission fails, the symbol will
remain. The pending measurement data will be
temporarily kept in the scale and transmitted to your
iPhone when next measurement is complete.
Data ready to transmit to
Data transmitting
the App:
-If SUCCEED, the symbol
disappears;
-If FAIL, the symbol
remains.
Data Transmission
CAUTION
Interference may occur in the vicinity of equipment marked with the following symbol . And
the Analyzer may interfere vicinity electrical equipment.
To enable the data transmission function, this product should be paired to a Bluetooth end at
2.4 GHz.
How to mitigate possible interference?
1. The range between the Analyzer and the Bluetooth end should be reasonably close, from 1
meter to 10 meters. Please ensure no obstacles between the Analyzer and the Bluetooth end
so as to obtain quality connection.
2. To avoid interference, other electronic devices (particularly those with Bluetooth transmission
/ Transmitter) should be kept at least 1 meter away from the Analyzer.
Bluetooth Module No.: AW8001
RF Frequency Range: 2402MHz to 2480 MHZ
Output Power Range: 4 dBm
Supply Voltage: 3V to 3.6V
CAUTION
The normal measuring time cannot over 1 minute.
After the measurement, please don’t stand on the scale for a long time.
Continous to use the scale for a long time, the surface temperature may exceed 41℃,
this will cause burns.
Page 7
10
11
TroubleshootingTroubleshooting
Error Prompt
Error Description Solution
Overload. The device will
power off in four seconds.
Stop using this scale for
measurement.
Low Battery. The device
will power off in four
seconds.
Replace all four batteries in the
same time. Please purchase the
authorized batteries for
replacement.
Please check below items:
-Bluetooth is ON.
-App is ON.
-Both devices are within the
transmission distance of Bluetooth.
Failure of pairing up your
scale with your iPhone.
Problem Root Cause Solution
Incorrect posture
Abnormal
measuring results:
- Too high; OR
- Too low; OR
- Huge difference
between two recent
measurement.
Please step on the platform
barefooted and stand still.
The device is located on
the soft ground such as a
carpet OR on a rugged
surface.
Please place the device on a
flat, hard surface.
Warm up your hands and feet to
resume blood circulation and then
measure again.
Cold body that may
results in bad blood
circulation.
When Measuring ...
Problem Root Cause Solution
Cold Electrodes.
Either your hands or your
feet are too dry.
Abnormal
measuring results:
- Too high; OR
- Too low; OR
- Huge difference
between two recent
measurement.
No display on
LCD when the
device powers on.
CANNOT proceed
to analyze body fat
The device
powers off
automatically.
Place the device in a warm
room for a while and then
measure again.
Wipe your feet with a damp
cloth, keeping them slightly damp
when starting measurement.
Batteries not yet installed.
Worn batteries.
Low battery.
Install the batteries.
(Please refer to Insert the
Batteries)
Replace all four batteries in the
same time. Please purchase the
authorized batteries for
replacement.
Step onto the platform
wearing socks or shoes.
Please keep barefooted during
the measurement, and keep full
contact with the electrodes as
well.
Replace all four batteries in the
same time. Please purchase the
authorized batteries for
replacement.
Page 8
12
SpecificationsTroubleshooting
13
Problem Root Cause Solution
Bluetooth is OFF.
Data transmission
failed.
Turn ON the Bluetooth via
“Setting >> General >> Bluetooth”.
App is OFF.
Press the icon to turn ON your
app.
Place your iPhone closer to the
scale.
Out of range of Bluetooth
transmission.
When Data Transmitting ...
