Page 1

MYOTRAC INFINITI™
User Guide
Page 2

MyoTrac Infiniti™ User Guide 2
Page 3
The Manufacturer:
Thought Technology Ltd.
2180 Belgrave Avenue
Montreal, Quebec, Canada
H4A 2L8
Product Name:
MyoTrac Infiniti System
Product #:
T9800
Device Name:
MyoTrac Infiniti Encoder
Device #:
SA9800
EC
REP
EMERGO EUROPE
Molenstraat 15
2513 BH, The Hague
The Netherlands
Tel: +31.70.345.8570
Fax: +31.70.346.7299
MyoTrac Infiniti™ User Guide 3
Page 4
Type BF Equipment
Internally powered equipment
Continuous operation
Read Instruction Manual
The pins of the connectors identified with the ESD warning symbol should not be touched
unless ESB precautionary procedures are used.
CAUTION
US Federal Law restricts this device to sale by, or on order of, a physician or any other
practitioner licensed by the law of the state in which he or she practices to use or order the
use of this device.
WARNING
Do not operate Active Sensors within 10 feet of an operating cellular phone, similar radio
transmitting device, other powerful radio interference producing sources such as arc
welders, radio thermal treatment equipment, x-ray machines, or any other equipment that
produces electrical sparks. Portable and mobile RF communication equipment can affect
this equipment.
With the MyoTrac Infiniti Encoder SA9800 use only with supplied power supply. GlobTek
Part Number WR92B2500LF9P-Y-MED (WR95/WR93/WR97) or GS889
The PC used with MyoTrac Infiniti must be placed outside the patient/client environment
(more than 3 meters or 10 feet) or the PC must comply with EN60601-1 (system safety).
After use, the Batteries or the Battery pack must be disposed of in accordance with local,
state and federal regulations and laws.
After use, the Disposable Electrodes may be a biohazard. Handle, and when applicable,
dispose of these materials in accordance with accepted medical practice and any applicable
local, state and federal laws and regulations.
Reusable electrodes present a potential risk of cross-infection especially when used on
abraded skin, unless they are restricted to a single patient or sterilized between patients. If
sterilizing electrodes, employ only gas sterilization.
Radiated radio frequency electromagnetic fields can cause performance degradation in the
MyoScan EMG sensor. In the worst case, an RF field strength of 22mV/M can cause an
increase of 1V in the signal reading from a MyoScan sensor. Be sure to keep in mind that
a very relaxed muscle should provide an EMG reading of approximately 1-3V.
This device is capable of generating current densities exceeding 2mA r.m.s./cm² this may
require special attention of the operator.
Do not exceed 0.1watts/cm² with recommended electrodes, assuming a load of 500.
1. 1’’ Round = Max 69mA
2. 2’’ x 2’’ Square = Max 100mA
3. 3’’ x 4’’ Rectangle = Max 100mA
4. St Cloud = Max 100mA
5. Femelex = Max 100mA
Avoid accidental contact between connected but unused applied parts and other conductive
parts including those connected to protective earth.
Explosion Hazard; Do not use in the presence of a flammable anesthetic mixture with air, or
with Oxygen or Nitrous Oxide.
Not to be immersed in water.
Take care in arranging patient and sensor cables to avoid risk of patient entanglement or
strangulation.
The operator is responsible for ensuring the safety of any devices controlled or triggered by
Infiniti equipment or software, or by any software or hardware receiving data from Infiniti
equipment. Infiniti equipment must not be configured or connected in such a way that
failure in its data acquisition, processing or control functions can trigger patient feedback
stimulus that poses an unacceptable level of risk.
Use of any equipment in a biofeedback or stimulation context should be immediately
MyoTrac Infiniti™ User Guide 4
Page 5

terminated upon any sign of treatment-related distress or discomfort.
Not to be connected to a patient undergoing MRI, Electro surgery or defibrillation.
Simultaneous connection of a patient to a high frequency surgical equipment may result in
burns at the site of the stimulator electrodes and possible damage to the stimulator.
Operations in close proximity (e.g. 1 m) to shortwave or microwave therapy equipment may
produce instability in the stimulation output.
The long-term effects of chronic electrical stimulation are unknown.
Stimulation should not be applied over the carotid sinus nerves, particularly in patients with
a known sensitivity to the carotid sinus reflex.
Stimulation should not be applied over the neck or mouth. Severe spasm of the laryngeal
and pharyngeal muscles may occur and the contractions may be strong enough to close the
airway or cause difficulty in breathing.
Patients with an implanted electronic device (for example a cardiac pacemaker) should not
be subjected to stimulation unless specialist medical opinion has first been obtained.
Stimulation should not be applied transthoracically in that the introduction of electrical
current into the heart may cause cardiac arrhythmias.
Stimulation should not be applied transcerebrally.
Stimulation should not be applied over swollen, infected, or inflamed areas or skin
eruptions, e.g., phlebitis, thrombophlebitis, varicose veins, etc.
Stimulation should not be applied over, or in proximity to, cancerous lesions.
Not for use with patients with undiagnosed pain conditions.
Only use the unit for which it was prescribed.
Do not immerse the unit in water or any other liquid substance.
Do not use if you have symptoms of bladder infection.
Do not use with diminished mental capacity or physical competence limiting the use of the
device.
Safety of powered muscle stimulators for use during pregnancy has not been established.
Caution should be used for patients with suspected or diagnosed heart problems.
Caution should be used for patients with suspected or diagnosed epilepsy.
Caution should be used in the presence of the following:
1. When there is a tendency to hemorrhage following acute trauma or fracture;
2. Following recent surgical procedures when muscle contraction may disrupt the
healing process;
3. Over the menstruating or pregnant uterus; and
4. Over areas of the skin which lack normal sensation.
Some patients may experience skin irritation or hypersensitivity due to the electrical
stimulation or electrical conductive medium. The irritation can usually be reduced by using
an alternate conductive medium, or alternate electrode placement.
Electrode placement and stimulation settings should be based on the guidance of the
prescribing practitioner.
Powered muscle stimulators should be kept out of the reach of children.
If damage is evident of the unit or accessories, discontinue use and contact your supplier
for further information on repair.
Powered muscle stimulators should be used only with the leads and electrodes
recommended for use by the manufacturer.
Portable powered muscle stimulators should not be used while driving, operating
machinery, or during any activity in which involuntary muscle contractions may put the user
at undue risk of injury.
The system should not be used adjacent to or stacked with other equipment, if used
adjacent or stacked the unit should be observed to verify normal operation in the
configuration in which it will be used.
Use of accessories, transducers or cables other than those specified by Thought
Technology ltd may result in increased emissions or decreased immunity of the equipment
to electromagnetic energy.
ATTENTION
Sensors and equipment damaged by static electricity are not covered under warranty. To
prevent static discharge from damaging the sensor and/or encoders, use anti-static mats or
sprays in your working area. A humidifier may also be used to prevent static environments
MyoTrac Infiniti™ User Guide 5
Page 6

by conditioning hot, dry air. It is recommended that all staff involved with the unit receive an
explanation of the ESD symbol and the precautions described above as a minimum.
Do not apply any electrode gel or equivalent directly on the sensor snaps. Always use
electrodes as a medium between the sensor and the client.
Not for diagnostic purposes, not defibrillator proof, not for critical patient monitoring.
To prevent voiding warranty by breaking connector pins, carefully align white guiding dot on
sensor plug with slot on sensor input.
Make sure to remove electrodes from sensor snaps immediately after use.
Do not plug third party sensors directly into instrument inputs. Plug only Thought
Technology Active Sensor cable connectors into instrument inputs. All electrodes and third
party sensors must be connected to active sensors, either directly or through an adapter.
Remove batteries when the device is not being used for an extended period of time. Please
dispose of battery following local regulations.
ADVERSE REACTIONS
Skin irritation and burns beneath the electrodes have been reported with the use of powered muscle
stimulators.
CONTRAINDICATIONS
Powered muscle stimulators should not be used on patients with cardiac demand
pacemakers.
INTENDED PURPOSE
Biofeedback, Relaxation & Muscle Re-Education purposes
Relaxation of muscle spasms
Prevention or retardation of disuse atrophy
Increasing local blood circulation
Stroke Rehab by Muscle re-education
Immediate post-surgical stimulation of calf muscles to prevent venous thrombosis
Maintaining or increasing range of motion
Acute and ongoing treatment of stress, urge or mixed urinary incontinence and where the
following results may improve urinary control: Inhibition of the detruser muscle through
reflexive mechanisms.
Incontinence treatment for assessing EMG activity of the pelvic floor and accessory muscles such
as the abdominal or gluteal muscles
Powered muscle stimulators should only be used under medical supervision for adjunctive
therapy for the treatment of medical diseases and conditions
NOTE
No preventative inspections required; maintenance must be performed by qualified personnel.
Factory re-calibration can be requested.
The supplier will make available, upon request, circuit diagrams, component parts lists and
description or other information required for the repair of product by qualified personnel.
The operator must be familiar with typical characteristics of signals acquired by this
equipment, and be able to detect anomalies in the acquired signal that could interfere with
treatment effectiveness. Depending on the importance of signal integrity, it may be advisable
to continuously monitor the raw signals, in time and/or frequency domain, while the device is
being used for biofeedback or other purposes. If anomalies are observed on acquired signals,
and if you suspect a problem with electromagnetic interference, contact Thought Technology
for a technical note on identification and remediation.
This product conforms to standards EN60601-1, EN60601-2-10 and EN60601-2-40; some
encoder labeling may indicate superceded standards.
MAINTENANCE AND CALIBRATION
Wipe encoder with a clean cloth
Factory testing and calibration ensure equipment accuracy and frequency response. Contact
Thought Technology for factory re-calibration if necessary.
STORAGE
Store in its original case at up to 90% humidity / 30C°
TRANSPORTATION
Transport in its original case
Manual # SA9810US Rev 5
MyoTrac Infiniti™ User Guide 6
Page 7

