Page 1

Page 2

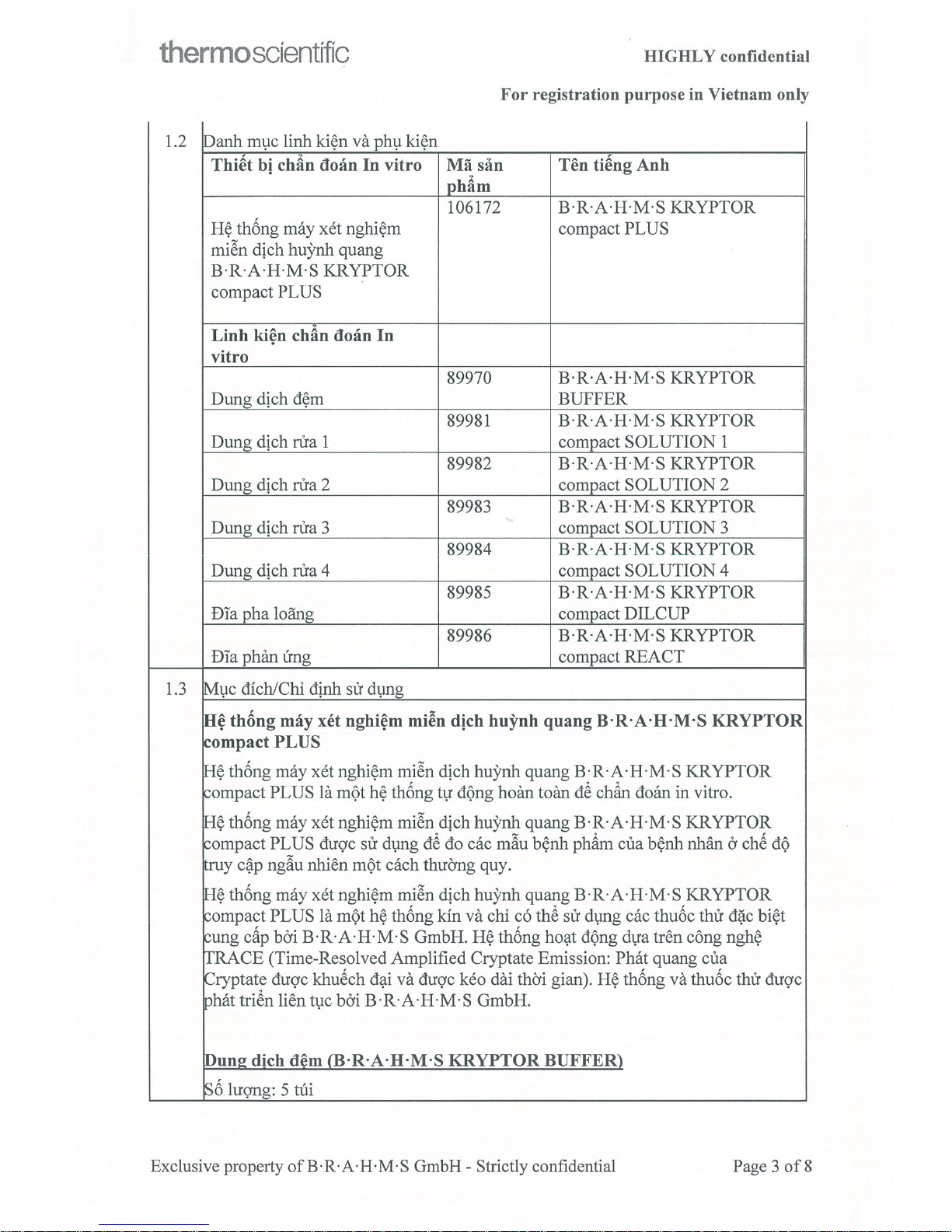
Page 3
Page 4

Page 5

Page 6
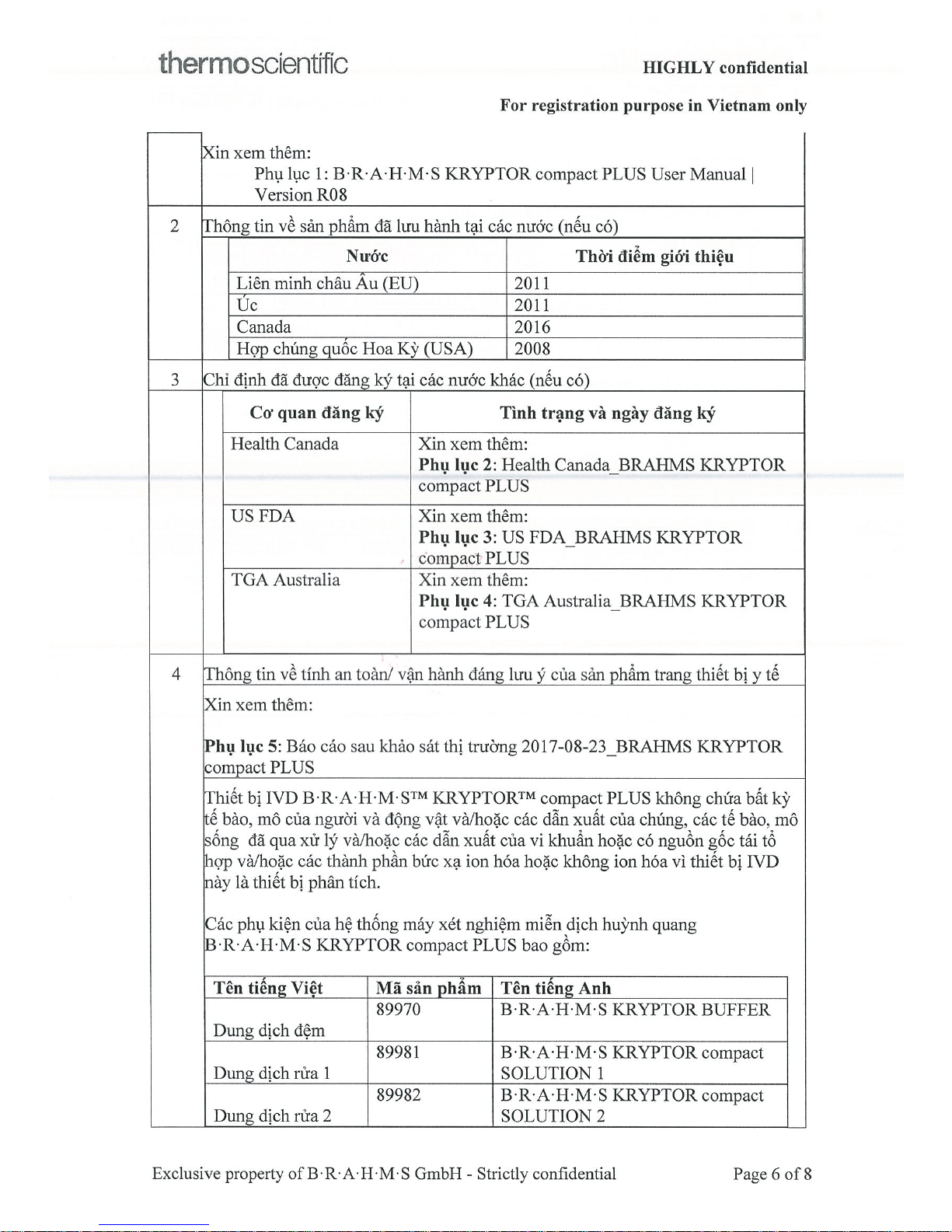
Page 7
Page 8

Page 9

Page 10

B·R·A·H·M·S KRYPTOR
compact PLUS
User Manual | Version R08
106172 I SW 7.06.00 I Date 21.02.2018
Page 11

