Transcend EZEX, Sleep Apnea Therapy System User Manual

EZEX
TM
User Manual
Page ii Transcend EZEX Sleep Apnea Therapy User Manual
Somnetics In ter national, Inc.
Notices
Revised Transcend EZEX User Manual 103435 Rev B
Published January 30, 2013 and supersedes all previous version s .
Notice The information c ontained in this docu ment is subject to cha nge without notice.
®
Trademark Somnetics
International, Inc.; all rights reserved.
Copyright © Copyright 2013 Somnetics International, Inc.; all rights reserved.
Contact Somnetics International, Inc.
and Transcend® are registered trademarks of Somnetics
Corporate headquarters Somnetics In ter national, Inc.
Email info@somnetics.com
Web http://www.mytranscend.com/
Telephone 651.621.1800
Toll-free telephone 877.621.9626
Fax 651.204.0064
33 5th Avenue NW, Suite 500
New Brighton, Minnesota 55112 US A
Authorized Representative (MDD 93/42/EEC)
Methodize Europe
Rx Only
Drieblad 11
NL-2811EG Reeuwijk
The Netherlands
Telephone +31 6 2129 4150l
Page iii Transcend EZEX Sleep Apnea Therapy User Manual
About this user manual
Note This user manual is designed to be duplex-printed (printed on both sides of each
sheet). If not printed in this manner, you will find blank pages in your printout.
Note For purposes of this manual, some software screen images may differ from the
actual screen display. This is only for clear printing and on-screen display of this
manual.

Page iv Transcend EZEX Sleep Apnea Therapy User Manual
Contents
Introduction ................................................................................................. 7
Indications for use .............................................................................................. 7
Contraindications ................................................................................................ 7
Precautions for use .............................................................................................. 7
Warnings ....................................................................................................... 8
Cautions ........................................................................................................ 9
Symbols .......................................................................................................... 10
Components of the Transcend EZEXTM System ................................................ 11
What’s not included (all sold separately) ......................................................... 12
Description of Transcend EZEXTM System components ..................................... 13
Transcend EZEX™ Device ................................................................................... 13
Control panel ............................................................................................... 13
Power connection jack and USB port ............................................................... 14
Air Inlet Filter .............................................................................................. 15
Assembling the Transcend EZEXTM System ..................................................... 16
Assembling the Transcend EZEX
TM
System ........................................................... 16
Powering the Transcend EZEXTM ................................................................... 17
Using the Multi-plug Universal Power Supply ........................................................ 18
Using the Transcend P
Using the Transcend P
Using the Transcend P
or P8 Battery ................................................................... 19
4
OvernightTM or P8 Multi-nightTM Battery with AC line power 19
4
OvernightTM or P8 Multi-nightTM Battery with the Transcend
4
Mobile Power Adaptor ............................................................................... 20
Explanation of the optional Transcend P
OvernightTM and P8 Multi-nightTM Battery
4
LED lights ............................................................................................... 21
Charging the Transcend P
Using the optional chest or arm strap with a Transcend P
TM
night
Battery ........................................................................................ 22
OvernightTM or P8 Multi-nightTM Battery ...................... 21
4
OvernightTM or P8 Multi-
4
Using the Transcend Mobile Power Adaptor (MPA1) ............................................... 23
Using the Transcend EZEXTM ........................................................................ 24
Standard user modes ........................................................................................ 24
Starting therapy ............................................................................................... 25
Using the ramp function ..................................................................................... 25
Using the EZEX function ..................................................................................... 26
Ending therapy ................................................................................................. 26
Drying mode .................................................................................................... 26
Replacing the Filter Media .................................................................................. 27
Caring for your Transcend EZEXTM and components ........................................ 28
Cleaning the exterior ......................................................................................... 28
Cleaning of accessories……………………………………………………………………………………….. ......... 29
Cleaning the Filter Media and filter frame ............................................................. 30
Cleaning for Multiple Users ................................................................................. 32

Page v Transcend EZEX Sleep Apnea Therapy User Manual
Fault, alert and reminder codes .................................................................... 33
Fault codes....................................................................................................... 33
Alert codes ....................................................................................................... 34
Troubleshooting .......................................................................................... 35
Appendix: Part numbers .............................................................................. 35
Disposable parts ............................................................................................... 36
Accessories ...................................................................................................... 36
Replacement parts ............................................................................................ 36
Appendix: Specifications .............................................................................. 38
Transcend EZEX
AC power supply PSA2 ...................................................................................... 38
Mobile power adaptor (optional) — MPA1 ............................................................. 38
Batteries (optional) — P
Transcend EZEX
Manufacturer’s declaration ................................................................................. 40
Electromagnetic emissions ............................................................................. 40
Electromagnetic immunity ............................................................................. 40
IEC 60601-1 compliance................................................................................ 42
TM
............................................................................................. 38
Overnight™ and P8 Multi-Night Battery ............................. 38
TM
performance .......................................................................... 39
4
Appendix: Limited warranty ......................................................................... 44


