Tianjin Empecs Medical Device MM1000BT User Manual

Call Customer Service Toll-Free
24 hours a day, 7 days a week
1-888-885-6677
or visit our website
www.empecs.com
Medisign MM1100 BT
Blood Glucose Monitoring System
User Manual
Manufactured by:
Tianjin Empecs Medical Device Co.,Ltd.
No.35 and 37, Yingcheng Street, Hangu,
Binhai New Area, 300480 Tianjin China
Read this User Manual carefully before you start the test and do a quality
control test. If you have any questions about the control test consult with your
healthcare professional. Confirm the measuring unit on the display is correct
with ever test result.

Sign of Trouble
Important Safety Instruction
If you are experiencing any of the issues below, call customer service at
1-888-885-6677.
lThe meter doesn't power on when the new test strip is inserted into the meter.
lThe meter doesn't power on when you replace the batteries.
lThe meter doesn't show the test result after the test is completed.
lThe LCD does not display correctly or is distorted after changing the batteries.
If you encounter any issues including the ones mentioned above, call
customer service at 1-888-885-6677.
lBlood glucose meters are at high risk of contamination with blood-borne pathogens
such as Hepatitis B Virus (HBV), Hepatitis C Virus (HCV), and Human
Immunodeficiency Virus (HIV).Transmission of these viruses from user to user has been
documented due to contaminated blood glucose devices. Accordingly, cleaning
and disinfecting meters between users can prevent the transmission of these viruses
through indirect contact.
lThe meter and lancing device are for single patient use. Do not share them with
anyone including other family members. Do not use on multiple patients.
lAll parts of the kit are considered as biohazards. The system may transmit infectious
diseases, even after it has been cleaned or disinfected.
lIf the meter is being operated by a second person who is providing testing assistance
to the user, the meter and lancing device should be disinfected prior to use by the
second person.
lReferences
1. FDA Public Health Notification: Use of Fingerstick Devices on More than One Person
Poses Risk for Transmitting Blood Pathogens:Initial Communication (2010)
http://www.fda.gov/MedicalDevices/Safety/AlertsandNotices/ucm224025.htm
2. CDC Clinical Reminder: Use of Fingerstick Devices on More than One Person Poses a
Risk for Transmitting Blood-borne Pathogens (2010)
http://www.cdc.gov/injectionsafety/Fingerstick-DevicesBGM.html
I ii

Contents
nAbout Your New System
Welcome
Intended Use
Application
Test Principle
Special Features
Usage and storage
Important Information
System Components
nTesting Your Blood Glucose
Disinfection Before test
Performing a Blood Glucose Test
Flagging Test Results
Handling the Used Test Strips
Alternate Site Testing (AST)
nMeter Setup, Memory and Downloading
Setting up Your Meter
.........................................................................................................
...................................................................................................
.....................................................................................................
...................................................................................................
.............................................................................................
........................................................................................
....................................................................................
......................................................................................
..................................................................................
.................................................................
......................................................................................
.........................................................................
...........................................................................
....................................................................................
01
01
02
02
03
04
05
09
13
14
22
23
24
28
Reviewing Test Results
Downloading Test Results to a PC
nPairing Your Meter
How to Pair Your Meter
nControl Test
Performing a Control Test
Understanding Control Test Results
nMaintenance and Troubleshooting
Cleaning and Disinfecting
Replacing the Batteries
Screen Messages
Troubleshooting
nTechnical Information and Warranty
Specifications
Warranty
Disposing of your Meter
Additional Supplies Available
...........................................................................................................
.....................................................................................
...................................................................................
...............................................................................
...............................................................
.............................................................................
..................................................................................
............................................................................................
...............................................................................................
..................................................................................................
.................................................................................
........................................................................
.................................................................
28
34
37
40
44
45
50
52
56
58
60
61
63

About Your New System
Welcome
Thank you for selecting the Medisign MM1100 BT Blood Glucose Monitoring
System. This user manual is designed to provide you with information on how to
use the Medisign MM1100 BT Glucose Monitoring System. Follow the instructions
carefully that are detailed in this manual. Before using your monitoring system
for the first time, please read this manual carefully and If you have any
questions, contact Customer Service at 1-888-885-6677, anytime-24 hours a
day. You can also obtain information at www.empecs.com.