Specifications
Product Name
Dimension
Glass Body Fat Analyzer (LS203-B)
Scale: 321x321x23.5mm
Panel: 321x321x6mm
Net Weight
Approximately 2kg ( Excluding the dry cells)
Display
Blue LCD with White Backlight
V.A.: 74x53mm
Measurement Unit
Kilogram / Stone / Pound
Measurement Range
5kg to 200kg / 0st: 11lb to 31st: 7lb / 11lb to 441lb
Division
0.1kg / 0.2lb
Accuracy
0-50kg: ±0.3kg;
100-150kg: ±0.5kg;
Working Environment
Temperature: 0 to 40
Humidity: ≤90% RH
Storage Environment
Temperature: -20 to 60
Power Source
6V (Four AAA-size Alkaline Batteries)
Auto-ON
SENSE ON technology
Auto-OFF
About 10 seconds while showing 0.0
About 15 seconds after the weight data is locked
Accessories
1. Four AAA-size Alkaline Batteries
2. User Manual
About the Accuracy of This Product
This product passes strict inspection before delivery and therefore its accuracy is guaranteed by the manufacturer.
Please refer to the above table for the descriptions on accuracy.
This product is specially designed for body fat analysis as well as weight measurement. It should NOT be used by
anyone during the process of transaction for verification of goods’ weight.
50-100kg: ±0.4kg;
150-200kg: ±0.7kg
Humidity: 10%RH to 93% RH
Mode of Operation
Continuous Operation
Protection Against
Ingress of Water
IPX0
Software Version 1.0
℃ ℃
℃ ℃
Pressure: 80kPa to 105kPa
Protection against
electric shock
Internally powered
ME equipment
Applied part Type BF applied part, including the whole top surface
Note: Not intended to be sterilized. Not for use in an oxygen rich environment.
Page 9
14
FCC Regulations
15
When carrying out usual maintenance, please ensure practice of the following Do’s and
Don’ts:
Transtek warrants its products free of defects in materials and workmanship in normal
use for a period of TWO years from the date of retail purchase.
This warranty does NOT cover damages caused by misuse or abuse, including but not
limited to:
Failure caused by unauthorized repairs or modifications;
Damage caused by shock or drop during transportation;
Failure caused by improper operation inconsistent with the instructions stated in this user
manual;
Malfunction or damage from failure to provide the recommended maintenance;
Damage caused by improper use of power supply.
Should this device require maintenance (or replacement at our option) under warranty,
please deliver the original package to GUANGDONG TRANSTEK MEDICAL
ELECTRONICS CO., LTD prepaid. Please return the store receipt (with the retail
purchase date) and a note with reasons to return on it as well.
Maintenance
Warranty
GUANGDONG TRANSTEK MEDICAL ELECTRONICS CO., LTD
Zone A, 5/F., Investment Building, No. 12 Huizhan East Rd., Torch Development District,
Zhongshan, 528437, Guangdong, China
Tel: 86-760-88282982
Website: http://www.transtek.cn
DO use a dry soft cloth to wipe the dust.
DO use a wet soft cloth, dipped into water and wrung out, to wipe the dirt. Then use a
dry soft cloth to dry up the device.
DON’T wash the device with water or immerse it in water.
DON’T use propellant, abrasive or other chemicals to wipe the dirt in avoidance of
discolor or malfunction.
DON’T disassemble this device. If you have any problems, please contact Transtek.
(Please refer to Warranty for contact information)
FCC User Guide Information
Radio Frequency Interface Requirements - FCC
Note: This equipment has been tested and found to comply with the limits for a Class B
digital device, pursuant to Part 15 of the FCC rules. These limits are designed to provide
reasonable protection against harmful interference in a residential installation. This
equipment generates, uses and can radiate radio frequency energy and, if not installed
and used in accordance with the instructions, may cause harmful interference to radio
communications. However there is no guarantee that interference will not occur in a
particular installation. If this equipment does cause harmful interference to radio or
television reception, which can be determined by turning the equipment off and on, the
user is encouraged to try to correct the interference by one or more of the following
measures:
Reorient or relocate the receiving antenna;
Increase the separation between the equipment and receiver;
Connect the equipment into an outlet on a circuit different from that to which the
receiver is connected;
Consult the dealer or an experienced radio / TV technician for help.
Radio Transmitters (Part 15)
This device complies with Part 15 of the FCC Rules. Operation is subject to the following
two conditions: (1) this device may not cause harmful interference, and (2) this device
must accept any interference received, including interference that may cause undesired
operation.