B·R·A·H·M·S GmbH
B·R·A·H·M·S KRYPTOR compact PLUS User Manual (Version R08en) Page 2 of 30
Name REF
B.R.A.H.M.S KRYPTOR compact PLUS 106172
B.R.A.H.M.S KRYPTOR compact PLUS Reading Module 106174
B.R.A.H.M.S KRYPTOR compact PLUS Pipetting Module 106173
B.R.A.H.M.S KRYPTOR BUFFER 89970
B.R.A.H.M.S KRYPTOR compact SOLUTION 1 89981
B.R.A.H.M.S KRYPTOR compact SOLUTION 2 89982
B.R.A.H.M.S KRYPTOR compact SOLUTION 3 89983
B.R.A.H.M.S KRYPTOR compact SOLUTION 4 89984
B.R.A.H.M.S KRYPTOR compact DILCUP 89985
B.R.A.H.M.S KRYPTOR compact REACT 89986
B.R.A.H.M.S KRYPTOR compact PLUS:
Instrument and consumables
Content changes versus previous version
• Add new laser labels
• Add UDI, USA: Rx only and cTUVus labels
• Add information about preventive maintenance frequency by service engineer
• Modification of related software version
• Add CE and IVD label on back side
Page 12

B·R·A·H·M·S GmbH
B·R·A·H·M·S KRYPTOR compact PLUS User Manual (Version R08en) Page 3 of 30
Labelling ................................................................................................................................................................. 4
Safety Instructions ................................................................................................................................................ 6
Introduction ............................................................................................................................................................ 8
Instrument Components ....................................................................................................................................... 9
Description of the System Status Window ....................................................................................................... 11
Main Menus .......................................................................................................................................................... 12
The Tool Palette ................................................................................................................................................... 13
Work Surface Colour Codes ................................................................................................................................ 14
Sample Carousel .................................................................................................................................................... 14
Reagent Area ......................................................................................................................................................... 15
Reaction Area ........................................................................................................................................................ 16
Dilution Plate .......................................................................................................................................................... 16
B.R.A.H.M.S KRYPTOR compact SOLUTIONS 1 to 4 ........................................................................................... 17
Fluidic System ....................................................................................................................................................... 17
Routine Work ....................................................................................................................................................... 18
Start-up .................................................................................................................................................................. 18
Maintenance ......................................................................................................................................................... 18
Reagent registration .............................................................................................................................................. 19
Reconstitution of reagents .................................................................................................................................... 19
Perform a calibration .............................................................................................................................................. 19
Run controls ........................................................................................................................................................... 20
Run patient samples with barcode ........................................................................................................................ 20
End of day .............................................................................................................................................................. 20
Analytes with Pre-incubation ............................................................................................................................. 21
Important User Information ............................................................................................................................... 23
Barcodes Specifications ...................................................................................................................................... 25
Troubleshooting Guide ....................................................................................................................................... 26
Result Window Messages ..................................................................................................................................... 26
Degraded Mode ..................................................................................................................................................... 28
Contents
Page 13
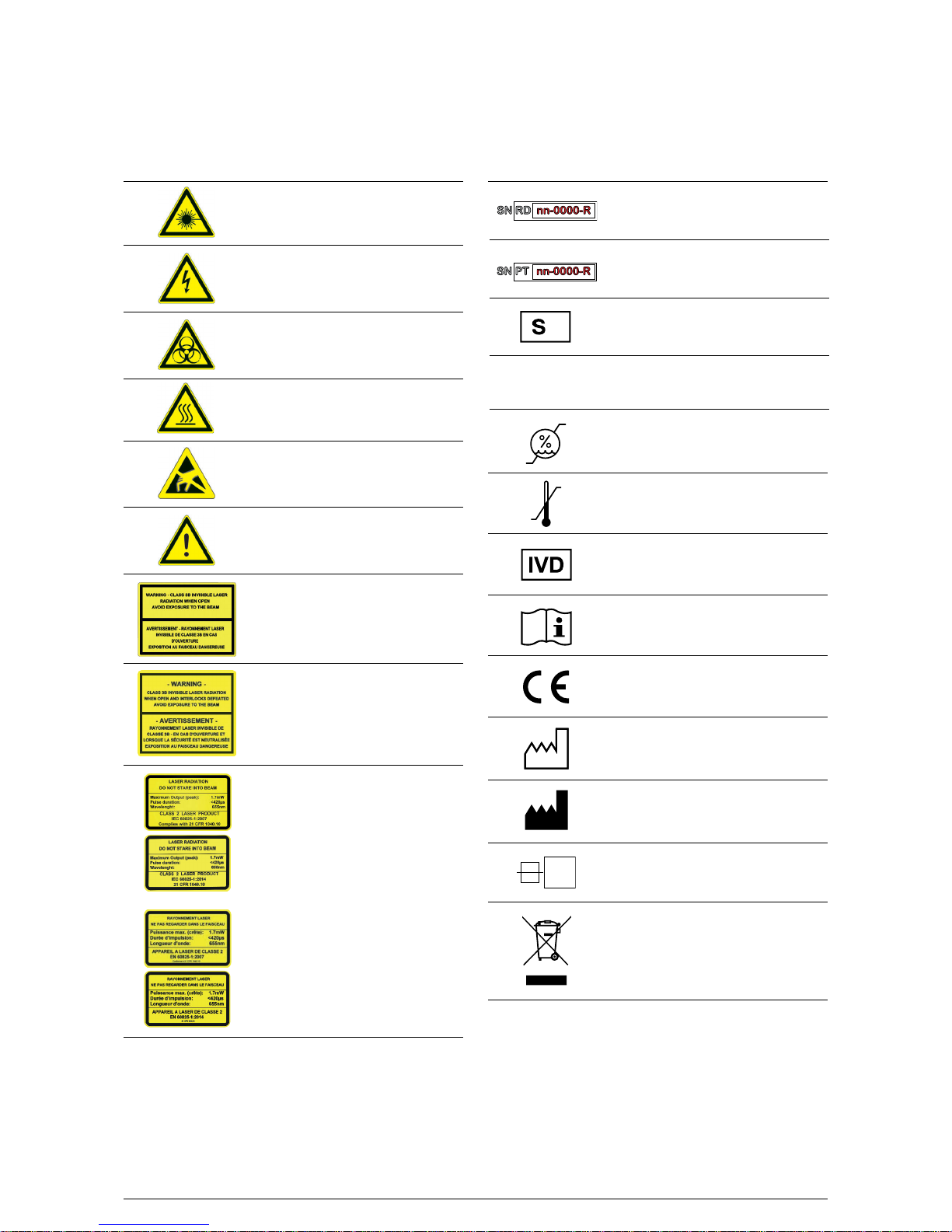
B·R·A·H·M·S GmbH
B·R·A·H·M·S KRYPTOR compact PLUS User Manual (Version R08en) Page 4 of 30
Labelling
Symbol Explanation
Laser, danger to eyes
Location: on fluidic hood,
on barcode readers
Electrical risk
Location: high voltage power supply,
inside electronic boxes
Biohazard
Location: waste bottle plug, wash station,
waste pump, clot detection board
Thermal risk
Location: reaction area entrance
Electrostaticsensitive part
Location: inside electronic boxes
User hazard
Location: on sample and cooling trays
Caution - Class 3B invisible laser
radiation when open.
Avoid exposure to the beam
Location: on reader head shielding
Caution - Class 3B invisible laser
radiation when open and
interlocks defeated.
Avoid exposure to the beam
Location: on side panel, access to the laser
Laser light
Do not stare into the beam.
Class 2 laser product
Location: on fluidic hood
Symbol Explanation
Serial number
of Reading Module
Serial number
of Pipetting Module
Identification
of the machine
Alternative current (AC)
Humidity range
Temperature range
Medical device conforms to
IVD Directive 98/79/EC
Consult instructions for use
Medical device is CE certified
to conform with the
IVD Directive
Date of manufacturing
Name and address of the
Manufacturer
Protective nominal value
(Rating of electrical fuse)
Electric and electronic equipments
have to be selectively collected
under the manufacturer
respons ibility B·R·A·H·M·S GmbH
V~
T 5A
+18 °C
+30 °C
N
20 %
85 %
Page 14