Introduction
The Transcend EZEX provides positive airway pressure to users in the range of 4 to 20
H2O as prescribed by the clinician. Buttons and LED lights facilitate control and provide
cm
operational feedback. A DC power jack and a USB port are also incorporated into the
Transcend EZEX.
Indications for use
The Transcend EZEX provides positive airway pressure for treatment of obstructive sleep
apnea (OSA) in adults weighing over 66 pounds (30 kg). The device is intended for home
and hospital/institutional use.
Contraindications
The Transcend EZEX is contraindicated in patients with the following conditions:
• Bullous lung disease
• Pathologically low blood pressure
• Pneumothorax or pneumomediastinum.
• Pneumocephalus has been reported in some users using nasal PAP.
Caution should be used when prescribing PAP for susceptible users such as those with any
of these conditi on s :
• Cerebral spinal fluid (CSF) leaks
• Abnormalities of the cribriform plate
• A prior history of head trauma
• Pneumocephalus
Precautions for use
This section describes the warnings and cautions associated with use of the Transcend
EZEX System. The following guidelines apply to this document:
Warning Indicates the possibility of serious injury or death to yourself or others.
Caution Indicates the possibility of minor injury or damage to the equipment.

Page 8 Transcend EZEX Sleep Apnea Therapy User Manual
Note Indicates a tip, explanation or feature to aid in understanding, or efficient operation
of the device.
Warnings
• Do not allow water to enter this device. Transcend EZEX should not be exposed t o
environmental conditions where the system may get wet.
• This device is not intended for life support.
• The Transcend EZEX System must be set up and adjusted by a trained provider
before being used for therapy ramp and pressure.
• The air temperature produced by this device can be as much as 10ºF higher than
the temperature of the room. Exercise caution if the room temperature is warmer
than 90ºF (32ºC).
• Do not block or otherwise obstruct the exhalation ports of the interface. Follow the
manufacturer’s instructions included with your interface.
• This equipment is not suitable for use with oxygen or in the presence of a
flammable anesthetic mixture with air or oxygen, or with nitrous oxide.
• The Transcend EZEX system is only to be used with the supplied or recommended
accessories. Use of accessories not recommended may result in increased
electromagnetic emissions or decreased electromagnetic immunity of the PAP
system and may be potentially unsafe.
• The Transcend EZEX system is not defibrillation proof.
• Do not attempt to sterilize Transcend EZEX.
• If the device is to be used by multiple patients a main flow bacteria filter should be
installed in-line between the device and the breathing circuit tubing to prevent
contamination.
• The device should be used only with masks and connectors recommended by
Somnetics or a health care professional. A mask should not be used unless the
device is turned on and is properly delivering ramp or therapy pressure. The
exhalation port(s) associated with the mask should never be blocked. Explanation
of the Warning: The device is intended t o be used w ith masks or connectors
specifically designed to have exhalation ports to allow continuous flow of air out of
the mask. When the device is turned on and functioning properly air flow from the
device flushes the exhaled air out through the mask exhalation port. When the
device is not operating, however, fresh air will not be provided through the mask
and exhaled air may be rebreathed. Rebreathing of exhaled air for longer than
several minutes can, in some circumstances, lead to suffocation.