Changes or modifications not expressly approved by the party responsible for
compliance could void the user’s authority to operate the equipment.
FCC RF Exposure Guidelines
Safety Information
Reducing RF Exposure - Use Properly
Only operate the device in accordance with the instructions supplied.
This device complies with FCC radiation exposure limits set forth for an uncontrolled
environment.
FCC Regulations
Maintenance
Page 10

16
Appendix
17
Appendix
Fat is essential for human body. It can not only store energy and protect viscera, but also
regulate body temperature and maintain normal physiological function of human body.
However, too much body fat is harmful to human body. It is always accompanied by Fatty
Liver, diabetes, coronary heart disease, etc.
Therefore self-measuring and self-monitoring body fat level are beneficial to your health.
Since we can’t judge body fat level simply by our weight, this body fat analyzer LS203-B ,
with BIA (Bio-impedance Analysis) technology applied, is an accurate device that offers a
quick and comfortable way to obtain your body fat level.
Source: Derived fr. Wang & Deurenberg: “Hydration of fat-free body mass”. American
Journal Clin Nutr 1999, 69833-841
Table of Body Fat Level (Unit: %)
Man / Sportsman
Age
20-29
30-39
40-49
<13 13.1-20 20.1-23 >23 <19 19.1-28 28.1-31 >31
14.1-21 21.1-24 >24 <20 20.1-29 29.1-32 >32
16.1-23 23.1-26 >26 <22 22.1-30 30.1-33 >33
<14
<16
50-59 17.1-24 24.1-27 >27 <23 23.1-31 31.1-34 >34<17
60+ 18.1-25 25.1-28 >28 <24 24.1-32 32.1-35 >35<18
Low Normal High V. High Low Normal High V. High
Woman / Sportswoman
Health Tips - About Body Fat
Gender
Men
Women
Body Fat
Percentage Range
Optimal Total Body Water
Percentage Range
4-14 70-63
15-21 63-57
22-24 57-55
≥25 55-37
4-20 70-58
21-29 58-52
30-32 52-49
≥33 49-37
Table of Body Water Level (Unit: %)
Guidance and manufacturer’s declaration – electromagnetic emissions
Emission test Electromagnetic environment – guidance
Group 1
Class B
Not applicable
Not applicable
Compliance
The device is intended for use in the electromagnetic environment specified
below. The customer or the user of the device should assure that it is used in
such an environment.
Harmonic emissions
IEC 61000-3-2
Voltage fluctuations/
flicker emissions IEC
61000-3-3
RF emission
CISPR 11
RF emission
CISPR 11
The device uses RF energy only for its
internal function. Therefore, its RF
emissions are very low and are not likely
to cause any interference in nearby
electronic equipment.
Table 1 – Guidance and MANUFACTURER’S declaration – ELECTROMAGNETIC
EMISSIONS – for all ME EQUIPMENT and ME SYSTEMS
EMC Guidance
Page 11
18
Appendix
19
Appendix
Table 2 – Guidance and MANUFACTURER’S declaration – electromagnetic IMMUNITY –
for all ME EQUIPMENT and ME SYSTEMS
The device is intended for use in the electromagnetic environment specified
below.
The customer or the user of the device should assure that it is used in
such an
environment.
Guidance and manufacture’s declaration – electromagnetic immunity
Immunity test
±6 kV contact
±8 kV air
±6 kV contact
±8 kV air
±2 kV for
power supply lines
Not applicable
Not applicable
Not applicable
±1 kV line(s)
to line(s)
<5% U
T
(>95% dip in UT)
for 0.5 cycle
40% U
T
(60% dip in UT)
for 5 cycles
70% U
T
(30% dip in UT)
for 25 cycles
<5% U
T
(>95% dip in UT)
for 5 sec
3A/m
NOTE UT is the a.c. mains voltage prior to application of the test level.
IEC 60601
test level
Compliance
level
Electrostatic
discharge (ESD)
IEC 61000-4-2
Electromagnetic
environment - guidance
Floors should be wood,
concrete or ceramic tile. If
floor are covered with
synthetic material, the
relative humidity should be
at least 30%.