B·R·A·H·M·S GmbH
B·R·A·H·M·S KRYPTOR compact PLUS User Manual (Version R08en) Page 5 of 30
Symbol Explanation
Product reference number
of the assembly
Barcode that provides UDI
information according to FDA
regulations
Federal law restricts this device
to sale by or on the order of a
licensed healthcare practitioner
(applicable to USA classification
only)
cTUVus logo
106172
USA: Rx only
Labelling of
B.R.A.H.M.S KRYPTOR compact SOLUTIONS
SGH 05 Corrosion
SGH 07 Exclamation Mark
Page 15

B·R·A·H·M·S GmbH
B·R·A·H·M·S KRYPTOR compact PLUS User Manual (Version R08en) Page 6 of 30
The safety instructions of the User Manual allow the
user to avoid injury to persons, material damages and
environmental contamination.
It is mandatory for users of Thermo Scientific™
B.R.A.H.M.S™ KRYPTOR™ compact PLUS to pay
particular attention to SAFETY INSTRUC TIONS written
in the B.R.A.H.M.S KRYPTOR compact PLUS Manual.
Installation of the B.R.A.H.M.S KRYPTOR compact
PLUS can only be performed by a properly trained service engineer. At the time of installation all performance specifications will be verified. Any attempt to
install, repair or modify the instrument by unauthorized
personnel is not allowed.
The system has to be maintained every 6 months by
an authorized and qualified engineer following the
procedures described in the service manual that is
provided only to the qualified engineers.
This in vitro device must only be used by qualified and
trained personnel according to GMP guide lines. Local
health and safety regulations must also be taken into
account.
Reading and interpretation of results must be done by
a qualified user.
B.R.A.H.M.S KRYPTOR compact PLUS must only be used
with materials, equipment and accessories specified in the
B.R.A.H.M.S KRYPTOR compact PLUS Manual.
B.R.A.H.M.S KRYPTOR compact PLUS analyser is a
Class 2 laser product.
Caution: Use of controls or adjustments or performing
any procedures other than those specified herein may
result in hazardous radiation exposure.
Precaution for installation
Indoor use only
Altitude Up to 2000 m
Temperature range 18
…30 °C
Humidity 20-85% (non condensing)
Corrosion Protection against 1N HCl,
1N NaOH, decontamination
solution, bleached reagent
Electrical mains 100-240 V~, mains supply
voltage fluctuations up to
± 10% of the nominal voltage
50-60 Hz
Power 465 VA
Transient overvoltage: category II
Placement Clearance distance:
Behind: 5 cm
Side-left: 10 cm
Side-right: 20 cm
Disconnecting device should be
accessible.
Transport conditions -20
…70 °C
Long term storage 0
…50 °C
Input and output connections
Caution:
On the serial communication ports, connect only a
RS-232 link (Very low safety voltage).
On the USB port, connect only the specific USB cable
provided (Very low safety voltage).
On Waste bottle cap (liquid level and mechanical
sensor), connect only the specific cable provided (Very
low safety voltage).
Electromagnetic compatibility
Changes or modifications not expressly approved by
B·R·A·H·M·S GmbH could void the user’s authority to
operate the equipment.
B·R·A·H·M·S KRYPTOR compact PLUS is compliant
with class B product requirements as defined in IEC
61326-2-6 standard.
B·R·A·H·M·S KRYPTOR compact PLUS complies with
the emission and immunity requirements describ ed
in EN 61326-2-6. The electromagnetic environment
should be evaluated prior to operation of the device.
Do not use this device in close proximity to sources
of strong electromagnetic radiation (e.g. unshielded
intentional RF sources), as these may interfere with
the proper operation.
Electrical security
Do not connect B.R.A.H.M.S KRYPTOR compact PLUS
to a power supply before ensuring that the voltage
setting is correct.
The analyzer can be used with a power supply (mains)
voltage of 100-240 V~ (50-60 Hz). Verify the voltage of
the local power supply (mains) to be used. Always plug
the analyzer into a grounded outlet. Operating tech ni cians and maintenance personnel are urged to follow
sound electrical safety practices at all times.
Although all metal parts of the analyzer are at ground
potential (zero voltage), they should never be touched
with one hand while touching a plumbing fixture, radiator, AC-operated device or other grounded object with
the other.
Before opening the analyzer, remove the power cable
from the power outlet. Do not replace components or
attempt any repairs with the analyzer switched on.
Do not operate the analyzer in an atmosphere contain ing explosive gases since components of the analyzer
could generate sparks.
Avoid spilling fluid on or into the analyzer at any time.
All spills should be wiped up promptly.
If the equipment is used in a manner not specified by
Safety Instructions
Page 16

