Page 1
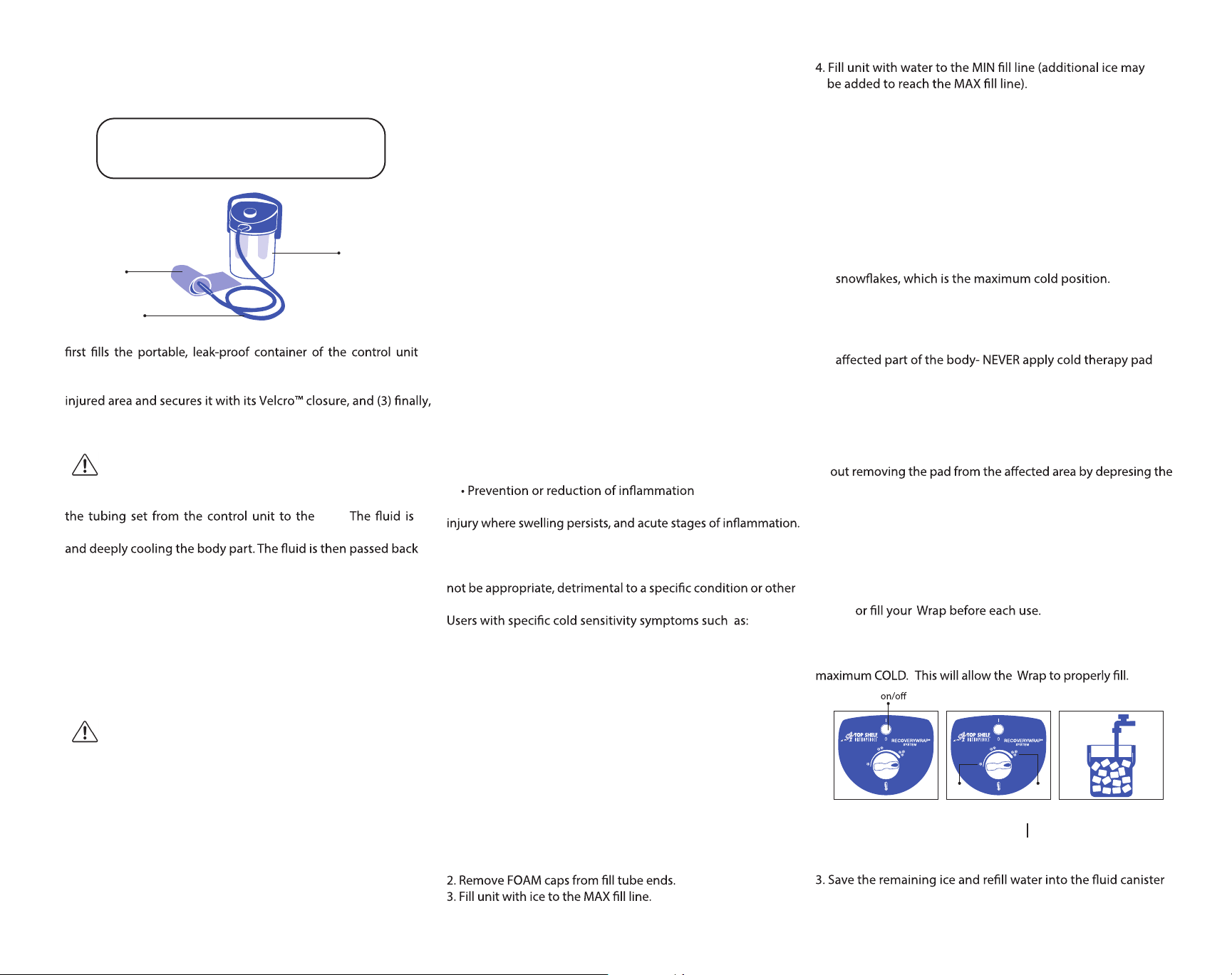
RecoveryWrap System
Instructions for Use
Please read these instructions completely and
carefully before applying this product. Correct
application is important for proper function.
Control Unit
Pad
Tubing Set
Setting up the system is a simple three-step process. (1) The user
with ice and water, (2) then applies the RecoveryWrap Pad with
an insulation barrier between the pad and skin, around the
attaches the control unit to the pad via the tubing set.
Warning
NNO part of the pad should directly touch the skin.
Once the control unit is turned on, cold water is passed through
pad.
then circulated through the pad, drawing heat from the body
through the tubing set to the control unit where it is re-cooled
and then re-circulated back to the pad.