Intended Use
Medisign MM1100 BT Blood Glucose Monitoring System is intended for the
quantitative measurement of the glucose in fresh capillary whole blood drawn
from fingertip, palm, and forearm by a single patient (lay user) as an aid in the
management of diabetes. It is intended for self testing by persons at home, for
single patient use only, and should not be shared.
It is intended for use outside the body (in-vitro diagnostic use only) and not for
the diagnosis of or screening of diabetes or for neonatal use.
The alternative site testing (palm and forearm) should be done only during
steady state times (when glucose in not changing rapidly).
Application
Medisign MM1100 Blood Glucose Test Strips are for in-vitro diagnostic use and
for self testing only. The Medisign MM1100 BT Blood Glucose Meter is not a
substitute for your physician or Healthcare Professional. Use the Medisign
MM1100 BT Blood Glucose Meter in conjunction with your Healthcare Program.
Test Principle
Glucose in the blood sample reacts with glucose oxidase on the test strip and a
harmless electrical current is produced. The strength of these currents change
with the amount of glucose in the blood sample and the Medisign MM1100 BT
Blood Glucose Meter automatically interprets this reaction.
0201

Usage and Storage
Glucose measurements are reported as plasma equivalents. This system uses
plasma equivalent methodology. Results that use a plasma equivalent method
are approximately 11% higher than those obtained with whole-blood
equivalent test strips.
lBefore using your monitoring system, please place it in the environment of the
system's normal operating temperature range of 50-104 ℉ for about
twenty (20) minutes.
lDo not allow dirt or dust into the test strip port in order to avoid affecting the
monitoring system's accuracy.
Special Features
lAccurate result in 5 seconds using only 0.5㎕ blood sample.
lThe Medisign MM1100 BT Glucose Meter identifies the test strip code
automatically.
lStores up to 300 test results.
lBlood glucose measurement units are pre-set in mg/dL.
lDo not attempt to repair or alter your monitoring system.
lDo not put the monitoring system near any electromagnetic field. (e.g. TV,
microwave oven, mobile phone)
lHandle your monitoring system with care.
lKeep away from direct sunlight and humid conditions.
lPlease use a soft cotton cloth to wipe the system.
lDo not use a corrosive product (e.g. Benzene, Acetone) to clean your
monitoring system.
03 04

Important Information
lMake sure to use your test strip immediately after retrieving it from the vial,
be sure to keep the test strip vial closed tightly at all times.
lFor the most accurate results, make sure your hands are clean and dry
before removing the test strips from their vial.
lDo not touch test strip slit.
lDo not use test strips that have expired, using expired test strips may cause
inaccurate test results.
lNever reuse a test strip that has had either blood or control solution
applied. The Medisign MM1100 Test Strip is for single use only.
lThis product is not recommended for pregnant women, newborns or if you
are severely dehydrated.
lBefore the test you must make sure that the code number displayed on
the meter matches the code number on the test strip vial.
lKeep your Medisign MM1100 BT Blood Glucose Meter, lancing device,
lBe sure to check the glucose measurement unit on the meter before the
test.
- This meter is preset to mg/dL.
- mg/dL is the unit of measurement used in the US.
lMake sure you close the test strip vial is closed tightly and store at indoor
area between 39 ~ 86℉,10~90%RH. Do not freeze.
lKeep test strips away from direct sunlight and heat.
lUse this system only for blood glucose test.
Before using the Medisign MM1100 BT Blood Glucose Monitoring System,
l
please read this manual carefully.
lThe meter and test strips should be handled at the same temperature.
lFor accurate test results, keep the Medisign MM1100 BT Blood Glucose
Monitoring System operating temperature at a range between 50-104 for
℉
more than twenty (20) minutes before testing.
lancets and control solutions out of reach of children or pets.