Page 9 Transcend EZEX Sleep Apnea Therapy User Manual
Cautions
• Federal law (United States) restricts this device to sale by, or on the order of, a
physician.
• Power the Transcend EZEX System only with the Somnetics-supplied power
supplies, mobile power adaptor, or batteries. See Appendix: Part Numbers.
• Discontinue use of the Transcend EZEX and contact your physician if respiratory or
skin irritations occur.
• Do not introduce objects into the Transcend EZEX air inlet or air outlet.
• Inspect the power cord for signs of wear or damage before each use. Replace the
power cord if necessary.
• Somnetics recommends replacing the air delivery tubi ng (hose) after every three
months of use.
• To protect the environment, some parts and accessories of the Transcend EZEX
System, including optional batteries, must be disposed of in accordance with local
regulations.
Page 10 Transcend EZEX Sleep Apnea Therapy User Manual
the order of a physician or properly licensed practitioner.
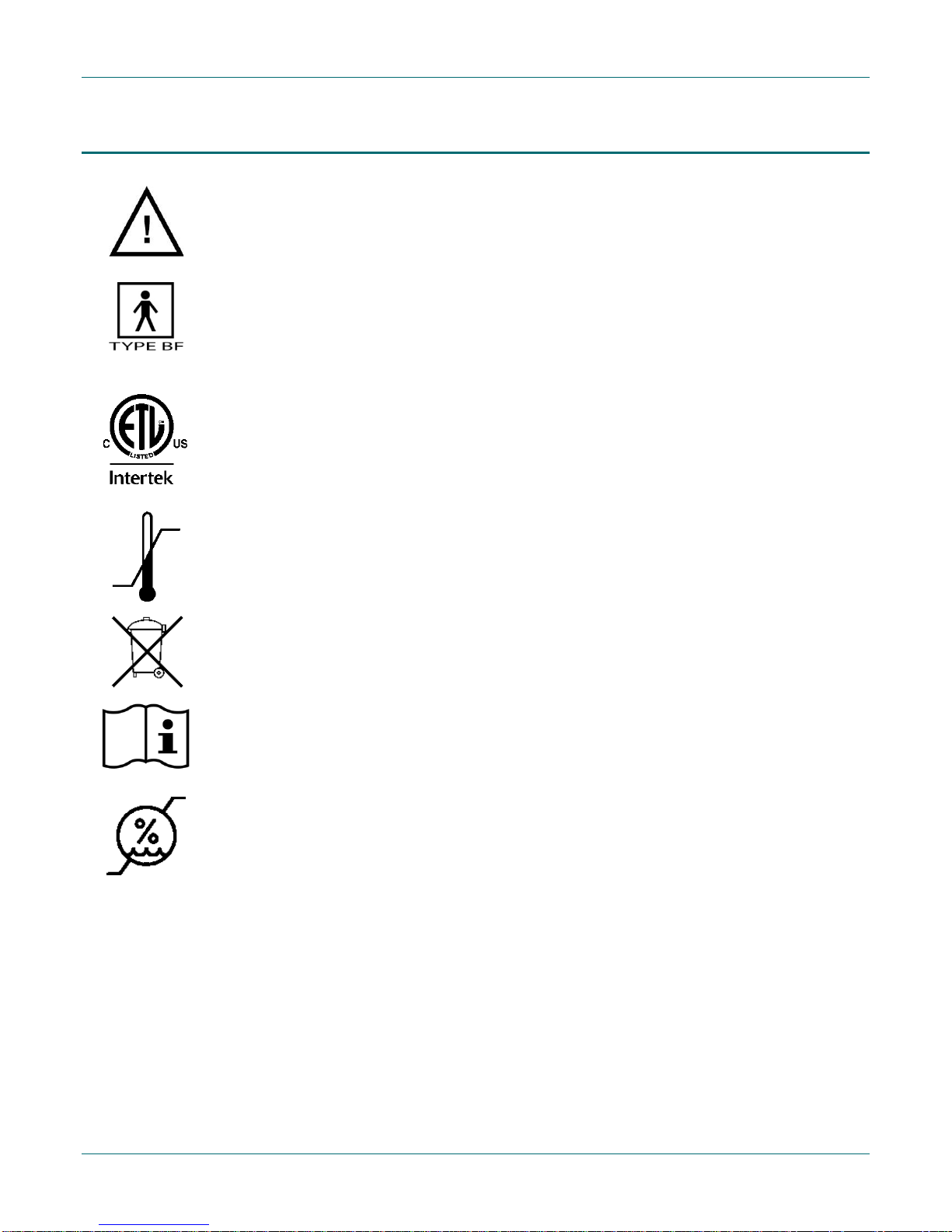
Symbols
Attention: Consult accompanying documents
Type BF Applied Part
ETL Classified
ETL Seal of Approval demonstrating qu ality, safety and professional manu facturing of
medical product
Upper and lower temperatu r e limits
Separate collection for ele c tric a l and electronic equipment per E C Directive 2002/96/EC. –
Waste Electrical and Electronic Eq uipment (WEEE)
Consult instru ctions for use
Upper and lower humidity limits
NonCondensing
Rx Only
Prescription on ly. U.S. federal law restricts this device to sale by or on

Page 11 Transcend EZEX Sleep Apnea Therapy User Manual
Components of the Transcend EZEX System
Begin by unpacking all items from the Transcend EZEX travel bag and inspect them to
ensure they were not damaged during shipment. Report any missing or damaged items to
the home healthcare provider that provided the Transcend EZEX System to you.
• Transcend EZEX
• Standard 6-foot hose (Note: not
compatible with optional H
Waterless Humidification Systems)
B or H9M
6
• Universal hose adaptor
• Multi-plug universal power supply set
(PSA2)
• Travel bag
• CD containing software
• CD containing user manuals
• Quick start guide
• USB Cable