When using this system, please check the skin where the
pad is applied periodically. Discontinue use if continued
numbness, skin discoloration, blisters, etc. are present. Please
refer to your health care professional prior to applying this
system and/or using the system for extended periods of time.
Warning
NO part of the pad should directly touch the skin.
Patient Discharge Instructions
1. Assess Patient: Screen the patient for any contraindications
and/or associated risk factors. If the patient has any contrain dications, do NOT dispense the RecoveryWrap System for treat ment. Consult a healthcare practitioner regarding the use of the
RecoveryWrap System.
2. Quick Guide: Refer to the quick guide for setup and directions
to apply the pad correctly. Instruct the patient on how to
properly use the RecoveryWrap System.
3. Protocol: Instruct the patient on the prescribed healthcare practi
tioner protocol (frequency and duration of use). Instruct patient
on periodic assesment of the skin area and the treatment period.
4. Potential for Injury: Instruct patient that improper use can
result i
n serious inury. Emphasize proper use, pad placement,
skin inspection, and following the prescribed protocol.
5. Pad Application: DO NOT apply the RecoveryWrap Pad direct ly to the skin. There should be a wound dressing or insulating
material between the skin and the pad. NO part of the pad
should directly touch the skin.
6. Skin Inspection: Instruct the patient to visually inspect the skin
per the healthcare practitioners instructions, typically every 1
to 2 hours.
7. Discontinue use of the RecoveryWrap System if you experience
any adverse reactions such as: burning, increased pain, increas ed swelling, increa
sed redness, discoloration, itching, welts, blis ters or any other changes to the skin appearance or condition.
Contact your healthcare practitioner immediately.
Indications and Contraindications
General Indications for Cold Therapy
There are general indications for using cold therapy across the
body and in various situations:
• First aid after trauma
• Relief of pain
• Prevention or reduction of swelling of traumatic origin
Cold is preferred during treatment of acute injury, sub-acute
Contraindications for Cold Therapy
Users should be aware of situations where cold therapy may
-
wise contraindicated for use.
• Cold Uticaria
• Cryoglobulinemia
• Raynaud’s Syndrome
• Proximal cold hemoglobinuria
• Vasospastic disease
• Cold hypersensitivity
• Peripheral neuropathy (as in some cases in diabetes)
• Compromised local circulation
Users should take caution in applying cold therapy over open
sores and abrasions. At a minimum these areas should be cleaned
and bandaged.
Directions For Use:
1. Unlock and remove lid by rotating handle away from the
vertical position.
5. Replace lid onto the unit- make sure to line up the cutout
with the notch on the lid.
6. Lock lid onto the unit by returning the handle to the
vertical position.
7. Connect BLUE connector hose to the unit by aligning the
white dots and pressing into the slot.
8. Connect BLUE connector hose (opposite end) to the cold
therapy pad by aligning the white dots and pressing together.
9. Attach the power cord to the unit.
10. Turn unit on with the green button on the top of the lid.
11. Adjust temp control knob to the image showing 3
12. Allow unit to PRIME for 3 minutes- additional ICE/WATER
may need to be added after this process – Turn unit OFF
before removing the lid to add ICE/WATER to the unit.
13. Over a dressing or moisture barrier apply the pad to the
directly to the skin.
14. Adjust the temp knob based on physician recommendation.
Patient Use Tips
1. Use cubed ice for best performance.
2. You may disconnect the RecoveryWrap pad from the unit
blue tabs on the hose coupling and gently pulling the hose
from the connector. The pad will seal itself and will not leak.
3. Do NOT run the unit without water in the container. The
pump is designed to run WITH water. Unplug the unit
before removing the lid from the water container.
Priming Information
When using your RecoveryWrap System it is important to
PRIME
This can be done by operating the RecoveryWrap System
with the new pad attached for at least 3 min set to
switch
ON
OFF
70˚F
1 2 3
ON
ON
OFF
OFF
40˚F
1. Press the Power Switch to the ON “ ” position.
2. Prime the unit: Adjust the Temperature Control Knob to a
comfortable setting.
to continue.
Page 2

The Caution or Warning symbol precedes an operational
step that could damage the instrument if the user does not take
certain precautions. Cautions or Warnings are located in the
main text, are preceded by a Caution or Warning statement and
are accompanied by this symbol in the left margin.
Caution: Risk of electric shock.
“OFF” (only for a part of EQUIPMENT)
“ON” (only for a part of EQUIPMENT)
Safety Symbols And Warnings
DANGER –To reduce the risk of elect
ric shock:
1. Always unplug this appliance from the electrical outlet
immediately after using and before cleaning.