05 06

lDo not use any anticoagulants or preservatives other than heparin when
collecting capillary blood into test tubes.
lBe sure to clean and disinfect your Medisign MM1100 BT Blood Glucose
Meter regularly.
lDo not reuse the test strips, test strips are single use only.
lYou may use this system at altitude up to 10,000 feet.
lClean and disinfect your Medisign MM1100 BT Blood Glucose Meter at
least once per week.
lThe Medisign MM1100 BT Blood Glucose Monitoring System is for testing
outside of the body (in-vitro diagnostic use).
l
Inaccurate results may occur for individuals or patient in shock.
l
Inaccurate results may occur for individuals experiencing a
hyperglycemic-hypersmolar state with or without ketosis. Critically ill
l Testing under out of the specification range may cause inaccurate results.
l Patients undergoing oxygen therapy may yield false low results.
l Lipemic samples (triglycerides) in excess of 1,500 mg/dL may produce
elevated results.
l Hematocrit is the percentage of red blood cells in the blood. HCT levels of
30-55% do not affect glucose measurements with this meter. If you do not
know your hematocrit level, consult with your healthcare professional.
l This test strip is not for use with arterial, venous, neonatal, serum or plasma
samples.
l Testing out of HCT level range of (30~55%) and recommended storage
conditions may cause inaccurate results.
lDispose of used test strips and lancets according to instructions from your
healthcare provider.
patients should not be tested with this blood glucose meter.
l
Interferences: Acetaminophen, salicylates, uric acid, ascorbic acid
(vitamin C) and other interferent substances in normal blood or normal
therapeutic concentrations do not significantly affect results, however
CAUTION: Use only Medisign MM1100 Test Strips with your Medisign MM1100
and Medisign MM1100 BT Blood Glucose Meters.
abnormally high concentrations in blood may cause inaccurate results.
07 08
The Complete Medisign MM1100 BT
Blood Glucose Monitoring System
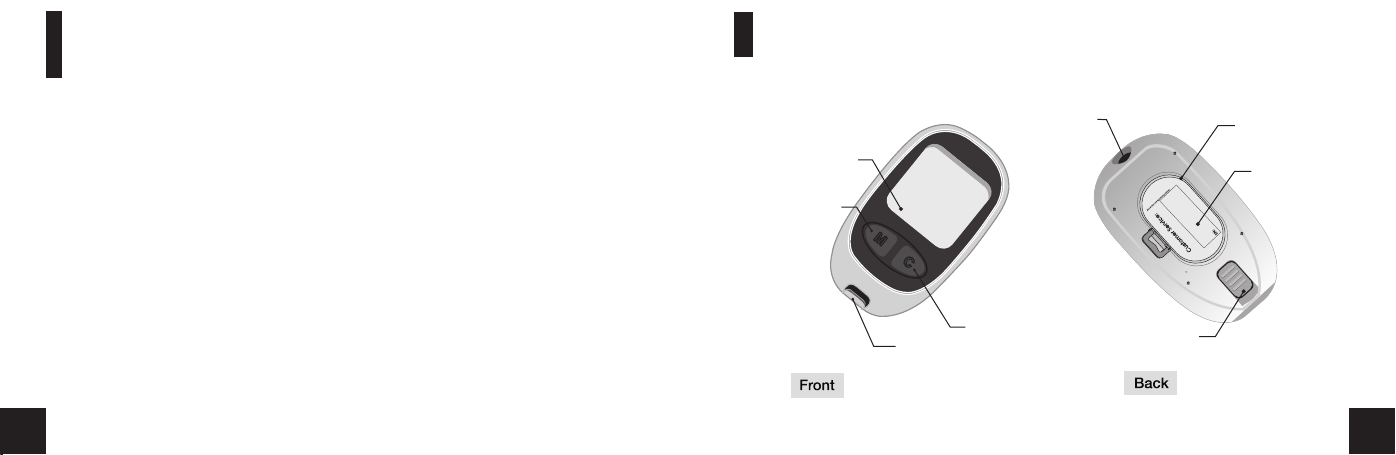
Medisign MM1100 BT Blood Glucose Meter
The Medisign MM1100 BT Blood Glucose Monitoring System includes the
Medisign MM1100 BT Meter, Ten(10) Medisign MM1100 Test Strip, Lancing
Device, Lancets, Carrying Bag, User Manual, Warranty Card, (2) 3.0 Volt CR2032
Lithium Batteries and Logbook.