Guidance and manufacturer’s declaration – electromagnetic immunity
The MyoTrac Infiniti is intended for use in the electromagnetic environment specified below. The customer or the
user of the MyoTrac Infiniti should assure that it is used in such an environment, and that precautions regarding
that environment are heeded.
Immunity test
IEC 60601
test level
Compliance level
Electromagnetic environment –
guidance
Electrostatic
discharge (ESD)
IEC 61000-4-2
6 kV contact
8 kV air
6 kV contact
8 kV air
Floors should be wood, concrete or
ceramic tile. If floors are covered with
synthetic material, the relative humidity
should be at least 30 %.
Electrical fast
transient/burst
IEC 61000-4-4
2 kV for power
supply lines
1 kV for input/output
lines
2 kV for power
supply lines
1 kV for input/output
lines
Mains power quality should be that of a
typical commercial or hospital
environment.
Surge
IEC 61000-4-5
1 kV differential
mode
2 kV common mode
1 kV differential
mode
2 kV common mode
Mains power quality should be that of a
typical commercial or hospital
environment.
Voltage dips, short
interruptions and
voltage variations
on power supply
input lines
IEC 61000-4-11
<5 % UT
(>95 % dip in UT)
for 0,5 cycle
40 % UT
(60 % dip in UT)
for 5 cycles
70 % UT
(30 % dip in UT)
for 25 cycles
<5 % UT
(>95 % dip in UT)
for 5 sec
<5 % UT
(>95 % dip in UT)
for 0,5 cycle
40 % UT
(60 % dip in UT)
for 5 cycles
70 % UT
(30 % dip in UT)
for 25 cycles
<5 % UT
(>95 % dip in UT)
for 5 sec
Mains power quality should be that of a
typical commercial or hospital
environment. If the user of the
MyoTrac Infiniti requires
continued operation during power
mains interruptions, it is recommended
that the MyoTrac Infiniti be
powered from an uninterruptible power
supply or a battery.
Power frequency
(50/60 Hz)
magnetic field
IEC 61000-4-8
3 A/m
3 A/m
Power frequency magnetic fields
should be at levels characteristic of a
typical location in a typical commercial
or hospital environment.
NOTE UT is the a.c. mains voltage prior to application of the test level.
MyoTrac Infiniti™ User Guide 7
Page 8
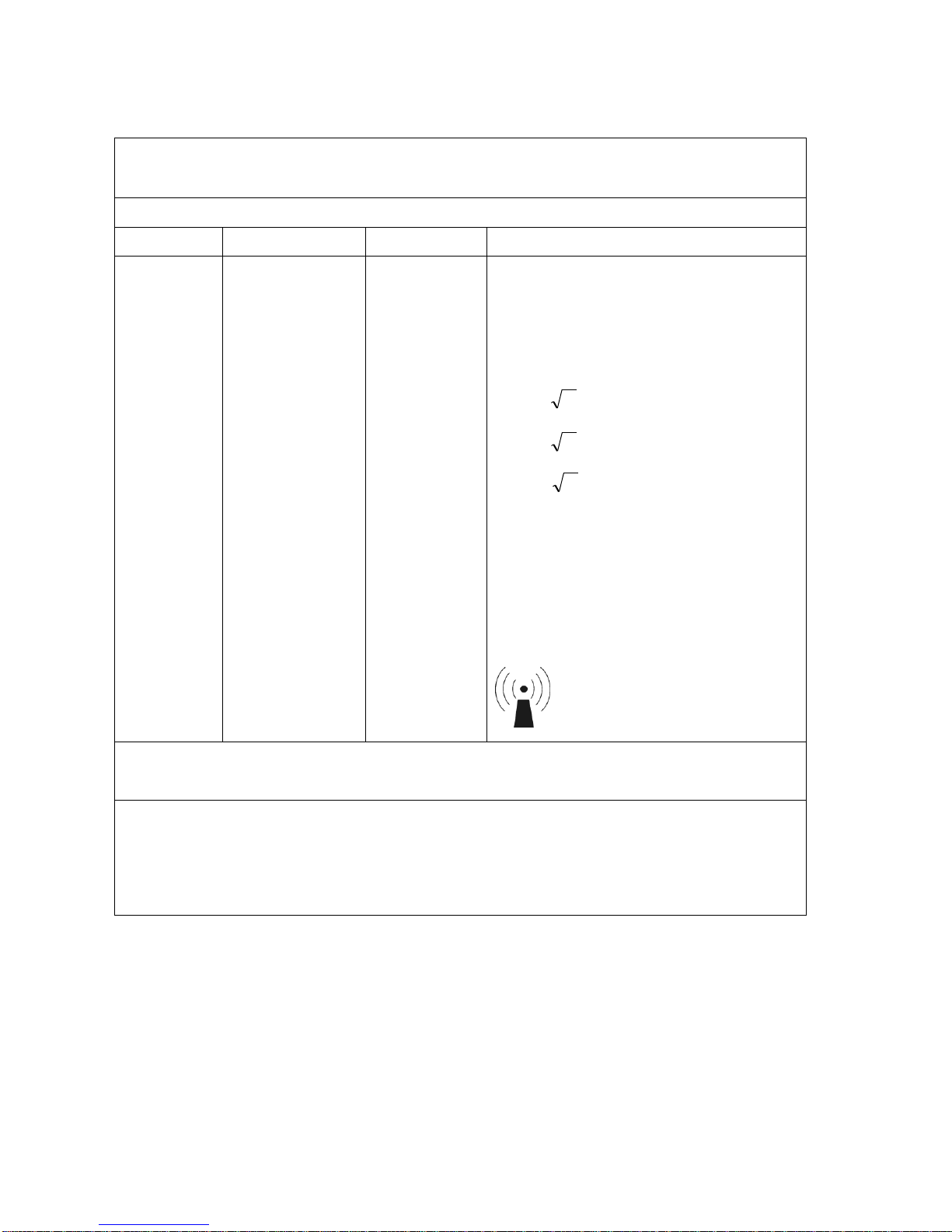
Guidance and manufacturer’s declaration – electromagnetic immunity
The MyoTrac Infiniti is intended for use in the electromagnetic environment specified below. The customer or the user of
the MyoTrac Infiniti should assure that it is used in such an environment.
Immunity test
IEC 60601 test level
Compliance
level
Electromagnetic environment – guidance
Portable and mobile RF communications equipment
should be used no closer to any part of the
MyoTrac Infiniti, including cables, than the
recommended separation distance calculated from
the equation applicable to the frequency of the
transmitter.
Recommended separation distance
Conducted RF
IEC 61000-4-6
3 Vrms
150 kHz to 80 MHz
3 Vrms
150 kHz to 80
MHz
Pd 2.1
80 MHz to 800 MHz
Radiated RF
IEC 61000-4-3
3 V/m
80 MHz to 2,5 GHz
3 V/m
80 MHz to 2,5
GHz
Pd 2.1
80 MHz to 800 MHz
Pd 3.2
800 MHz to 2.5GHz
where P is the maximum output power rating of the
transmitter in watts (W) according to the transmitter
manufacturer and d is the recommended separation
distance in metres (m).
Field strengths from fixed RF transmitters, as determined by an electromagnetic site survey,a should be
less than the compliance level in each frequency
range.
b
Interference may occur in the vicinity of equipment
marked with the following symbol:
NOTE 1 At 80 MHz and 800 MHz, the higher frequency range applies.
NOTE 2 These guidelines may not apply in all situations. Electromagnetic propagation is affected by absorption
and reflection from structures, objects and people.
a
Field strengths from fixed transmitters, such as base stations for radio (cellular/cordless) telephones and land mobile
radios, amateur radio, AM and FM radio broadcast and TV broadcast cannot be predicted theoretically with accuracy.
To assess the electromagnetic environment due to fixed RF transmitters, an electromagnetic site survey should be
considered. If the measured field strength in the location in which the MyoTrac Infiniti is used exceeds the applicable
RF compliance level above, the MyoTrac Infiniti should be observed to verify normal operation. If abnormal
performance is observed, additional measures may be necessary, such as reorienting or relocating the MyoTrac
Infiniti.
b
Over the frequency range 150 kHz to 80 MHz, field strengths should be less than [V1] V/m.
MyoTrac Infiniti™ User Guide 8
Page 9

Recommended separation distances between
portable and mobile RF communications equipment and the MyoTrac Infiniti
The MyoTrac Infiniti is intended for use in an electromagnetic environment in which radiated RF disturbances
are controlled. The customer or the user of the MyoTrac Infiniti can help prevent electromagnetic interference by
maintaining a minimum distance between portable and mobile RF communications equipment (transmitters) and the
MyoTrac Infiniti as recommended below, according to the maximum output power of the communications
equipment.
Rated maximum output
power of transmitter
W
Separation distance according to frequency of transmitter
150 kHz to 80 MHz
80 MHz to 800 MHz
800 MHz to 2,5 GHz
Pd 2.1
Pd 2.1
Pd 3.2
0,01
0.12
0.12
0.23
0,1
0.38
0.38
0.73
1
1.2
1.2
2.3
10
3.8
3.8
7.3
100
12
12
23
For transmitters rated at a maximum output power not listed above, the recommended separation distance d in
metres (m) can be estimated using the equation applicable to the frequency of the transmitter, where P is the
maximum output power rating of the transmitter in watts (W) according to the transmitter manufacturer.
NOTE 1 At 80 MHz and 800 MHz, the separation distance for the higher frequency range applies.
NOTE 2 These guidelines may not apply in all situations. Electromagnetic propagation is affected by absorption and
reflection from structures, objects and people.
Guidance and manufacturer’s declaration – electromagnetic emissions
The MyoTrac Infiniti is intended for use in the electromagnetic environment specified below. The customer or the user of
the MyoTrac Infiniti should assure that it is used in such an environment.
Emissions test
Compliance
Electromagnetic environment – guidance
RF emissions
CISPR 11
Group 1
The MyoTrac Infiniti uses RF energy only for its internal function.
Therefore, its RF emissions are very low and are not likely to
cause any interference in nearby electronic equipment.
RF emissions
CISPR 11
Class B
The MyoTrac Infiniti is suitable for use in all establishments,
including domestic establishments and those directly connected to
the public low-voltage power supply network that supplies
buildings used for domestic purposes.
Harmonic emissions
IEC 61000-3-2
Not applicable
Voltage fluctuations/
flicker emissions
IEC 61000-3-3
Not applicable
MyoTrac Infiniti™ User Guide 9
Page 10