B·R·A·H·M·S GmbH
B·R·A·H·M·S KRYPTOR compact PLUS User Manual (Version R08en) Page 7 of 30
B·R·A·H·M·S GmbH, the protection provided by the
equipment may be impaired.
Biohazardous
When working with human serum, controls and calibrators all accessible parts of the analyzer must be
consi dered as biohazardous. The pipette tip, sample
cassettes, reagent cassettes, carousel drip pan, and
analyzer deck should be routinely disinfected.
Concerning all areas exposed to patient potential
infectious material or to user contact follow the clean ing requirements and use 5% Hypochloride solution
for decontamination. (See B.R.A.H.M.S KRYPTOR compact PLUS Manual, chapter maintenance.)
It is strongly recommended to wear gloves and a
special coat.
Waste
The reagents and the waste must disposed of as
potential infectious laboratory waste according to local
regulations.
Dilution plate, reaction plate
Before disposing of the dilution plate as well as the
reaction plate stick the adhesive cover film with
bio hazard sign on the plate. The adhesive covers are
supplied with the plates.
B.R.A.H.M.S KRYPTOR compact SOLUTIONS 1 to 4
For detailed information read the safety instructions for
use inserts.
Safety data sheets are available at International Product
Support on request.
Laser
Concerning the sample carousel barcode reader (Class
2 laser) and the manual barcode reader do not look at
the laser. Avoid direct eye exposure.
Class 2 lasers are limited to a maximum output power
of 1 milliwatt and the beam must have a wavelength
be tween 400 and 700 nm. A person receiving an eye
exposure from a Class 2 laser beam, either accidentally
or as a result of someone else’s deliberate action (mis use) will be protected from injury by their own natural
aversion response. This is a natural involuntary response that causes the individual to blink and avert their
head thereby terminating the eye exposure. Repeated,
deliberate exposure to the laser beam may not be safe.
The laser inside the instrument (Class 3B laser) emits
an invisible radiation whose characteristics are the following:
Laser Type LTB SRS
Beam Deviation (mrad) 3*3 5*8
Pulse Length (ns) 2.5 <3.5
Max Peak Power (kW) 100 45
Repetition Rate (Hz) 20 20
Class 3B lasers may have sufficient power to cause an
eye injury, both from the direct beam and from reflections. The higher the output power of the device the
greater the risk of injury. Class 3B lasers are therefore
considered hazardous to the eye.
The laser is only accessible by opening the instrument
cover. The instrument cover (side access panel) is
equipp ed with a safety interlock, which stops laser
beam im me dia tely.
For further information see latest version of
B.R.A.H.M.S KRYPTOR compact PLUS Manual and
latest version of B.R.A.H.M.S KRYPTOR instructions for use for reagents and consumables.
Page 17
B·R·A·H·M·S GmbH
B·R·A·H·M·S KRYPTOR compact PLUS User Manual (Version R08en) Page 8 of 30
Intended use
B.R.A.H.M.S KRYPTOR compact PLUS is a fully automated system for in vitro diagnostic. B.R.A.H.M.S
KRYPTOR compact is an autoanalyser which can
perform biochemical investigations.
It is used for measurement of patient samples in
random access mode routinely.
B.R.A.H.M.S KRYPTOR compact PLUS is a closed
system and can only operate utilising special reagents
offered by B·R·A·H·M·S GmbH. The system is based
on TRACE technology (Time-Resolved Amplified Cryptate Emission). The system and the reagents are under
con tinuous develop ment by B·R·A·H·M·S GmbH.
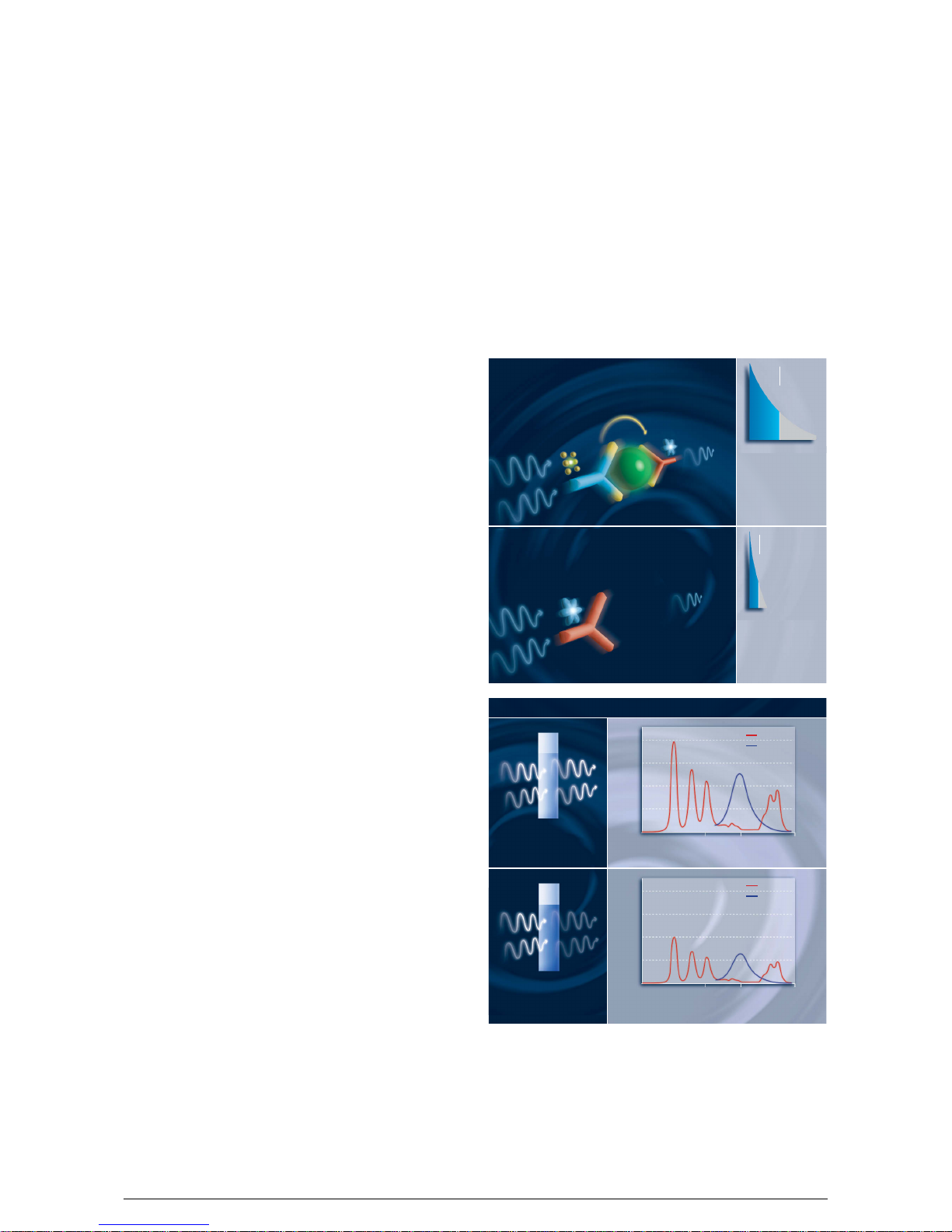
TRACE – the unique measuring principle
of B.R.A.H.M.S KRYPTOR compact PLUS
The measurement principle of B.R.A.H.M.S KRYPTOR/
KRYPTOR compact is based on TRACE technology,
which measures the signal that is emitted from an
immuno complex with time delay.
The basis of the TRACE technology is non-radiative
energy transfer from a donor to an acceptor.
The proximity of donor and acceptor when they are
part of an immunocomplex and the spectral overlap
between donor emission and acceptor absorption
spectra on the one hand, intensify the fluorescent
signal of the cryptate and on the other hand they
extend the life span of the acceptor signal, permitting
the measure ment of temporally delayed fluorescence.
Precise measurement of analyte concentration
When the sample is excited with a nitrogen laser at
337 nm, the donor emits a long-life fluorescent signal
in the milli-second range at 620 nm, while the acceptor
generates a short-life signal in the nanosecond-range at
665 nm or 707 nm depending on the type of acceptor.
When the two components are bound in an immunocomplex, both the signal amplifi cation and the prolong ed life span of the acceptor signal occur at 665 nm or
707 nm, so that it can be measured over µ-seconds.
This long-life signal is proportional to the concentration
of the analyte to be measured.
Introduction
Reliable prevention of interference
Non-specific signals are eliminated by the internally
calc ulated ratio of the intensities at these wavelengths
(665/620 or 707/620).
The signal generated by the cryptate at 620 nm serves
as an internal reference and is measured simul t ane ously with the long-life acceptor signal at 665 nm or
707 nm. Interfering influences, e.g. from turbid sera,
are automatically corrected.
Amplification
by energy
transferDonor
Bound
acceptor
Unbound acceptor
Life span of the
bound acceptor
signal / time-resolved
Life span of the
unbound acceptor signal
Unbound acceptor
Immunocomplex
Excitation
of the sample
at 337 nm
Excitation
of the sample
at 337 nm
10 nsec
µsec
Long-life
acceptor signal
at 665 or 707 nm
Time in µsec
Time in nsec
Intensity
Short-life
acceptor signal
at 665 or 707 nm
Intensity
0.5
620 665 λnm
1
Signal relation 665/620 = 1
Fluorescence intensity
Donor
Acceptor
0.5
620 665 λnm
1
Signal relation 665/620 = 1
Fluorescence intensity
Donor
Acceptor
T = 100%
T = 50%
Internal correction of interfering influences
Page 18
B·R·A·H·M·S GmbH
B·R·A·H·M·S KRYPTOR compact PLUS User Manual (Version R08en) Page 9 of 30
2
3
4
1
Instrument
Components
presence. Levels of buffer and distilled water are
managed by float switches within the main body of
the instrument. When the bottle becomes red on the
VDU screen, it is possible to refill the bottles without
interruption to the current run. There is no fluid sensor
on these two bottles.
Carousel
The carousel is di vi d ed into 5 posi tions.
Positions 1, 2 and 3
are hybrid locations
which allow to put
reagent or sample
trays. Positions 4
and 5 are dedicated
only for sample trays. When the hood is open, it is
possible to turn the carousel by hand to simplify
access to each position.
3
This instrument has two parts: pipetting module
and reading module.
Pipetting Module
This part comprised a pipetting unit , fluidic system
and carrousel .
Pipetting unit
The pipetting unit consists of a heated tip sample
probe and a wash station. The heated tip uses a
capacitive liquid level detection system to aspirate
all components required to perform the biological
analysis. This includes the sample, diluent and
reagents. After aspirating the sample and reagent
components into the tip, the tip is heated to reaction temperature and acts as a pre-reaction incubator. This ensures that the fluids are at reaction temperature before dispensing in to the reaction area.
At the end of dispense, the tip is washed to avoid
carry-over contamination between samples.
Caution, any fluidic hood opening stops tip motion.
Fluidic system
The fluidic system includes 3 bottles of 5 liters.
One for the PBS wash liquid (BUFFER; order code for
the buffer: B.R.A.H.M.S KRYPTOR BUFFER) used to
wash the tubing system after pipetting. A second
bottle with distilled or deminerialized water used as
liquid system (DISTILLED WATER) by priming before
pipetting and to keep the tubing line in water during
instrument stand by or switch off. A third bottle is
used to collect liquid waste (WASTE). Only the waste
bottle contains a float sensor on the cap to manage
the liquid level and a mechanical sensor for bottle
2
1
2 3
1
Distilled or demineralized
water tank with floating
switch
PBS Tank with floating
switch
Page 19