2. DO NOT use while bathing or in a shower.
3. DO NOT place or store appliance where it can fall or
be pulled into a tub or sink. DO NO
T place in or drop
into water or other liquid.
4. DO NOT reach for a products that has fallen into water.
Unplug immediately.
Periodically check skin where pad is applied for skin
discoloration, numbness, blisters, etc., which can indicate a potential burn. If detected, discontinue use and consult a physician.
An appliance should never be left unattended when
plugged in. Unplug from outlet when not in use, and before
Close supervision is necessary when this appliance is used
by, on, or near children, invalids, or disabled persons. This appliance
should not be used by or on children without parental supervision.
Use this appliance only for its intended use as described
in this manual. DO NOT use attachments not recommended by
the manufacturer.
Never operate this appliance if it has a damaged cord
or plug, if it is not working properly, if it has been dropped or
damaged, or dropped into water.
Warnings
1. Ensure the top of the appliance is properly installed and the
handle is fully engaged prior to carrying the appliance.
2. DO NOT carry this appliance by supply cord or use cord
as a handle.
3. Keep the cord away from heated surfaces.
4. Never operate the appliance without the Fluid Canister, the Top
Assembly, the Tubing Set, and the pad completely connected.
5. Never operate the appliance with any foreign objects
in the Fluid Canister.
6. Never drop or insert any object into any opening.
remove plug from outlet.
8. Use cooling surfaces carefully. Do not use over insensitive skin
areas or in the presence of poor circulation on an incapacitated
person may be dangerous. Consult your physician.
NO part of the pad should directly touch the skin.
Leaks
NOTE: Some condensation(wetness) on the lines and pads is
unavoidable, especially in warmer climates.
1. If during the use of a pad you experience a leak (other than
condensation) disconnect the pad coupling from the
hose by depressing the blue tabs. Reconnect the pad to the hose
2. If the coupling continues to leak or the leak is demonstrated
in the pad itself, STOP using the unit. Contact customer service.
Cleaning the RecoveryWrap Pad:
The RecoveryWrap pad can be washed in cold water using
gently cycle in a conventional laundry machine.
*Top Shelf Orthopedics recommends a maximum of three
machine washes per Recovery wrap.
Cleaning the Control Unit
To clean your RecoveryWrap System, please follow these simple
steps:
1. Remove electrical cord, fold down handle, and remove
top assembly.
fresh, room temperature water.
3. Replace top assembly, electrical cord, and raise handle to lock.
4. Turn system ON for 10 minutes with setting on maximum COLD.
5. Repeat these actions periodically.
Warranty
Top Shelf Orthopedics guarantees the RecoveryWrap System
free from defects in material and workmanship for a period of
6 months from the date of purchase except as noted below.
This warranty extends only to the original retail purchaser.
This Top Shelf Orthopedics product warranty does not cover
damage caused by misuse or abuse; accident; the attachment
of any unauthorized accessory; alteration to the product; or any
other conditions whatsoever that are beyond the control of
Top Shelf Orthopedics.
Note: For single patient use only.
RecoveryWrap
Physical
Size (approximately) 8”di ame ter x 11.8”H (20.3cm x 30.0cm)
Weight (dry) 5 l bs . (2.27 kg)
Fluid Tether 8 . (2.4m)
Control System
Type Man ual us er control
Thermal System
Range 40°F to 70°F (4.4°C to 21.1°C)
Operating Period 2-6 ho urs typ i ca l con nuou s us e
Circulating System
Reservoir Capacity 1.1 ga ll on s (4.1 l ite rs)
Reservoir Fluid Ice water
Flow Rate (through Vital Wrap) 5.5 gph (21 l ph)
Maximum Pressure 18 ps i
Electrical System
Voltage 1 V C
Max Po wer 18W
Leakage Current Und er 300µA
Regulatory
tion Cla ss II Equi pme nt
Classi ca
Type of Equipment Type BF
Operating Environment
Atmospheric Pressure 525 to 795 m mHg
Humidity 30% to 70% re la ve non-con dens i ng
Temperature 10°C to 40°C
Transport & Stor age Environment
Atmospheric Pressure 179 to 760 m mHg
Humidity 10% to 95%
Temperature 0°C an d +50°C
Regulatory Approvals
EN60601-1-2(2007)
20 A , 60HZ
1851 East Paradise Road Suite A
Tracy, CA 95304
Phone: 1.866.592.0488
www.topshelforthopedics.com
02419 Rev A
 Loading...
Loading...