Table of Contents
About This Guide..................................................................................................................................................................... 11
Chapter 1 .................................................................................................................................................................................. 12
Introduction to your MYOTRAC INFINITI™ Encoder ............................................................................................................ 12
System Requirements .......................................................................................................................................................... 13
MyoTrac Infiniti Components ................................................................................................................................................ 14
Connection to the Client ....................................................................................................................................................... 17
Connection to the PC ........................................................................................................................................................... 22
Screen Elements .................................................................................................................................................................. 23
Thought Support .................................................................................................................................................................. 23
Settings Menu ...................................................................................................................................................................... 24
Chapter 2 .................................................................................................................................................................................. 28
SEMG sessions on your MYOTRAC INFINITI™ Encoder ..................................................................................................... 28
Open SEMG Sessions ......................................................................................................................................................... 28
Script SEMG Sessions ......................................................................................................................................................... 30
Chapter 3 .................................................................................................................................................................................. 32
Stim sessions on your MYOTRAC INFINITI™ Encoder ................................................................................................ ........ 32
Open Stim Session .............................................................................................................................................................. 33
Script Stim Sessions ............................................................................................................................................................ 36
Chapter 4 .................................................................................................................................................................................. 37
EMG-STIM on your MYOTRAC INFINITI™ Encoder ............................................................................................................ 37
Chapter 5 .................................................................................................................................................................................. 41
Data Management on your MYOTRAC INFINITI™ Encoder ................................................................................................. 41
MyoTrac Infiniti Review ........................................................................................................................................................ 42
Chapter 6 .................................................................................................................................................................................. 43
Display Options on your MYOTRAC INFINITI™ Encoder ..................................................................................................... 43
Displays ............................................................................................................................................................................... 43
Chapter 7 .................................................................................................................................................................................. 47
Flow on your MYOTRAC INFINITI™ Encoder ................................................................ ..................................................... 47
Chapter 8 .................................................................................................................................................................................. 48
Reference ............................................................................................................................................................................ 48
Technical Support and Order Placing ................................................................................................................................... 49
Technical Support ................................................................................................................................................................ 49
Product Numbers & Accessories .......................................................................................................................................... 50
Placing Orders ..................................................................................................................................................................... 51
Specifications ....................................................................................................................................................................... 52
MyoTrac Infiniti Hardware Copyright Notice ......................................................................................................................... 56
MyoTrac Infiniti™ User Guide 10
Page 11

About This Guide
Welcome to the MYOTRAC INFINITI™ encoder. This guide is designed to help you get up and
running quickly with your new encoder. It will describe the operation of the encoder, and how it
interfaces to the host personal computer (PC).
It walks you through:
Physical Operation of the encoder.
EMG, Stimulation and EMG-STIM sessions.
Data management.
Display options.
After you have become familiar with the key concepts of your new encoder, you can use the rest of
this guide as a reference for less common tasks, and also as a source of information if you have
problems operating it.
MyoTrac Infiniti™ User Guide 11
Page 12

Chapter 1
Introduction to your MYOTRAC INFINITI™ Encoder
This chapter explains the physical interface with the MyoTrac Infiniti Encoder, how to use it for the
first time, and how to transfer data to the host PC.
Getting to know your MyoTrac Infiniti Encoder
What is a MyoTrac Infiniti Encoder?
The MyoTrac Infiniti is the cutting edge in handheld, dual channel Surface Electromyography
(SEMG) combined with Muscle Stimulation (STIM), for contraction of muscles and for urinary
incontinence. With it you will be able to deliver targeted and customized treatment directly to the
client’s clinically relevant areas. The total integration of the two modalities of SEMG and STIM in a
single device provides a third modality of treatment-SEMG-STIM, with features such as EMGTriggered Stimulation and Alternating EMG and STIM.
Note: EMG triggered STIM is not available for incontinence application.
A simple first approach has been adopted in the design of the MyoTrac Infiniti to make it as easy
and fast as possible to get the clinical results desired from this powerful device.
Customizing the MyoTrac Infiniti to your clinical needs couldn’t be easier; all users input is directed
through a series of intuitive and guided screens using touch screen technology.
The partnership of the MyoTrac Infiniti with the BioGraph Infiniti PC software enhances yet further
the power and flexibility of the MyoTrac Infiniti. This link enables you to transfer session data to the
PC for further viewing, analysis and reporting, in real time or post session.
MyoTrac Infiniti™ User Guide 12
Page 13
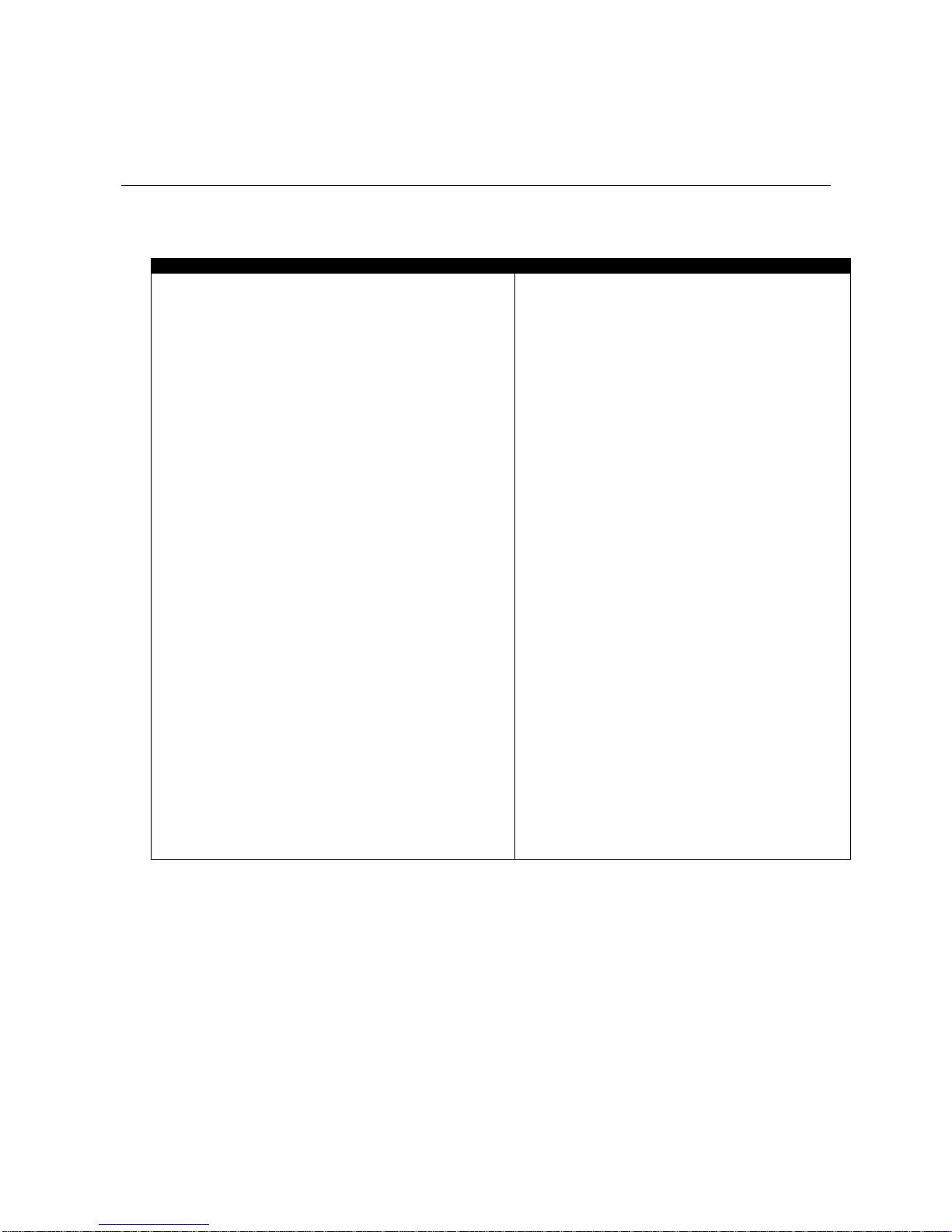
System Requirements
Recommended
Minimum
IBM PC compatible
(Intel/Pentium/Celeron family or AMD
K6/Athlon/Duron family, CPU P4 speed 3
GHz or higher), Desktop or Laptop with
two monitor capability
Windows 2000/XP Professional or Home
edition.
50 - 60 gigabytes hard disk space for
video recording and processing. (The
software needs 2.5 gigabytes to install
and run on available hard drive space)
Memory, 512 MB of RAM or more
CD ROM or DVD drive
SVGA graphic card (1024 x 768) or higher
resolution adapter & monitor
32 bit Sound Blaster compatible sound
card & speakers
1 to 4 USB ports, depending on the
desired number of MyoTrac Infiniti
encoders
Mouse or compatible pointing device
MS Word 97 or higher (for printing
purposes)
Compact Flash Reader (For use with
compact flash card only)
Webcam 30 frames per second (for video
purposes only)
NOTE: When using certain more complex
screens, you must adhere to the
Recommended Computer Requirements.
IBM PC compatible
(Intel/Pentium/Celeron family or AMD
K6/Athlon/Duron family, CPU P3
speed 1.8 GHz), Desktop or Laptop
Windows 2000/XP Professional or
Home edition.
10 - 20 gigabytes hard disk space
(The software needs 2.5 gigabytes to
install and run on available hard drive
space)
Memory, 256 MB of RAM or more
CD ROM or DVD drive
SVGA graphic card (1024 x 768) or
higher resolution adapter & monitor
16 Bit Sound Blaster compatible
sound card & speakers
1 to 4 USB ports, depending on the
desired number of MyoTrac Infiniti
encoders
Mouse or compatible pointing device
Word 97 or higher (for printing
purposes)
NOTE: For most recent computer
requirements contact Thought Technology
Ltd for MAR473
To install the BioGraph Infiniti software, your computer system must meet or exceed the following
requirements.
Update information
Periodically updates may become available for the BioGraph Infiniti software and for the MyoTrac
Infiniti Hardware. Please contact your local distributor or visit our website
www.thoughttechnology.com for further information on how to obtain updates.
MyoTrac Infiniti™ User Guide 13
Page 14