B·R·A·H·M·S GmbH
B·R·A·H·M·S KRYPTOR compact PLUS User Manual (Version R08en) Page 10 of 30
SOLUTIONS 1, 2, 3 and 4 (order code B.R.A.H.M.S
KRYPTOR compact SOLUTION 1-4)
The SOLUTIONS 1 and 2 are used for reconstitution
of freeze dried reagents. The SOLUTIONS 3 and 4 are
needed for different washing steps between pipetting reagents and samples. Their use depends on the
analytes. Do not top-up the bottles. Each solution
bottle is managed on the instrument by the software
through a barcode placed on the top of the bottle.
SOLUTIONS 1 and 2 are mandatory to launch reconstitution of reagent kits. SOLUTIONS 3 and 4 are
mandatory to launch pipetting sequence for samples.
DILUTION PLATES (order code B.R.A.H.M.S
KRYPTOR compact DILCUP)
The dilution plate, which consists of 24 wells, is used
by the system to reduce a high concentration or make
a recovery test by using a diluent or a specific reagent.
A barcode placed on the convex side of the plate,
allows the software to monitor the availability of wells
and lifetime on board the instrument.
Reading Module
Power on or off of the complete instrument is done
with the button O/ I placed on right side of reading
module. This module consists of the reaction area
and optical reading system to measure the signal
emitted by the immuno complex. The reaction
plate contains 96 wells (order code B.R.A.H.M.S
KRYPTOR compact REACT) and its life on board the
instrument is moni tored by the software through a
barcode placed on each reaction plate and transparent damper closing. The reaction plate is heated at
37 °C ± 0.5 °C.
Handheld barcode reader
All relevant information concerning calibrators, controls, reagent lots and reaction plate barcodes are
registered on B.R.A.H.M.S KRYPTOR compact PLUS
by the barcode reader as well as reaction plate barcode.
5
4
Reagents tray
This accessory contains up
to 4 reagent kits. The cool ing system (2...8 °C) is operational only when the tray
is installed on the carousel
and the ins trument is turned
on. An infrared system is
used to detect reagent segments during movement of the carousel. Barcoded
identification of reagent kits is automatically scanned
when the carousel rotates. Caution: Put the kit in
right order, barcode must be visible within the perspex window (refer to photo). Inventory for all
reagent kits and reagent segment temperature is
fully managed by the software with use of colour
codes.
Tray for samples, dilution plates and
SOLUTIONS 1-4
This tray has space for 16
sample tubes which can be
any combi nation of primary
or second ary tubes at a
height between 60 mm and
120 mm and a diameter
between 11 mm and 17 mm.
Calibrators, con trols and mi cro- cups in a specific metallic adaptor can also be
installed. This tray also has space to install SOLU TIONS 1-4 or dilution plates. Sample trays are
detected on board by barcode identific ation placed
on the top and the middle of the segment.
5
Page 20