Medisign MM1100 test strips and Medisign Glucose Control Solution can be
provided by contacting your supplier, or for additional information you can
contact Customer Support at 1-888-885-6677.
Display
'M' Bu tt on
'C' Bu tt on
Test Strip port
Serial port
Test Strip Ejector
Battery Lid
Label
09 10
Medisign MM1100 Blood Glucose Test Strip
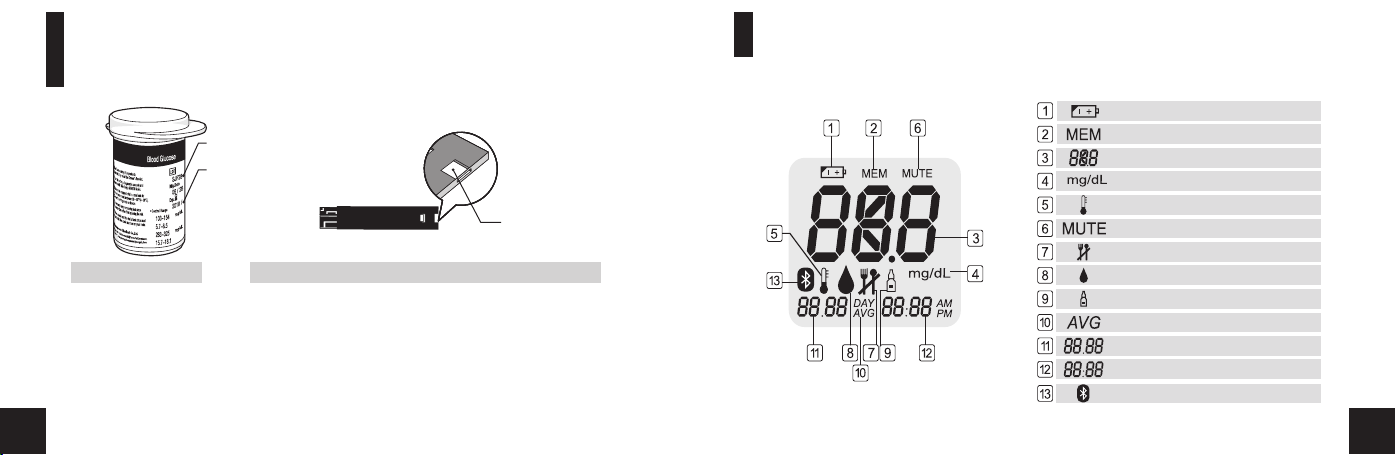
Explanation of Display
Low Battery Warning
Lot Nu mb er
Expi ra tion Date
Stored Test Results
Test Results
Unit of Measurement
A
B
Samp le
Chan ne l
Test Strip Vial Medisign MM1100 Blood Glucose Test Strip
lUse Medisign MM1100 Blood Glucose Test Strips with the Medisign MM1100
and Medisign MM1100 BT Blood Glucose Meter.
lCheck the expiration date of your test strips before using.
lWrite the opening date of the new test strip vial in order to avoid using any
expired products.