MyoTrac Infiniti Components
Compact Flash for increased memory capacity and one method for transfer of data to the PC.
USB for real time transfer of data to the PC.
Touch screen enables graphically guided navigation through the software.
Rugged Ergonomic Case, easy to hold or attach to the subject and will withstand the rigors of
daily use.
Battery Charging jack for wall connection enables fast built-in battery charging.
Headphone Jack for stereo sound feedback (or use the built-in speaker).
Push button On/Off switch to prevent accidental switching.
2 Channels of Surface EMG and stimulation through the same electrodes; saves time and
increases flexibility.
MyoTrac Infiniti™ User Guide 14
Page 15

Power
There are three basic methods to power the MyoTrac Infiniti unit: Inserting batteries into the battery
compartment of the unit, plugging it into the wall using the supplied AC adapter, or plugging it into a
powered up computer using a USB cable.
The MyoTrac Infiniti is available with battery charging capabilities. It will work with four standard
Alkaline AAA batteries available in all consumer electrical stores. It is also possible to run the unit
on removable, externally rechargeable batteries. A rechargeable battery pack is supplied with the
MyoTrac Infiniti and can be charged while still inside the unit.
Note: When changing batteries it is recommended to plug the unit into external power, either USB
or wall transformer so that data is not lost. Failure to supply external power will result in data and
script loss.
The battery compartment cover slides open by pushing up using the notch provided. Place four
AAA batteries in the slots, observing the polarity as illustrated. Please note that a diagram of the
correct battery polarity is embossed on the inside surface of the compartment.
Alternatively it is possible to use a rechargeable battery pack (Thought Technology Part Number
MI1028). This battery pack is plugged into the connector in the battery compartment marked
BATT. The pack then fits into the normal battery area. Note: only use battery packs from Thought
Technology or authorized representative, as use of other battery packs will damage the device.
A wall mounted AC power adapter, supplied with the MyoTrac Infiniti, is used to connect the unit to
an electrical outlet. This can be used in conjunction with the batteries or without.
The unit can also be powered from the computer via the USB cable. The cable is connected to the
unit on one side and on the other side to the USB port of the computer. This can be used in
conjunction with or without the batteries.
Charging the Batteries
MyoTrac Infiniti™ User Guide 15
Page 16

64MB
1.75 hours
128MB
3.5 hours
256MB
7 hours
512MB
14 hours
1GB
27.5 hours
2GB
55.5 hours
Note: exact power supply subject to change without notice.
Internal Charger
If your MyoTrac Infiniti was supplied with a wall mounted AC adapter it is possible to charge the
battery pack while it is inserted in the device.
Note: Only use Thought Technology Ltd supplied wall mounted chargers with this device. Failure
to do so could result in potential injury. Use only GlobTek Part Number WR92B2500LCP-Y-MED
To start the charging plug in either the wall mounted AC adaptor or the USB cable. A full charging
cycle from fully empty to fully charged will take approximately 2hrs for AC adaptor and 5.5hrs for
the USB cable. The unit can be used while plugged in to either power source. The charging cycle
does not need to be completed in full; it can be stopped at anytime by removing the connector.
When the unit is turned off while plugged into an external power source, the screen displays a
battery symbol. Charging action is shown with an animation of the battery filling up. When the
battery is fully charged, the symbol shows a full battery.
If the unit is plugged into an external power source while it is turned off, it will start charging within
one minute.
The state of the battery charging is available by going to the power menu in the settings menu of
the device. It indicates the current mode of power and whether the unit is currently charging the
batteries.
Note: The rechargeable batteries must be fully charged prior to initial use. In order for the batteries
to reach full capacity it may be necessary to charge them several times (~2-8) after initial use.
Memory
Recorded data can be saved using three methods - choose the one which most closely matches
your usage needs. To select saving method, select the Settings menu from the main menu, and
tap on the Save icon. Note data can only be recorded from EMG sessions not from Stim sessions.
Internal Memory – Limited size, only the statistical summaries are recorded. Specifically, the
statistics for 13 open sessions or 9 training sessions (work/rest) or 6 assessment sessions
(work/rest + fast-flick + endurance) can be recorded. Data can be lost if the batteries are
removed from the unit for longer than a few minutes.
Compact Flash Card – Most flexible method of data saving: save all the raw data for review
on the encoder or for download to the PC. Available in most electronics stores in a range of
memory sizes. Since all EMG data is recorded, the amount of data that is saved to the
compact flash card depends on the size of the card:
The encoder is delivered with a protective insert in the compact flash slot. To remove it, push
the button next to the slot once to eject the card. The CF card can then be inserted; you will
MyoTrac Infiniti™ User Guide 16
Page 17

notice that the CF card can only be inserted one way into the encoder to protect from incorrect
insertion. When inserted properly it will be flush with the encoder rear. Follow the procedure
above to remove this card when no longer required, and re-insert the protective insert. CF
cards require a CF card reader to transfer data to the PC. The CF cards and reader can be
purchased from most computer stores. Before its first use in the encoder, a CF card requires
PC formatting using the file manager, then format the card using the BioGraph Infiniti Main
Application. Formatting and transferring CF data to the PC is covered in depth in the
BioGraph Infiniti software manual.
Real Time PC Transfer – Connect to the PC via the USB and save and display the data on
the PC in real time. See the following section “Connection to the PC”.
Attention: Do not remove the CF card without first stopping recording. If the CF card is removed
during recording, you will lose all the data for the current session.
Tapping
Like using a mouse on a computer screen the MyoTrac Infiniti allows you to use your finger or a
stylus to tap the buttons directly on the screen. The first time you start your handheld unit, or if the
power has been disconnected for a while, you will be guided through a set of welcome screens
including calibration, time and date setting. The calibration aligns the internal circuitry of the
encoder with its touch sensitive screen so that when you tap a button on the screen, the handheld
unit can detect exactly which button is being pressed.
Note: Always use a stylus for tapping the screen. Never use a pen, pencil or other marking or
sharp object on the screen. Damage resulting from misuse of the screen is not covered by the
warranty.
As necessary wipe screen with a dry cloth to clean.
Connection to the Client
Depending on the type of session you are going to record there are different ways to connect the
two channels to the client.
EMG only – Either plug the extender cable into the device directly and connect to the client
with EMG or Stimulation electrodes, or plug them into the pre-amplifier and the pre-amplifier
into the MyoTrac Infiniti.
Stim only – Plug the extender cable into the device directly and connect to stimulation
electrodes, selecting the appropriate size stim electrode for the treatment to be delivered.
EMG-Stim – As the session contains a stimulation element follow the stim instructions,
connecting only using the extender cable, ensure that you use stim electrodes not SEMG
electrodes.
Attention: Do not use SEMG type electrodes for STIM or EMG-STIM
Sessions.
MyoTrac Infiniti™ User Guide 17
Page 18

Attention: When you insert the extender cable (lead wire) into the electrode
connector, MAKE SURE THAT NO BARE METAL OF THE PINS IS EXPOSED.
Attention: When using stimulation ensure that the intensity is set below the maximum for the
selected electrode size. This is assuming an electrode impedance of 500 or 1k respectively.
1’’ Round = Max 69mA / 49mA
2’’ x 2’’ Square = Max 100mA / 100mA
3’’ x 4’’ Rectangle = Max 100mA / 100mA
St Cloud Vaginal = Max 100mA / 74mA
Femelex = Max 100mA
Note: If an external pre-amplifier is connected to the device all stim settings are disabled to protect
the circuitry of the amplifier.
MyoTrac Infiniti™ User Guide 18
Page 19

Arms and Shoulders
Head and Neck
Abdominals
Back and Buttocks
Legs and Hips
Incontinence
When connecting a sensor or extender
cables, be sure to properly line up the
guiding dot on the top of the plug with the
notch in the encoder's input socket. Forcing
the plug into the jack in any other position
may damage your equipment.
Before applying electrodes, be sure the skin surface is cleaned and dried. Make sure the
electrodes are placed firmly to the skin and make good contact between the skin and electrodes.
Please consult the clinical guide for information on electrode selection for different placements.
The illustration below shows the division of the body into six areas of treatment.
MyoTrac Infiniti™ User Guide 19
Page 20