B·R·A·H·M·S GmbH
B·R·A·H·M·S KRYPTOR compact PLUS User Manual (Version R08en) Page 11 of 30
Description of the System Status Window
ACCESS TO MAIN MENUS
B.R.A.H.M.S KRYPTOR compact PLUS
TOOL BAR
WINDOWS COMMAND BAR
WINDOWS START BUTTON
WINDOWS TASK BAR
STATUS BAR
B.R.A.H.M.S KRYPTOR compact PLUS
WORK SURFACE
7
2
3
4
5
6
1
Waste
Distilled or demineralized water: Liquid system
Buffer PBS: Phosphate Buffer Saline: Wash
liquid
B.R.A.H.M.S KRYPTOR compact
Wash Solutions
Dilution plates
Sample tray
Reagents tray
Reaction plate
8
7
6
5
4
3
2
1
1
2
3
4
5
6
7
1
2
3
4
6
5
7
8
Page 21

B·R·A·H·M·S GmbH
B·R·A·H·M·S KRYPTOR compact PLUS User Manual (Version R08en) Page 12 of 30
Main Menus
SYSTEM
* Logon
* Shutdown
* Maintenance
* Maintenance Log
* Session Log
* Service Diagnostics
* Printer Setup
* Close
DATA
* Worklist
* Results
* QC
Functions
* Work
Analysis
INSTRUMENT
* Rescan Carousel
* Start Processing
* Pause Processing
* Query All
* Prime
* Reconstitute Kits
* Reaction Plate
* Sample Carousel
* Reagents
REGISTRATION
* Calibrator/Standard
* Control
* Reagent Lot
ADMINISTRATION
* Panels
* Analytes
* Combined Analytes
* Reflex Testing
* User Accounts
* Instrument Params
* LIS Interface
* Preferences
HELP
* User Manual
* About
These menus are available by clicking on:
The main menus bar appears
To close it, click again on:
Page 22

B·R·A·H·M·S GmbH
B·R·A·H·M·S KRYPTOR compact PLUS User Manual (Version R08en) Page 13 of 30
The Tool Palette
ICON COMMAND
Scan Carousel
Start Processing
Pause Processing
Manage
Worklist
Validate
Results
QC Functions
Reagent Lot
Manager
Calibrator Lot
Manager
Prime Instrument
Reconstitute
Reagents
Advanced Menu
User Manual
Load/unload the
Reaction plate
System Logon
procedures
System Shutdown
procedures
FUNCTION
F1
F2
F3
F5
F6
F7
F8
F9
F10
F11
MENU OPTION
Instrument
Rescan Carousel
Instrument
Start Processing
Instrument
Pause Processing
Data
Worklist
Data
Results
Data
QC Functions
Registration
Reagent Lot
Registration
Calibrator/Standard
Instrument
Prime
Instrument
Reconstitute Kits
Help
Instrument
Reaction Plate
System
Logon
System
Shutdown
DESCRIPTION
Identification of samples without starting a run
This icon disappears after click on it to give access
to Pause processing.
This icon appears after Processing has started.
Addition of more samples to the carousel.
If you push the blue button on the front of the
instrument, at the end of pause, carousel hood is
open automatically.
If you use this icon, at the end of pause it is needed
to click on the blue button to open the hood.
To close it, click again on the icon.
Allow to load reaction plate by plate ID manual entry.
Allow to unload reaction plate.
Page 23

B·R·A·H·M·S GmbH
B·R·A·H·M·S KRYPTOR compact PLUS User Manual (Version R08en) Page 14 of 30
COLOUR EXPLANATION
Black: no sample present
Dark green: the sample is ready to be tested
Dark green with a white point: sample during pipetting process
Dark green with a red circle: STAT sample
Blue: sample to be removed (all the tests of this sample have processed successfully including
the out of range detection)
Yellow: sample with barcode without test in worklist or presence on carousel of same ID on different sample tube
Red: problem with sample processing
(e.g. reagent related, consumables related; clot, insufficient sample volume)
Red with a white point: sample during pipetting process with at least one test with problem
Work Surface Colour Codes
Sample Carousel
At the end of carousel scan, all samples with a barcode and tests in the worklist will be shown in green, blue, yellow or
red. If the system is connected to LIS, there are different colours for the sample status (see Administration Menu/
LIS interface in B.R.A.H.M.S KRYPTOR compact PLUS Manual).
An information window is available by clicking with the left button of the mouse on a selected sample.
In case of sample tubes without barcodes, it is possible to identify a location in the sample tray by manual entry
through the Carousel Status window, or the worklist.
Double click on sample tube to open Carousel Status window.
Page 24

B·R·A·H·M·S GmbH
B·R·A·H·M·S KRYPTOR compact PLUS User Manual (Version R08en) Page 15 of 30
Reagent Area
Each reagent cassette has its own identification. An information area appears by clicking (left button of the mouse)
on reagent tray screen image, this shows cassette ID and temperature in degrees of the kit.
A colour code on the circle portion exists as a quick visual check of the right temperature in the reagent area or a
reagent cassette’s fan fault.
All reagent kits detected by barcode are shown on screen in the appropriate reagent cassette. A more detailed information Window is available by clicking (left button of the mouse) on a reagent kit.
The colour of the background indicates the availability of the kits and the colour of the rectangle indicates the number
of remaining tests.
Double clicking anywhere in the reagent area opens the Reagent Status window. This window enables you to
display information about each reagent kit installed in the reagent area of the instrument and to request calibration
for each reagent kit.
TEMPERATURE BAR ON THE REAGENT AREA
Green: 2.0…8.0 °C
Blue: < 2.0 °C
Red: > 8.0 °C or communication failed
Yellow: one or more reagent cassette’s fan is out of order
COLOUR
White background: Kit OK (calibration and reconstitution OK)
Red rectangle: Number of tests remaining: ≤ 5
White background: Kit OK (calibration and reconstitution OK)
Yellow rectangle: Number of tests remaining: > 5 – ≤ 10
White background: Kit OK (calibration and reconstitution OK)
Green rectangle: Number of tests remaining: > 10
Yellow background: Kit or calibration expires tomorrow
Green rectangle: Number of tests remaining: > 10
Red background: Kit to reconstitute, to calibrate or expired kit or no test remaining
COLOUR
EXPLANATION
Page 25

B·R·A·H·M·S GmbH
B·R·A·H·M·S KRYPTOR compact PLUS User Manual (Version R08en) Page 16 of 30
Reaction Area
Each reaction plate is barcode labelled to allow the loading of the plate. The reaction plate has to be loaded to
activate its preheating and to allow the dispensing of samples.
Click on the plate to view the exact number of tests dispensed and completed. When the reaction plate is full,
there is no automatic unloading, it is necessary to request by clicking on reaction plate. A reaction plate not used
but unloaded could be loaded again during 2 hours after its first loading.
There is no automatic shutdown (machine remains in ready stage). As long as the reaction plate is on board, it is
ready to be used, until 7 days are passed or plate is full. After 7 days, the plate is automatically unloaded at the
next end of day (first ready or initialized stage after midnight).
Time validity status of reaction plate is available when clicking with the left mouse button on the reaction plate on
the screen. If there is an “xy position error”, the plate is automatically unloaded, and cannot be used any more.
Pipetting sequence does not start if the reaction area temperature is below to 35.7 °C or over 38.5 °C.
Dilution Plate
Each dilution plate is barcode labelled to manage the wells used and the shelf life on board (365 days). Check
dilution plate cleanness during daily maintenance.
COLOUR
EXPLANATION
White well = available well / coloured well = used well
Green: More than 10 wells available
Yellow: From 5 to 10 wells available
Red: Less than 5 wells available or expired
COLOUR EXPLANATION
Green: Proportion of tests running and not completed
Blue: Proportion of tests completed
Green: 36.2…38.0 °C
Blue: < 36.2 °C
Red: > 38.2 °C
Page 26