Power frequency magnetic
fields should be at levels
characteristic of a typical
location in a typical
commercial or hospital
environment.
3A/m
Power
frequency
(50Hz) magneti
c
field IEC
61000-4-8
Voltage dips,
short interruptions and voltage
variations on
power supply
input lines IEC
61000-4-11
Electrical fast
transient/burst
IEC 61000-4-4
Surge IEC
61000-4-5
Table 4 Guidance and manufacture’s declaration – electromagnetic immunity –
for ME EQUIPMENT and ME SYSTEMS that are not LIFE-SUPPORTING
Guidance and manufacture’s declaration – electromagnetic immunity
Immunity test
3 Vrms
3 V/m
The device is intended for use in the electromag netic environment specifi ed below. The
customer or the user of the device should as sure that i t is used in such an en vironment.
Compliance
level
IEC 60601
test level
Conducted RF
IEC 61000-4-6
Radiated RF
IEC 61000-4-3
3 V/m 80 MHz
to 2.5 GHz
3 V
rms
150 kHz to
80 MHz
Electromagnetic environment -
guidance
Portable and mobile RF communications
equipment should be used no closer to
any part of the device including
cables, than the recommended
separation distance calculated from the
equation applicable
to the frequency of
the transmitter.
Recommended separation distance
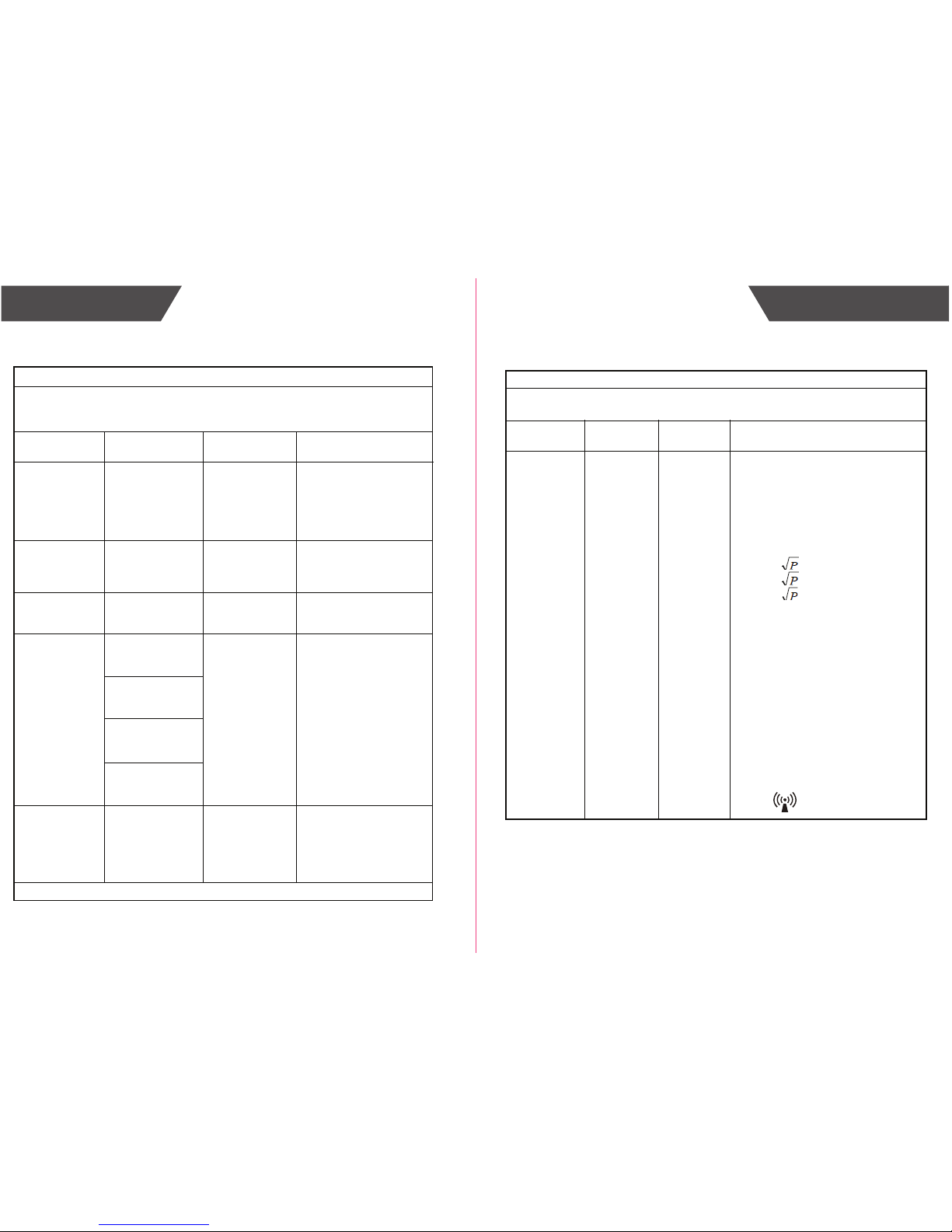
d = 1.167
d = 1.167
80 MHz to 800 MHz
d
= 2.333
800 MHz to 2.5 GHz
where P is the maximum output power
rating of the transmitter in watts (W)
according to the transmitter manufacturer and d is the recommended
separation distance in metres (m).
Field strengths from fixed RF transmitters, as determined by an
electromag-
netic site su
rvey,
a
should be less than
the compliance level in each frequency
range.
Interference may occur in the vicinity of
equipment marked with the following
symbol:
Page 12

20
Appendix
21
NOTE 1 At 80 MHz and 800 MHz, the higher frequency range applies.
NOTE 2 These guidelines may not apply in all situations. Electromagnetic
propagation is affected by absorption and reflection from structures, objects and
people.
a Field strengths from fixed transmitters, such as base stations for radio
(cellular/cordless) telephones and land mobile radios, amateur radio, AM and FM
radio broadcast