Page 12 Transcend EZEX Sleep Apnea Therapy User Manual
What’s not included (all so ld separately)
• H
• H
• Nasal seal (mask cushion for use with H
B Waterless Humidification System
6
M Waterless Humidification System
9
Systems)
B or H9M Waterless Humidification
6
• Transcend Mobile Power Adaptor
• Transcend P
• Transcend P
Overnight Battery
4
Multi-night Battery
8
• Battery pouch and chest or arm strap system
• Transcend Base Station
• Transcend Portable Solar Charger (to be used as an alternative charging source for
Transcend P
and P8 batteries)
4
Page 13 Transcend EZEX Sleep Apnea Therapy User Manual
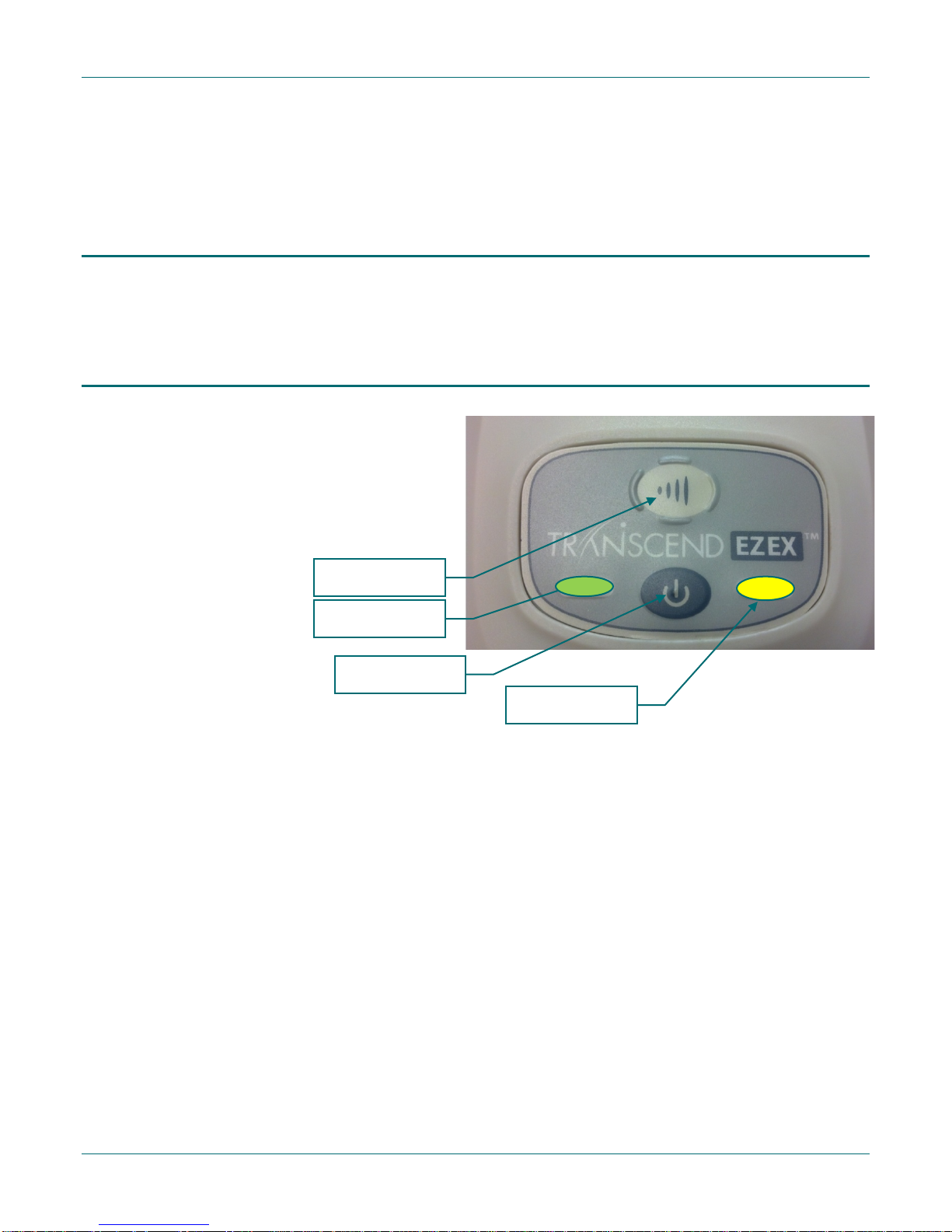
The Transcend EZEX control panel has
Ramp button
Green LED
Power button
Yellow LED
Description of Transcend EZEX System
components
Transcend EZEX Device
The Transcend EZEX comes ready to generate and regulate continuous positive airway
pressure therapy for delivery to the interface. An external power source connects to the
Transcend EZEX to supply power to the device.
Control panel
two pushbuttons used to activate the
blower and the pressure ramp feature.
There are also two LED lights, including a
green LED for indicating normal
operational modes and a yellow LED that
indicates fault conditions.
 Loading...
Loading...