B·R·A·H·M·S GmbH
B·R·A·H·M·S KRYPTOR compact PLUS User Manual (Version R08en) Page 17 of 30
B.R.A.H.M.S KRYPTOR compact SOLUTIONS 1 to 4
For SOLUTIONS 1 and 2, the dead volume has been defined to finish a reconstitution started even if red level is
detec t ed; it is normal to have around 12 mL in SOLUTION 1 and approximately 8 mL in SOLUTION 2 when red
status appears on the interface screen. For SOLUTIONS 3 and 4 the dead volume is around 4 mL, defined to finish
wash step.
COLOUR
EXPLANATION
Green: OK
Yellow: Take care to change the bottle soon / solution expires tomorrow
Red: Bottle to be replaced = dead volume reached or solution expired
Fluidic System
An intermediate tank system enables you to refill B.R.A.H.M.S KRYPTOR BUFFER or distilled/demineralized water
bottle when red status is detected without stopping the current run by use of dead volume in intermediate tank
system. If the dead volume is used then waste red level is detected and the instrument stops at the end of current
run or prime. A prime is mandatory after refilling a bottle.
COLOUR EXPLANATION
Green: OK
Red: Waste bottle full (void), buffer or water bottle empty (filling), intermediate waste collector full (Call Hot Line)
Page 27

B·R·A·H·M·S GmbH
B·R·A·H·M·S KRYPTOR compact PLUS User Manual (Version R08en) Page 18 of 30
Routine Work
Start-up
Fill the appropriate bottles with distilled or demineralized water and buffer PBS, empty the waste bottle.
Ensure B.R.A.H.M.S KRYPTOR compact SOLU TIONS 1
to 4 are on board. If B.R.A.H.M.S KRYPTOR compact
SOLUTIONS 1 to 4 are closed, open the caps. Put caps
into specific loca tions and inscribe the caps with
number of solution.
Check the fluidic and carousel hoods are closed.
After a power off
- Check if carousel hood is closed.
- Switch on the instrument with O/I (ON/OFF) button
found on right side of the instrument.
- Switch on the XPC, monitor, printer.
- Click on Windows Start button and choose
icon to launch the B.R.A.H.M.S
KRYPTOR compact software.
2 programs will be start ed:
1) XPC program is the user interface.
2) Check if XIPC program is active by icon presence on
clock bar.
After a Shutdown start here
- Check if carousel hood is closed.
- Click on
- Select Name and Password (e.g. user: Admin and
pass word: Admin) in the System LogOn window.
- An automatic scan is launched after motor initialization. System is ready to use at the end of scan.
- An automatic scan is done every two minutes when
carousel hood is closed to manage reagent tray
tempera ture.
- Do maintenances as requested.
- Place a new reaction plate, close the transparent
damper and load it by scanning the barcode.
- Request carousel hood opening by pressing on the
blue button in front of carousel.
- If there is no reagent cassette, load one or more on
specific positions.
- Check status of dilution plate and B.R.A.H.M.S
KRYPTOR compact SOLUTIONS 1 to 4 on interface,
according to necessity, change or load new consum ables.
- Load kits on reagent tray when temperature status is
green.
- Ensure the reagent lid is well closed.
After an automatic change of day
The automatic change of day procedure is launched every
day, on the first ready or intialized stage after midnight.
This will perform automatically all the initializations (init
pipetor + init reader).
Nevertheless, the user will have a logon to perform
manual maintenances and database maintenance.
User is warned that he has to logon with the message
“KRYPTOR compact – Change of day made – Maintenance needed” in the title of the window.
User can go on using the analyser, without logon, in
case of use by night team, who wants that maintenance is performed by “morning team”.
Maintenance
Items on the maintenance screens only need to be per formed if they are marked *Expired*.
(!) These procedures will be performed automatically
when the system has been started.
Daily maintenance
- Initialize pipettor (!). To be done only if problems
occur during run on pipettor module.
- Initialize reader (!). To be done only if problems
occur during run on reader module.
- Prime Liquid Handling System (!). Check visually
the absence of leaks and bubbles during the priming
procedure.
- Check and clean reader head window by using a
cotton-swab first with water then with alcohol.
- Check and clean condensation in Reagent cassettes
with absorbent paper.
- LIS end of day. This action enables to purge LIS files
and avoid the occurrence of data transmission problems.
- Check dilution plate cleanness. Look at dilution
plate already on board if there is no dirty particles in
dilution well.
Weekly maintenance
- Check for liquid leaks. Open fluidic hood to check
the tubing system (tubing connectors, syringes).
When fluidic hood is closed, pipettor initialization is
automatically done.
- Tip path cleaning with decontamination solution
to clean up serum splashes with a paper towel
slightly wet with water, then repeat with alcohol or
specific product to decontaminate.
- Backup DataBases. Backup of all databases older
than 3 days.
Page 28

B·R·A·H·M·S GmbH
B·R·A·H·M·S KRYPTOR compact PLUS User Manual (Version R08en) Page 19 of 30
- Backup Log Files. Backup of log of run and main tenance log files.
- Remove messages from box office. This action
enables the purge of the box office and avoid the
occurrence of data transmission problems.
- Cleaning of water bottle. Empty and replace dis tilled/
demineralized water if it is more than seven days old.
- Clean carousel pan. Clean the tray under the sample
carousel with a paper towel slightly wet with water,
then repeat with alcohol or specific product to decontaminate.
Monthly maintenance
- Bottles decontamination. Empty the bottles and
pour in 1 liter of 5% sodium hypochlorite solution. Fit
a bottle cap and swirl the liquid inside the bottle so
that it comes into contact with all the internal sur faces. Allow to stand for 15 minutes, then empty and
rinse the bottle with water.
- Secure tip cleaning. Tip parking over night or if instru-
ment is not in use for a longer time. B.R.A.H.M.S
KRYPTOR compact SOLUTION 3 and 4 must be ava il able.
- Automatic check dot. Tip coming on dot point. If
adjustment is correct, tip coming back to wash cup.
In case of adjustment problem, user should open the
fluidic hood and check if tip can be slightly bent to
enter the hole.
Reagent registration
For the following working steps installation of valid
version of K-DISK ANA is needed.
Necessary when using new lots only:
Reagent
- Click on Reagent Lot Manager:
- Click on Register.
- Scan the barcode sheet.
- Click on OK and confirm the registration with Yes.
Calibrator
- Click on Calibrator/Standard Manager:
- Click on Register.
- Scan the barcode sheet.
- Click on OK and confirm the registration with Yes.
Control
- Click on:
- Click on:
- Click on Register new Controls.
- Scan the barcode sheet.
- Click on OK.
Reconstitution of reagents
- Click on to start reconstitution process.
- All freeze dried reagent kits placed in the instrument
will be reconstituted.
- In the status bar the progression of the reconstitution
in % is visible.
Perform a calibration
Calibrator
- Double click on the kit you want to calibrate.
- Select a calibrator lot from the drop-down arrow list.
- Click on Add to worklist, OK, Close.
- Request a carousel hood opening by pressing on blue
button in front of instrument.
- Place the calibrator tube on the sample car ousel making sure the barcode fills the slot.
- Close the carousel hood.
- Start the instrument:
Results
- Open results list:
- Select the calibrator result and click on Validate curve.
- The software indicates if the new calibration curve is
acceptable or not:
if Yes click on:
if No click on:
Page 29