Temperature Error
No Beep
Pre meal / Post meal Mark
Ready to Test
Control Solution
Average Result
Month / Date
Time
Bluetooth (wireless RF on)
b
11 12
64 5
A01
7
8
9
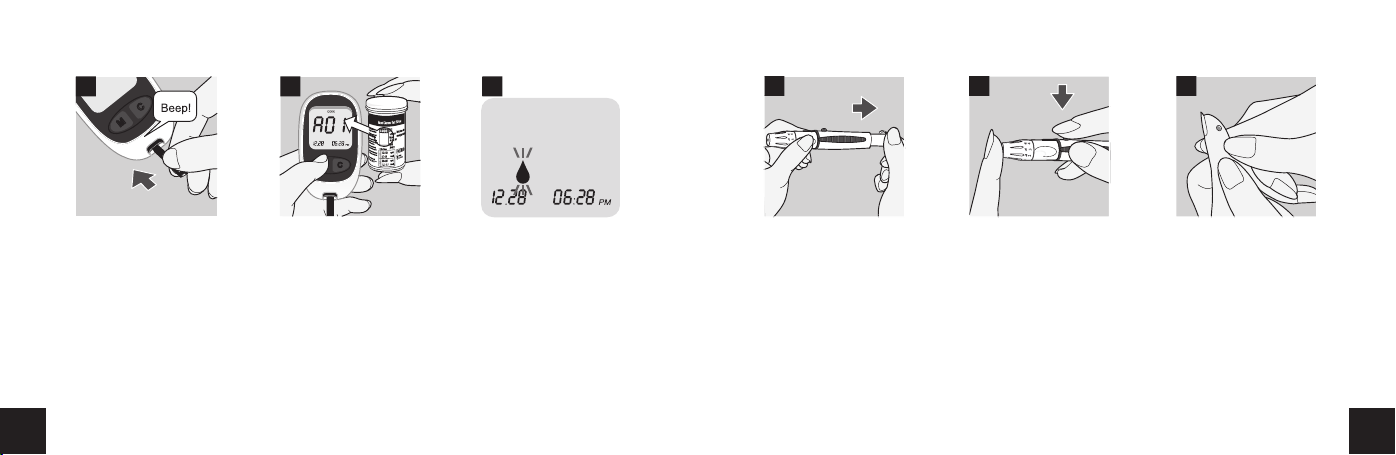
4. Insert a test strip to turn on the meter. Make sure it is inserted completely
without bending the test strip.
5. The meter identifies the code number automatically. Compare the code
number displayed on the LCD with the code number shown on the test strip
vial. If they do not match try again with an another test strip. If the problem
persists contact customer support at 1-888-885-6677.
6. When the blood symbol appears, you can proceed with your test.
7. Adjust the puncture depth setting if necessary by turning the lancing device
cap, number 1 is the shallowest depth while number 5 is the deepest. Slide
the ejection/cocking control barrel back until it clicks. If it does not click, the
lancing device may have been cocked when the lancet was inserted.
8. Hold the lancing device firmly against your finger. Press the release button.
9. Gently squeeze your finger to assist the flow of blood. Do not squeeze
excessively on the puncture site.
15 16
Testing Your Blood Glucose
Performing a Blood Glucose Test
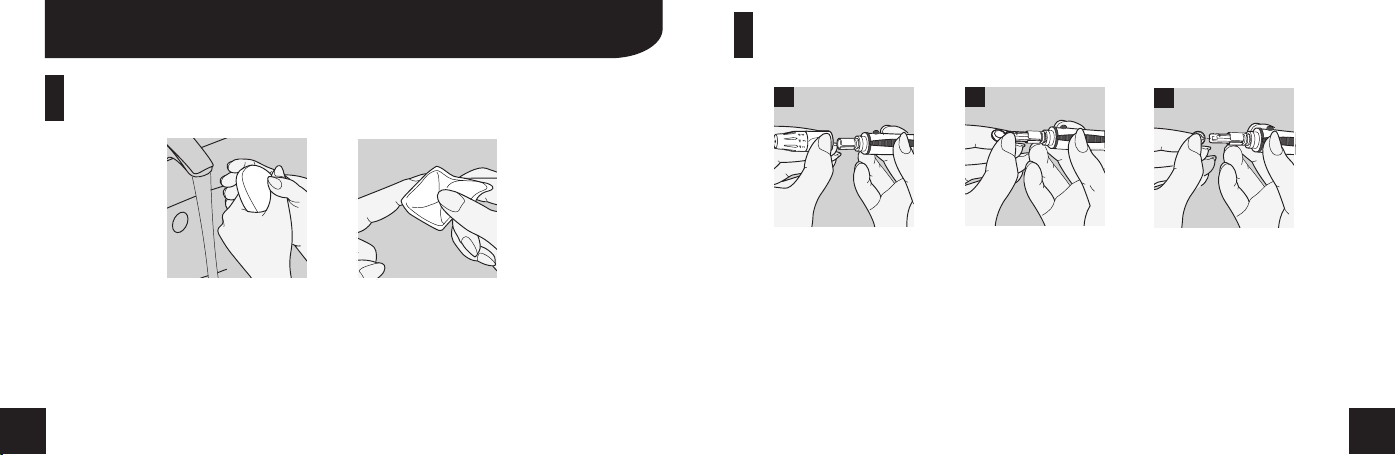
Disinfect Before Testing
lBefore testing, wash your hands with soap and water and dry thoroughly.