Using the MyoTrac Infiniti with AC Power Adapter or Connected to a PC
The MyoTrac Infiniti is designed for safe operation on ungrounded AC power sources. However, if
you are using the MyoTrac Infiniti while it is connected to an ungrounded AC power source, for
best results you may need to follow some simple guidelines for skin preparation and electrode
placement. These measures will help to avoid falsely elevated EMG readings while the muscle is at
rest.
If you notice elevated resting EMG levels not related to the patient’s condition, and if this occurs
only when the unit is connected to AC power (directly via the supplied AC adapter or indirectly via a
USB connection to the PC), and if it is necessary to run the MyoTrac Infiniti on ungrounded power
(i.e. no 3rd ground pin on the AC wall socket or on the PC power supply), try the following
techniques to improve the readings.
First, if you are using a PC with only 2 prongs on the wall plug and you have a grounded outlet (3
pin wall sockets with a working ground), plug the ac adapter into the MyoTrac-Infiniti and into the
grounded outlet to provide a ground for the system.
If you have no opportunity to ground either the PC or the AC adapter, use the following electrode
placement tips:
If the EMG site is located on an extremity or limb, be sure to place the REF (black colored)
electrode more proximally (on or closer to the trunk of the body) than the sense electrodes
(yellow and blue), and at least ten centimeters away from either sense electrode.
Prepare the skin under all three electrodes, using a product designed for skin preparation prior
to electrode application (mild abrasives such as NuPrep are effective).
If you are using Ag/AgCl (silver/silver chloride) electrodes, put some conductive electrode
paste or cream on them before applying them to the skin, or try using gel-type rather than dry
Ag/AgCl electrodes.
Resting EMG readings will not be affected by connection to AC power, in the following cases:
Running the MyoTrac Infiniti stand-alone, with no AC power adapter and no connection to the
PC (only on its rechargeable batteries).
MyoTrac Infiniti™ User Guide 20
Page 21

If the PC is portable, running the MyoTrac Infiniti unit off of the PC’s battery via the USB cable,
with the PC and the MyoTrac’s AC adapters both unplugged. Recharging using the AC
adapter may be necessary when the MyoTrac is not in use.
MyoTrac Infiniti™ User Guide 21
Page 22

The MyoTrac Infiniti can be connected to a
Personal Computer (PC). To do this, connect the
USB cable supplied to an available USB port on
the PC, and then plug the other end into the USB
connector marked with the USB Symbol on the top
edge of the MyoTrac Infiniti. As the device is plug
and play, when you switch on the encoder the
computer will automatically recognize it. Now
recognized it will be listed in the device manager as
a Human Interface Device (HID Compliant Device).
Once connected and ready to use, a “ding-dong”
sound will be played. If however a problem is
encountered, a “dong-ding” sound is played. This
sound is also played to alert the user that the USB
cable has been disconnected.
Once connected to the PC, the encoder is
available to the BioGraph Infiniti software.
In other words, the MyoTrac Infiniti
becomes a slave device and is controlled
from the PC. When you have selected
the screens you wish to record, the
BioGraph Infiniti software will establish a
connection. When the connection has
been made the screen of the MyoTrac
Infiniti will display a message saying that
it is connected to the software. Data will
then be available from the BioGraph
Infiniti Software. Note that if the device is
a home unit, it does not deliver live data
(only Compact Flash data). Follow the
instructions in the BioGraph Infiniti
software manual for detailed information
on how to record, replay, review and print
a session using the MyoTrac Infiniti.
Connection to the PC
MyoTrac Infiniti™ User Guide 22
Page 23

Screen Elements
Screens contain up to three element types:
Data displayed in a variety of methods such as line graphs, bar graphs, and digital displays.
Buttons to allow navigation and control of the software.
Thought Support help icon, available in the top left of every screen.
Thought Support
Thought Support is a help feature that is available in every screen of the MyoTrac Infiniti. To
access Thought Support tap the question mark in the top left corner of every screen. This will
present you with screen specific assistance explaining the buttons and their functions. Clicking
anywhere on the screen when the help is displayed will remove it and return you to the original
screen. As a safety feature tapping the question mark during stim will terminate the session that is
currently being recorded.
MyoTrac Infiniti™ User Guide 23
Page 24

Settings Menu
The settings menu is accessible from the main menu and give you control over the global settings
of the MyoTrac Infiniti.
Welcome Settings
If not performed when the unit was first switched on the calibration of the screen and the setting of
the internal clock can be done from the Settings/Welcome menu. Follow the on screen instructions
to make these settings.
Save
The device will automatically set the saving or transferring methods. This screen displays the
selected method and gives you the ability to override the default selections. It favors Compact
Flash Saving over Internal Memory. Information on the amount of memory used is also displayed
in this menu.
Power
Using the power menu it is possible to view the current power settings including the battery level
and charging state.
Lock
The lock feature enables you to lock the user interface, limiting the ability to change clinical
parameters. This gives you the ability to control the prescribed treatment Script when giving the
unit to a client. Using this menu it is possible to then set up the Script that you want to use. Once
the Script is selected you set a four-digit pass code to lock the interface. To unlock enter the pass
code again. If you inadvertently forget the pass code that you have set, enter 0911 and the
encoder will unlock.
MyoTrac Infiniti™ User Guide 24
Page 25

Sound
The sound setting screens allow you to set up the types of sound feedback that the device
provides. The three settings that can be modified are Volume, Mini-Pause, and Feedback.
MyoTrac Infiniti™ User Guide 25
Page 26

Volume
The top-most button (“Volume”) allows for
general sound setup. From this screen, the
touch sound (heard each time a button has
been tapped) can be muted, voice-prompt
(heard during scripted sessions) can be
disabled and the general volume level can
be controlled.
Mini-Pause
Mini-pause is used to monitor the behavior of
a patient during regular activity hence
enabling the reduction of muscle fatigue.
The “Mini-Pause” button allows the user to
set up parameters which will alert the user to
relax their muscles during an Open SEMG
session.
Feedback
The “Feedback” button allows you to change
the style of sound feedback for each of the
two channels independently. The audio
feedback corresponds to the EMG signal.
MyoTrac Infiniti™ User Guide 26
Page 27

No Feedback
Proportionally Increasing Above Threshold
The higher the EMG signal goes above the set
threshold, the quicker the tone of the audio feedback.
Used for muscle control.
Proportionally Decreasing Above Threshold
The higher the EMG signal goes above the set
threshold, the slower the tone of the audio feedback.
Used for muscle control.
Proportionally Increasing Below Threshold
The lower the EMG signal falls below the set threshold,
the quicker the tone of the audio feedback.
Used for muscle control.
Proportionally Decreasing Below Threshold
The lower the EMG signal falls below the set threshold,
the slower the tone of the audio feedback.
Used for muscle control.
Single sound when threshold is crossed above
A tone is heard at the moment the EMG signal crosses
above the threshold.
Used for muscle strength.
Single sound when threshold is crossed below
A tone is heard at the moment the EMG signal crosses
below the threshold.
Used for muscle relaxation.
Continuous sound when threshold is crossed above
A tone is heard continuously while the EMG signal
remains above the threshold.
Used for muscle strength.
Continuous sound when threshold is crossed below
A tone is heard continuously while the EMG signal
remains below the threshold.
Used for muscle relaxation.
The currently selected feedback option is highlighted black. You can choose from nine different
sound modes:
MyoTrac Infiniti™ User Guide 27
Page 28

Chapter 2
SEMG sessions on your MYOTRAC INFINITI™
Encoder
What is an SEMG session?
During a Surface Electromyography (SEMG) session, muscle activity is measured and can be
recorded by sensing the electrical activity that occurs during muscle contraction and relaxation
cycles.
Surface Electromyography (SEMG) sessions can be recorded in two formats -Open Sessions or
Script Sessions. Recorded data can be saved onto the internal memory of the MyoTrac Infiniti or
onto a Compact Flash Card (CF) inserted into the MyoTrac Infiniti, or transferred in real time to the
host PC. It is also possible to not save any data and just verify the signal.
Open Sessions, also known as free-running sessions, can proceed for an unlimited duration of time
without following any set protocols. Different display types (line graph, bar graph and digital
display) can be selected to view and/or record the data.
Script Sessions, also known as protocol sessions, run for a specified period of time. A script
session includes a combination of work, rest, fast flick and/or endurance type exercises. The
number of sets for each type and the respective duration has already been determined. The
MyoTrac Infiniti has a number of predefined script sessions and furthermore allows for creation of
customized script sessions.
Features of an SEMG session
Sound Options:
During the free-running portion (before recording begins) of both Open and Script SEMG sessions,
the sound options can be modified. Since the Mini-Pause alarm is only activated during an Open
session, it is only modifiable during that session. The other sound options are Volume and
Feedback. These options are discussed in detail in Chapter 1 -> Settings Menu -> Sound.
Auto-Threshold:
In a script SEMG session, the Auto-Threshold option is available. This allows for the system, as
opposed to the clinician, to determine the threshold value. This means that during the first 3
activities of a session (i.e. work or fast-flick), the threshold that the patient is able to reach is
automatically calculated and displayed. The patient must then continue to reach that target
throughout the remainder of the session.
Open SEMG Sessions
An Open Session can proceed for an unlimited duration of time without following any set protocols.
When the user has finished recording the data or verifying the signal, they can simply stop the
session by pressing the stop button, the next button, or the back button (depending on whether the
session is being recorded or not). SEMG sessions can be viewed using one of three display
modes; as visual signals in the line graph and the bar graph or as numeric data using the digital
display mode.
MyoTrac Infiniti™ User Guide 28
Page 29

To start an Open SEMG session:
Select New Open Session from the main menu.
Select Display type (line graph, bar graph or digital display).
Select Client to record this session for (if recording to On-Board Memory or Compact Flash
Card).
Press the Record button having verified the signal (if session is not being saved, then it is just
running).
When the session has been completed tap the Stop button and you will be returned to the
main menu.
MyoTrac Infiniti™ User Guide 29
Page 30

Script SEMG Sessions
A script session is a session where the type, number and duration of steps within the session are
pre-defined. A simple example would be a work/rest script that may contain five interspaced work
and rest steps each ten seconds in length. This would give the script a duration of 100 seconds.
The MyoTrac Infiniti comes with a number of pre-defined SEMG Scripts for you to use. To select
one, choose the body area that you are going to be working with and it will show you a list of scripts
that are applicable to that body area. Alternatively, use the Custom Wizard to define your own
script following the on screen instructions. It is possible to save up to five custom SEMG scripts on
board the device.
Scripts specific to the following parts of the body are included in the device:
Arm and Shoulder
Head and Neck
Abdominal and Chest
Back and Buttocks
Hip and Leg
Incontinence
To start an Script SEMG session:
Select New Session Script from the main menu.
Select EMG, and a client if you wish to save the data.
Select a body area to be presented with an area specific list of scripts.
Select a script from the list by scrolling using the arrow keys then the next button, if you need
more information on the selected script prior to selecting it press the settings button in the
button bar.
Choose whether or not the threshold should be set automatically.
Start the session by pressing the record button. Instructions guiding you through the script
appear in the message bar at the top of the screen.
MyoTrac Infiniti™ User Guide 30
Page 31