and TV broadcast cannot be predicted theoreticall
y with accuracy.
To assess the electromagnetic environment due to fixed RF transmitters, an
electromagnetic site survey should be considered. If the measured field strength in
the location in which the device is used exceeds the applicable RF
compliance level above, the device should be observed to verify normal
operation. If abnormal performance is observed, add
itional measures may be
necessary, such as reorienting or relocating the device.
b Over the frequency range 150 kHz to 80 MHz, field strengths should be less than 3 V/m.
0.01 N/A
N/A
N/A
N/A
N/A
0.117
0.369
1.167
3.690
11.67
0.233
0.738
2.333
7.377
23.33
0.1
1
10
150 kHz to 80 MHz
d = 1.167
80 MHz to 800 MHz
d = 1.167
800 MHz to 2.5 GHz
d = 2.333
Table 6 – Recommended separation distances between portable and mobile RF
communications equipment and the ME EQUIPMENT or ME SYSTEM –
for ME EQUIPMENT and ME SYSTEMS that are not LIFE-SUPPORTING
Recommended separation distances between
portable and mobile RF communications equipment at the LS203-B
The device is intended for use in an electromagnetic environment in which radiated
RF disturbances are controlled. The customer or the user of the device can help
prevent electromagnetic interference by maintaining a minimum distance between
portable and mobile RF communications equipment (transmitters) and the device as
recommended below, according to the maximum output power of the communications
equipment.
Rated maximum
output power of
transmitter
(W)
Separation distance according to frequency of transmitter
(m)
100
For transmitters rated at a maximum output power not l
isted above, the recommended
separation distance d in meters (m) can be estimated using the equation applicable to
the frequency of the transmitter, where P is the maximum output power rating of the
transmitter in watts (W) according to the transmitter manufacturer.
NOTE 1 At 80 MHz and 800 MHz, the separation distance for the higher frequency
range applies.
NOTE 2 These guidelines may not apply in
all situations. Electromagnetic propagation
is affected by absorption and reflection from structures, objects and people.
Appendix
 Loading...
Loading...