lUse an alcohol prep pad to wipe the area before testing.
lWait until the alcohol dries completely.
1 2 3
1. Remove your lancing device cap.
2. Install the new lancet into your lancing device.
3. Remove the protective cap from the lancet. Replace the lancing device
cap.
NOTICE: Refer to the lancing device instruction for additional detailed
information. The lancing device instruction depicted in this manual is for most
universal lancing devices.
13 14
10
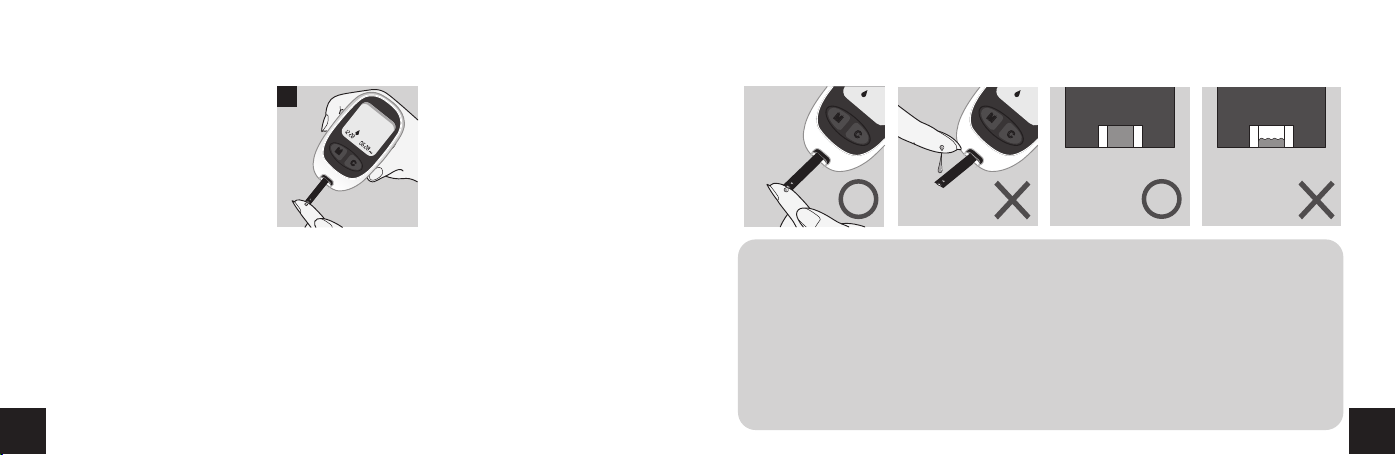
10. Hold the tip of the test strip to the drop of blood, the test strip will
automatically draw the blood into the test strip. When the test strip has
enough blood the meter will beep and count down from five seconds. After
five seconds your test result will appear.
NOTICE: When the blood sample is not sufficient, the display screen will show
the message of "Er 4"(Please refer to error messages in this manual.)
CAUTION:
lDo not drop the blood directly on the end of the test strip.
lThe sample channel at the end of the test strip should show full. When you
hear the beep sound, you have enough blood in the test strip.
lInaccurate results may occur for individuals or patient in shock.
Inaccurate results may occur for individuals experiencing a
hyperglycemic-hypersmolar state, with or without ketosis. Critically ill
patients should not be tested with a blood glucose meter.
17 18
 Loading...
Loading...