In the review mode the data saved from a script session is compartmentalized into each of the
types of steps recorded, for example statistics are available for the entire collection of work
segments. If this session is subsequently transferred to the host PC running BioGraph Infiniti then
the information corresponding to the script that was used to record the session will also be
transmitted, even if it was a unique custom script. For more information see Chapter 5.
MyoTrac Infiniti™ User Guide 31
Page 32

Chapter 3
Stim sessions on your MYOTRAC INFINITI™
Encoder
What is a Stim session?
A Neuromuscular Electrical Stimulation (NMES) session is used in order to stimulate muscle
contraction. During a stim session an electrical impulse is delivered to targeted muscles via
electrodes which cause the active motion in the muscles. Stimulation can be used for muscle rehab
as well as for incontinence treatment. Each of these can be run in two formats: Open Sessions or
Script Sessions. Data is not recorded during a stimulation session as no EMG is recorded.
Features of a Stim session
Below is a typical waveform for a stimulation pulse. There are a number of variables that can be
controlled when running a stimulation session based on your clinical objectives. These can be
manually adjusted or a pre-specified set can be selected by using a Script.
Pulse Width – The duration of each individual pulse.
Pulse Rate - The rate at which a number of pulses are delivered.
Intensity – The intensity in mA that is delivered by each pulse. The maximum available
intensity is 100mA, determined with an input impedance of 500. The maximum intensity that
you could use is determined by the electrode size that you are using and may be less than
100mA.
Ramp – The time it takes for the intensity of successive pulses to reach the preset maximum
or back to zero from the start or the end of a series of pulses.
As can be seen from the diagram above of a single stimulation pulse the stim is balanced thus
there is no DC component. This means that during stimulation session there is no residual build up
of energy at the electrode site.
One safety feature of the MyoTrac Infiniti is a “client detect”, that detects the connection of the
electrodes to the user. If there is not a good electrical connection the unit will not permit the stim to
start. Test this periodically by disconnecting the client cable and trying to increase the stim
MyoTrac Infiniti™ User Guide 32
Page 33

intensity. If no stim is felt when the stim is increased, stop use of the unit and contact technical
support.
Stimulation for Muscle Rehab
When NMES is used for muscle rehabilitation, the current intensity is incremented in steps of 1mA
and 2 channels of stimulation are possible.
Stimulation for Incontinence Treatment
The MyoTrac Infiniti can be used to treat 3 different types of incontinence: stress incontinence,
urge incontinence and mixed incontinence. Depending on the type of incontinence, the pulse rate
will vary. When setting the current intensity for incontinence it should be noted that the increment
step is 0.5mA and stimulation is only possible on one channel.
Open Stim Session
To start an Open Stim session:
New Open Session from the main menu.
Select Stim (or U-Stim for treatment of incontinence)
Confirm Settings to be used during this session by tapping the checkmark.
Start the Stim session: Increase the Stim intensity by using the arrows.
Attention: It is not possible to increase the intensity of the stimulation during the
ramps or during the rest phases. It is however possible to decrease during the
work or rest phases.
MyoTrac Infiniti™ User Guide 33
Page 34

Stim Settings
The Stim settings menu summarizes the
settings for the stimulation session. In the
Open Session mode there is a simple
structure of work and rest segments to divide
the Stim delivery with rest. The Ramp Up &
Down Time is the amount of time it takes the
stimulation to ramp up to full intensity at the
beginning of the work period and to ramp
down at the end of the work period,
respectively. The pulse rate is the frequency
of the individual pulses that go to make up the
total stimulation. The pulse width is the
duration of each of these individual pulses. To
make changes to any of these settings press
the edit button on the bottom left of the screen.
MyoTrac Infiniti™ User Guide 34
Page 35

Edit Wizard
The edit wizard guides you through each
of the parameters that are available to
change in the software – Work, Rest
durations, Ramp Time, Pulse Rate and
Pulse Width. Note that for Incontinence
stim sessions, Pulse Rate and Pulse
Width cannot be modified. Once the
changes have been made, review them in
the Stim settings overview before
continuing. Stim intensity is controlled
from the stimulation screen.
Stim Delivery Screen
The Stim delivery screen is the screen that
displays the current intensity, maximum intensity
and its controls for each of the two channels.
Tap the Start button followed by the up arrows to
incrementally control the current being delivered.
Current can only be increased during the work
period, not during the ramp times or during the
rest period. The current can be decreased during
the work or rest periods, but not the ramp times.
The maximum current set for the work period is
displayed for each channel in the diagram in the
middle of the screen. The actual supplied current
is shown as the larger numbers at the top of the
control arrows. This supplied current reading will
display the max mA during work, 0mA during rest
and an increasing and deceasing value during the
ramps. If you keep your finger on the control
buttons it will continue to increase until it reaches
the maximum of 100mA.
If at anytime you wish to stop the session press
the STOP button that covers the top portion of the
screen. As a safety feature if the MyoTrac Infiniti
detects the electrodes being removed while the
unit is stimulating, it will automatically stop the
Stim. The illustration of the stim cycle in the
middle of the screen indicates along with the label
at the top of the screen the period of the stim you
are currently in.
Displayed in the status bar of the stimulation
screen is the number of sets done, for an open
stimulation setting, or the number remaining for a
scripted session.
MyoTrac Infiniti™ User Guide 35
Page 36

Script Stim Sessions
A script session is a session where the type, number and duration of steps within the session are
pre-defined. A simple example would be a work/rest script that may contain five interspaced work
and rest steps each ten seconds in length. This would give the script a duration of 100 seconds.
The work segment of the stim script will deliver stimulation at the preset pulse frequency and
duration for a predetermined amount of time, at an intensity that you set.
The MyoTrac Infiniti comes with a number of pre-defined Stim Scripts for you to use. To select
one, choose the body area that you are going to be working with and it will show you a list of scripts
that are applicable to that body area. Alternatively, use the Custom Wizard to define your own
script following the on screen instructions. It is possible to save up to five custom Stim scripts on
board the device. Please consult the clinical guide for further information.
To start a Script Stim session:
Select New Session Script from the main menu.
Select Stim.
Select a body area from the illustration to be presented with a list of body area specific scripts.
Select a Script from the list by scrolling using the arrow keys then the next button. If you need
more information on the selected Script prior to selecting it, press the settings button in the
button bar.
When a Script has been selected, you are taken to the stimulation screen where you start the
session by pressing the start button. Instructions guiding you through the Script and a count
down of the number of sets appear in the message bar at the top of the screen.
MyoTrac Infiniti™ User Guide 36
Page 37

Chapter 4
EMG-STIM on your MYOTRAC INFINITI™ Encoder
EMG-Stim Sessions
EMG-Stim sessions are conducted in a Script format only. A script session is a session where the
type, number and duration of steps within the session are pre-defined. A simple example would be
a work/rest script that may contain five interspaced work and rest steps each ten seconds in length.
This would give the script a duration of 100 seconds. The work segment of the stim script will
deliver stimulation at the preset pulse frequency and duration for a predetermined amount of time,
at an intensity that you set. This stimulation will occur either above or below the set threshold after
a predetermined delay time.
MyoTrac Infiniti™ User Guide 37
Page 38

The MyoTrac Infiniti comes with a number of pre-defined EMG-Stim Scripts for you to use. To
select one, choose the body area that you are going to be working with and it will show you a list of
scripts that are applicable to that body area. Alternatively, use the Custom Wizard to define your
own script following the on screen instructions. It is possible to save up to five custom EMG-Stim
scripts on board the device. Please consult the clinical guide for further information.
Types of EMG-Stim
There are two different types of EMG-Stim sessions that can be used on the MyoTrac Infiniti: EMGTriggered Stim (ETS) sessions and Alternating EMG-Stim sessions. Since these modes involve
the measurement of SEMG and delivery of electrical stimulation, the MyoTrac Infiniti uses the
same electrodes for stimulation and for SEMG, thus making it possible to direct the stimulation to
the same place as the SEMG was been measured. EMG-Stim sessions are run as Script Sessions
and no data is recorded.
What is an EMG triggered Stim session?
An SEMG triggered Stim session is the combination of the two complementary modalities to form a
third treatment possibility. The client’s own volitionally activated SEMG is used as a guide to
determine when to automatically stimulate. This combination of passive and active rehabilitation
elements could be considered as getting the best of both worlds.
Note: EMG triggered STIM is not available for incontinence application.
Features of an EMG triggered Stim session
Trigger Modes:
Depending on the context, stimulation can occur either when crossing above threshold or when the
signal falls below the threshold. When the trigger is above threshold, it can be referred to as
“reward” mode. When the target threshold has been reached, an electrical impulse is delivered to
the patient in order to complete the contraction. This mode motivates the patient and involves
them in the rehabilitation process. When the trigger is set to activate below threshold, it can be
referred to as “assistance” mode. When the patient is unable to hold the contraction for a long
enough duration using this mode will assist them in finishing the contraction. The trigger mode can
be set when customizing a script session. In the case of a predefined script session the trigger
mode is already selected and can be verified by checking the script information.
Threshold Modes:
This threshold can be set either by the user (manual) or by the system itself (automatic). Automatic
threshold mode is different for trigger above threshold and trigger below threshold. When the
stimulation is triggered below threshold, then during the first 3 activities of a session (i.e. work or
fast-flick), the threshold that the patient is able to reach is automatically calculated and displayed.
The patient must then continue to reach that target throughout the remainder of the session. When
stimulation is triggered above the threshold then the threshold will be adjusted throughout the
session.
Script Structure
SEMG triggered Stim sessions are conducted in a Script format. A Script takes the form of a
number of work and rest steps. During a work step, the MyoTrac Infiniti will guide the client to
activate the muscle being monitored. In addition to the signal displayed on the graph we can also
see the trigger threshold defined at setup time. If during the work period the signal reaches the
trigger threshold for a time greater than the pre-determined delay period, then the stimulation is
triggered. This stimulation is delivered for the remainder of the work step. The rest step is just
MyoTrac Infiniti™ User Guide 38
Page 39

that, it is a period of time where the client rests following a period of activation of the muscle being
monitored. During the rest period it is not possible to stimulate, and the threshold trigger is
ignored. The illustration below shows a Script of 20 second work and rest steps, with a threshold
set to 40uV and a delay of 5 seconds on a positive crossing trigger. It shows two work segments:
one where the stimulation is not triggered, the other when it is.
What is an Alternating EMG-Stim session?
An Alternating EMG-Stim session is a repeated sequence of Stim and EMG. It is not the same
thing as an EMG triggered Stim session. In this mode the Stim occurs during the rest period and it
is used in order to make the patient understand what the muscle contraction should feel like.
During the work period, the patient will need to reproduce the contraction achieved during the
previous stim session. This combination of stimulation and reiterative behavior can strengthen the
muscles and help the patient relearn the natural reflexes.
Features of an Alternating EMG-Stim session
Threshold Mode:
For Alternating EMG-Stim sessions, automatic threshold mode is used to determine the value of
the threshold. This works in the same was as for script SEMG sessions. Auto-threshold mode
allows for the system, as opposed to the clinician, to determine the threshold value. This means
that during the first 3 activities of a session (i.e. work or fast-flick), the threshold that the patient is
able to reach is automatically calculated and displayed. The patient must then continue to reach
that target throughout the remainder of the session.
Script Structure
Alternating EMG-Stim sessions are conducted in a Script format. A Script takes the form of a
number of work and rest steps. During a work step, the MyoTrac Infiniti will deliver an electrical
impulse to the muscle being monitored. This stimulation will cause the muscle to contract. During
the rest step, the patient will volitionally contract their muscle in a manner similar to what was felt
when the electrical impulse was delivered during the previous work step. In this case the threshold
acts solely as a target that the patient must reach. The illustration below shows a Script of 20
second work and rest steps, with a threshold set to 40uV. It shows three rest segments and two
work segments: during each of the work segments, muscle contractions similar to the stim-induced
ones occur.
MyoTrac Infiniti™ User Guide 39
Page 40

To run an EMG-Stim Script select:
New Script Session from the main menu.
Select EMG-Stim
Select Body area from illustration for area specific scripts.
Select the threshold mode
o EMG-Triggered Stim (threshold manually set)
o EMG-Triggered Stim (threshold automatically set)
o Alternating Stim and EMG
Select Script by using the up, down and forward arrows. A number of pre-defined Scripts are
available or you can design your own using the custom design Wizard, covered later in the
chapter.
Threshold Setting: Confirm or change the threshold that will be used to determine if the
stimulation should be triggered.
Set Stim Intensity: The intensity that is set here will be delivered if the correct criteria are met
in the recording phase. Use the up and down arrows to set the intensity. When this is set
move forward to the recording screen.
Start the EMG Triggered Stim session: Start the Script driven session by tapping the record
button. To stop the session tap this button again, or, during the Stim delivery, press the stop
button.
Note: EMG triggered STIM is not available for incontinence application.
MyoTrac Infiniti™ User Guide 40
Page 41

Chapter 5
Data Management on your MYOTRAC INFINITI™
Encoder
This chapter explains how to manipulate the data that you have recorded to the MyoTrac Infiniti for
a number of clients and how to interface this data with the BioGraph Infiniti on the host PC.
What is Data Management?
The MyoTrac Infiniti can save sessions in various different ways depending on your clinical needs.
It can save summary data direct to its internal memory, full sessions to the Compact Flash Card, or
full sessions (via live acquisition) directly transmitted to the host PC via the USB cable. This leads
to a need to centralize data for long-term storage (archiving) and further analysis. The host PC
handles this central storage. Data saved on the MyoTrac Infiniti Compact Flash Card can be
transferred to the PC. When this data is downloaded to the PC you can add it to existing client files
or create new ones. For further information on BioGraph Infiniti and how it handles session data
from the MyoTrac Infiniti please refer to the software manual for the BioGraph Infiniti.
MyoTrac Infiniti™ User Guide 41
Page 42

MyoTrac Infiniti Review
Data can also be reviewed on the MyoTrac Infiniti as a stand alone piece of equipment. It is
possible to view the summary statistics or replay the session in either of two display types. To
access the data tap the Database button on the main menu. This will present you with the saved
sessions listed in order of date. Use the up and down arrows to scroll between sessions. When
you have selected the session that you are interested in, use the forward arrow to view it.
Once you have selected the data session that you wish to manage you are presented with the
summary screen. This screen gives you information on when the session was recorded and for
which client. As you can see in the illustration below there are three choices.
Replay – When selecting to replay a session it gives you further information on the recorded
Script and asks if you wish to replay it in the same screen type or change between the line
graph, and bar graph. This feature is only available if the session was saved to the compact
flash card.
Session Statistics – The session statistics comprise the whole session. If the session was
recorded in a Script it breaks down the data into step type statistics (work, rest, flick and
endurance).
Internal Session Delete – This feature enables you to permanently remove sessions that
have been recorded on the internal memory. The session saved to the CF card can only be
removed by using the BioGraph Infiniti Software.
MyoTrac Infiniti™ User Guide 42
Page 43

Chapter 6
Display Options on your MYOTRAC INFINITI™
Encoder
In this chapter we will take a closer look at each of the three major display types with which to view
SEMG data.
Displays
When recording an SEMG session, there are three types of data display available: Line Graph, Bar
Graph, and Digital Display.
Line Graph
The line graph display is a good choice if the signal is time dependant, and a history of the signal is
required. It is the most comprehensive of the display choices, showing in real time up to two
signals on a single set of axis. Some of the key features of the line graph are the thresholds, gain
and sweep controls.
Bar Graph
The bar graph display is designed to give a clear indication of the relative levels of each channel. It
can also be used in ratio mode to give information on the interplay of the two channels.
Digital Display
The digital display consists of two pages of numerical information computed from the two channels.
It is possible to change the automatic refreshing rate of the data displayed or to refresh manually.
MyoTrac Infiniti™ User Guide 43
Page 44

Line Graph
Record/Stop – When you initially move into the line graph screen the trace will appear on the
screen to allow you to confirm electrode placements and signals. To start the recording, press
on the circle. It will change to a square. To stop the recording press it once again. During the
recording, the word REC will flash in the status bar at the top of the screen.
Gain/Sweep – Expand or contract the X or Y axis scale to view the signal as the scale that
you want. When you tap on the gain and sweep button a sub menu appears. Use the first
button on the sub menu to select the Y or X axis to change. The second button on the sub
menu puts change focus on either the top digit or the bottom digit of the axis selected. The +
and – buttons increase or decrease the selected values.
Threshold – The threshold settings allow you to set a threshold for feedback. When you tap
on the threshold button you are presented with a sub menu. The first button on the sub menu
is used to select a threshold for Channel (A) or (B). With either A or B selected, use the + and
– buttons to move the position of the threshold up or down on the range. The button below
depicts an eye either open or closed. This is used to show or hide the selected threshold.
Line Display – This button enables you to select whether Channel A should be filled or
unfilled. Here you can also decide to show or hide the signal for each of the channels.
X and Y axis – Scales settings currently selected via the gain/sweep button.
Thought Support Help – Screen specific help. If you are not sure about the icons, this will
show you the way.
Ch A/B Legend – This is the legend for describing which line styles and displays the settings
from the line style button.
Thought Support Help – Screen specific help. If you are not sure about the icons, this will
help.
Sound Enabled – Use this button to disable the sound feedback being given in this screen.
Speaker symbol has a cross through it if the sound is disabled. Before the recording begins,
this button is also used to modify the sound settings (volume, mini-pause, if available and
audio feedback).
MyoTrac Infiniti™ User Guide 44
Page 45

Bar Graph
Record/Stop – When you enter this screen the signal from the inputs will appear, enabling
you to confirm electrode placements and signals. To start the recording, press on the circle.
It will change to a square. To stop the recording press it again. During the recording the word
REC will flash in the status bar at the top of the screen.
Gain/Sweep – Expand or contract the Y axis scale to view the signal at the scale that you
want. When you select the gain button a sub menu appears. The first button A/B in the sub
menu allows you to independently change the gain for the A or B bar graph; use it to select
the one to change. The second button changes focus from the top of the gain to the bottom
number for the gain. Then use the + and – buttons to increase or decrease the gain.
Threshold – Set one threshold per channel and move it up and down the scale. Tapping the
threshold button displays a sub menu enabling you to choose bar graph A or B to adjust, and
hide/reveal the thresholds.
Bar Displays – This button changes the bars displayed in the screen. There are four choices:
both bars A and B, one or the other, or the ratio of A/B and B/A.
Thought Support Help – Screen specific help. If you are not sure about the icons, this will
help.
Sound Enabled – Use this button to disable the sound feedback being given in this screen.
Speaker symbol has a cross through it if the sound is disabled. Before the recording begins,
this button is also used to modify the sound settings (volume, mini-pause, if available and
audio feedback).
MyoTrac Infiniti™ User Guide 45
Page 46

Digital Display
Record/Stop – When you enter this screen the digital tables will be updating enabling you to
confirm electrode placements and signals. To start the recording, press on the circle. It will
change to a square. To stop the recording, press it again. During the recording the word REC
will flash in the status bar at the top of the screen.
Second Page – To display the second page of statistics press the 2nd page button. On page
two there is the ability to use a threshold. Its settings are the same as for the Line and Bar
Graph.
Refresh – This button allows you to select the refresh rate between 1,5,10 seconds or manual
refresh.
Statistics – Stats displayed for both channels include:
Max value of signal during the current refresh period.
Min value of signal during refresh period.
Mean value of signal during refresh period.
% above is the percentage of time the signal was above the threshold during the refresh
period.
% vs. T (T being threshold value) is comparing the signal against the threshold as if the
threshold is 100% in real time.
Zero Xing (Abbreviated from Zero Crossings) is the number of times the raw signal crosses
from a negative value to a positive value and back averaged over a 1 second time period.
The Ratio A/B is displaying the ratio between the two signals in real time.
Refresh Rate – Displays currently selected refresh rate.
Thought Support Help – Screen specific help. If you are not sure on the icons, this will help.
Session Duration – Current session duration.
Sound Enabled – Use this button to disable the sound feedback being given in this screen.
Speaker symbol has a cross through it if the sound is disabled. Before the recording begins,
this button is also used to modify the sound settings (volume, mini-pause, if available and
audio feedback).
MyoTrac Infiniti™ User Guide 46
Page 47

Flow on your
MYOTRAC INFINITI™ Encoder
New Open Session
Chapter 7
MyoTrac Infiniti™ User Guide 47
Page 48

Reference
PC
Personal Computer
SEMG
Surface Electromyography
STIM
Stimulation
Stylus
A blunt pointed object used to tap the screen.
CF
Compact Flash
T
Template
A
Channel A
B
Channel B
REC
Record
-ve
Negative
+ve
Positive
Scripts Session
A Script is a sequence of events in a pre-determined session duration.
Open Session
An open session is defined on the fly by the clinician. Its length is governed by
the Record and Stop buttons.
Zero X-ing
(Zero Crossings)
Then number of times the raw signal crosses from positive to negative or the
reverse.
USB connector
Glossary of Terms, Abbreviations and Symbols
Chapter 8
MyoTrac Infiniti™ User Guide 48
Page 49

Technical Support and Order Placing
Returning Equipment
Be sure to call for an authorization number (RA) before returning any equipment!
Send the unit(s) postage prepaid and insured, with proof of purchase to one of the addresses
below.
If you are shipping from outside Canada or the USA to Canada, mark the package ‘Goods to be
repaired - Made in Canada’ to avoid unnecessary customs charges.
All customs and duties charges will be billed to you if incurred by sending the unit to the wrong
address.
Provide a detailed description of the problem you are experiencing, and your telephone/fax
number and email (see form on the last page of this manual).
In the USA, ship insured to:
Thought Technology Ltd.
Cimetra LLC
8396 State Route 9
West Chazy, New York
12992, USA
In Canada and all other countries, contact your dealer or ship insured to:
Thought Technology Ltd.
2180 Belgrave Avenue
Montreal, Quebec
Canada H4A 2L8
Technical Support
For technical support please refer to the Thought Technology Ltd website at
www.thoughttechnology.com for frequently asked questions. If your support issue is not covered
please e-mail or telephone at the number below.
(514) 489-8251 techsupport@thoughttechnology.com
Fax (514) 489 8255
MyoTrac Infiniti™ User Guide 49
Page 50

Product Numbers & Accessories
MYOTRAC INFINITI ENCODER & SENSORS
SA9570 ST CLOUD SENSOR
SA9800 MYOTRAC INFINITI
T6050 TTL INCONTINENCE SENSOR VAGINAL
T6051 TTL INCONTINENCE SENSOR RECTALSA9305 EEG SENSOR
T7900 BIOGRAPH INFINITI SOFTWARE
T9503M MYOSCAN EMG SENSOR
ACCESSORIES
MI0009-00 4 X AAA BATTERIES
MI1028 BATTERY PACK
MI1031 INFINITI CASE
MI1050 Rechargeable Battery Pack
SA3403 ELECTRODE EXTENDER CABLES (THREE PACK)
SA5000-14 AQUASENSE SMALL ARM PROTECTOR
SA5000-16 AQUASENSE MEDIUM ARM PROTECTOR
SA5000-18 AQUASENSE LARGE ARM PROTECTOR
SA5010-14 AQUASENSE SMALL LEG PROTECTOR
SA5010-16 AQUASENSE MEDIUM LEG PROTECTOR
SA5010-18 AQUASENSE LARGE LEG PROTECTOR
SA7713 USB CABLE 1.8M
SA9801 Sensor DIN Adaptor Cable
SA9804/06/11 STIMULATION ELECTRODES
SA9810 MYOTRAC INFINITI USER GUIDE
T3402M TRIODE DISPOSABLE ADHESIVE ELECTRODES (X 100)
T3404 SINGLE DISPOSABLE ELECTRODES (X 300)
T3425 UNI-GEL ELECTRODES (X 100)
T8720M SENSOR EXTENDER CABLE (PROTECTED PIN)
T9385M SENSOR REPLACEMENT CABLE KIT
SA2306 HEADBAND EMG
SA8740 DIN CABLES
T3470 NU-PREP GEL
T3450
MyoTrac Infiniti™ User Guide 50
Page 51

10-20 PASTE
Placing Orders
Outside USA
Tel: (514) 489-8251
Fax: (514) 489-8255
In USA Toll-Free
Tel: 1-800-361-3651
E-Mail: mail@thoughttechnology.com
MyoTrac Infiniti™ User Guide 51
Page 52

Specifications
MyoScan EMG Sensor (SA9503M)
Size (Approx.)
37mm x 37mm x 12mm (1.45” x 1.45” x 0.45”)
Weight
15g (0.5 oz)
Input Impedance
≥1012Ω in parallel with 10pF
Input Range
0 – 2000V
RMS
Sensitivity
<0.1V
RMS
CMRR
>130dB
Channel Bandwidth
10Hz – 1kHz
Signal Output Range
0 – 1.0V
RMS
Input / Output Gain
500
Supply Voltage
7.26V (± 0.02V)
Current Consumption
0.7mA (± 0.25mA)
Accuracy
0.3V
RMS
Plus 4% of reading @25C to 30C,
MyoTrac Infiniti Encoder (SA9800)
Size (approx.)
102mm x 152mm x 51mm (4” x 6” x 2”)
Weight (approx.)
330 g (11oz) with Batteries
Power Source
4AAA batteries, single use alkaline or Rechargeable battery pack
6V Medical Grade AC wall adaptor
5V USB
Battery Life, Alkaline cells (1200mAh)
3 – 4h typical
Battery Life, Battery Pack (700mAh)
3 – 4h typical
Low-battery warning
Before shut down
ADC output
14bits
Encoder sample rate
2048 samples/second nominal
Encoder channel bandwidth (3dB)
Internal EMG amplifier
External Sensor Input
20 – 500Hz
DC – 500Hz
Anti-aliasing Filter
4th order Butterworth -3dB at 500Hz
Alias rejection
24 dB typical
Gain accuracy
0.5 %
Accuracy including external sensor
5 % +0.6V RMS
STIM SETTINGS
VALID BETWEEN 500 to 1k load impedance.
Stimulation intensity
0mA – 100mA
MyoTrac Infiniti™ User Guide 52
Page 53

Stimulation Pulse Rate
2Hz – 100Hz
Pulse Width
50-400s
Ramp Time ( Up and Down )
0 – 10 sec
NOTE: MYOTRAC INFINITI and its sensors are sensitive electronic instruments and should be handled
as such. Be especially careful to avoid both pulling on the electrode cable and getting moisture or
electrode gel on the sensor snaps. If necessary, wipe the surface with a damp cloth or use a
moistened Q-tip to remove gel from inside the sensor snaps. Wipe with a dry cloth.
MyoTrac Infiniti™ User Guide 53
Page 54

Optional Extended Warranty
Please contact Thought Technology Ltd. for further details.
Warranty
The MYOTRAC INFINITI system and all equipment including optional items are guaranteed to be free
from defects in material and workmanship for 1 year from the date of purchase.
In the unlikely event that repair is necessary, call Thought Technology Ltd. to receive a Return
Authorization. Then send the unit back by a traceable method--Thought Technology will not be
responsible for items not received. We will repair or replace your unit(s) free of charge.
This warranty does not apply to damage incurred through accident, alteration, or abuse, nor to sensor
damage created by static electricity, or to screen damage resulting from misuse. Do not use this
equipment in dry, static area unless using an anti-static mat or anti-static spray on carpeted areas.
IMPORTANT: Remove dead batteries promptly to prevent corrosion damage.
MyoTrac Infiniti
™
User Guide 54
Page 55

Repair Return Form
Name
Company
Address
Phone No.
Fax No.
Date Purchased
From Whom
Model Name
Serial Number
Problem
Be sure to call for authorization before returning any equipment!
Remove or copy this form and include it with the unit(s).
Include copy of original invoice and return to the address in the Returning Equipment section.
MyoTrac Infiniti
™
User Guide 55
Page 56

MyoTrac Infiniti Hardware
Copyright Notice
This hardware contains proprietary embedded software code, which is the property of Thought
Technology Ltd.; it is provided under a license agreement containing restrictions on use and disclosure
and is also protected by copyright law. Reverse engineering of the software or the resulting output
data stream is prohibited.
Due to continued product development the embedded software may change without notice. The
information and intellectual property contained herein is confidential between Thought Technology
Ltd. and the client and remains the exclusive property of Thought Technology Ltd.
If you find any problems in the documentation, please report them to us in writing. Thought
Technology Ltd. does not warrant that this document is error-free.
No part of this publication may be reproduced, stored in a retrieval system, or transmitted in any form
or by any means, electronic, mechanical, photocopying, and recording or otherwise without the prior
written permission of Thought Technology Ltd.
MyoTrac-Infiniti™ and BioGraph Infiniti™ are trademarks of Thought Technology Ltd.
© Copyright Thought Technology 2005
MyoTrac Infiniti
™
User Guide 56
Page 57

MyoTrac Infiniti
™
User Guide 57
 Loading...
Loading...