Run controls
Control
Adding a Control through Worklist
- Select Add control in the Data, Worklist:
- Select a control from the list, press Add to worklist,
Close, Close.
Adding a Control to be tested on specific reagent
kit
- Double click on the kit which should be used to measure
the controls.
- Select Control lot from the drop-down arrow list.
- Select Low volume and/or Stat status for this control.
- Click on Add to worklist, OK, Close.
- Request a carousel hood opening by pressing on blue
button in front of instrument.
- Place the control tube on the sample carousel making sure the barcode fills the slot.
- Close the carousel hood.
- Start the instrument:
Results
- Open results list:
- If result is in “needs resolution” status related to concentration out of 2SD range (shown in red) or with
flag, check the information and validate, reject or
relaunch the test. Accept ing the control automatically
sends the result to the QC program.
- Select the B.R.A.H.M.S KRYPTOR compact Quality
control program to follow control life.
Run patient samples
with barcode
Patient Samples with barcode
- Select Add sample from Data, Worklist:
- Enter the Sample ID. Cassette ID and Tube position
are mandatory in case of sample without barcode.
- Select tests and dilutions from the displayed list, Add.
- Click on Stat to pipette a sample in priority.
- Click on Low volume sample vial if sample is in
microcup.
- Select Save sample and continue with required
samples, Close.
- Request a carousel hood opening by pressing on blue
button in front of carousel part.
- Place sample tubes on the carousel making
sure the barcode fills the slot.
- Close the carousel hood.
- Start the instrument:
Results
- Open results list:
- Select a test.
- It is possible to Cancel tests in detecting, or counting
step or in pending, only in “Ready/…” status.
- Results needing resolution can be: Accepted, Re-run
or Rejected.
- Select the results then Print Status or Print Report
to print results.
End of day
Shutdown (stand by modus)
- Check inventory of reagents on board for tomorrow’s
workload.
- Check the level of waste, distilled or demineralized
water and B.R.A.H.M.S KRYPTOR BUFFER.
- Check if the B.R.A.H.M.S KRYPTOR compact SOLUTION 3 and 4 is on board.
- Select and confirm with Yes.
- Unload the reaction plate and confirm the popup
window with OK.
- Close the transparent damper and confirm the popup
window with OK.
To power OFF the instrument
- Remove dilution plate(s), samples, and reagents.
- Close carousel hood.
- Select and confirm with Yes.
- Unload the reaction plate and confirm the popup window with OK.
- Close the transparent damper and confirm the popup
window with OK.
- Instrument is washing, wait for the completion of
steps (SOLUTION 3 and water washing) in case of
instrument power off.
- Close B.R.A.H.M.S KRYPTOR compact software:
- Close Windows by clicking on Start and Turn off.
- Switch OFF the PC, screen, printer and instrument.
B·R·A·H·M·S GmbH
B·R·A·H·M·S KRYPTOR compact PLUS User Manual (Version R08en) Page 20 of 30
Page 30

B·R·A·H·M·S GmbH
B·R·A·H·M·S KRYPTOR compact PLUS User Manual (Version R08en) Page 21 of 28
The assay of an analyte with a pre-incubation is composed of two phases:
Pre-incubation or Phase I: the system aspirates one
of the 2 conjugates and the antigen (sample). The
system dispenses the mixture into the reaction well
and the pre-incubation starts.
Phase II: the system aspirates the second conjugate
and dispenses it into the reaction well. At this moment
the Out of Range detection starts.
This leads to a specific operation of the interface
to avoid losing tests in Phase I distributed into the
reaction area.
The final test result will be achieved through Phase I
and Phase II using the same reagent kit.
To alert the user that a kit is in use for Phase I and is
required for Phase II, a padlock will appear on the user
interface at the edge of the reagent kit as shown
below. The kit should not be removed while the
padlock is displayed.
At the same time, a flashing red square will appear in
the top left corner of the screen.
The message behind the red square indicates the
estimated time in minutes and seconds remaining of
Phase I. There is also a number in parentheses to show
the number of tests in Phase I.
By reading this message the user can estimate the
time available to open and close the hood.
The padlock is removed when the reagent kit has no
tests remaining in Phase I, however the RED square
can remain active if other tests in Phase I are underway.
If the buffer bottle, water bottle, one of the solution
bottles or the waste bottle is detected in red status
during Phase I or Phase II, pipetting will stop and a
popup window on interface informs to replace the
solution. In Phase II the carousel hood will be opened
by the system software and a beeping sound will alert
the user.
A user request to open the carousel hood during Phase
I is subject to confirmation by the user via a message
appearing on the screen.
Clicking on the “Yes” button allows the hood to be
opened with a beeping sound to alert the user and a
new text message next to the red flashing square indicating the time available for the user to close the hood.
Clicking on “No” led to the abandonment of the application to open the carousel hood.
When closing the carousel hood, the text indicates
the estimated time in minutes and seconds before the
distribution of Phase II.
When closing the hood, a carousel scan is performed.
If a reagent kit containing a pre-incubation test was not
found on board of the instrument, the user is notified
via a message displayed on the interface:
If the kit is not returned before the start of Phase II, any
tests in progress with this kit will be declared failed. At
the results list, the user can view the status of a test.
Analytes with Pre-incubation
B·R·A·H·M·S GmbH
B·R·A·H·M·S KRYPTOR compact PLUS User Manual (Version R08en) Page 21 of 30
Page 31

B·R·A·H·M·S GmbH
B·R·A·H·M·S KRYPTOR compact PLUS User Manual (Version R08en) Page 22 of 30
During Phase I, tests appear in blue with the message
pre-incubating:
The start time of a test is given after the start of Phase II.
If the test is failed during Phase I, the test is indicated
in red with an alarm: “System error: Pre-incubation has
failed.”
Page 32

B·R·A·H·M·S GmbH
B·R·A·H·M·S KRYPTOR compact PLUS User Manual (Version R08en) Page 23 of 30
Detailed information about consumables and
patient samples
By clicking and holding the left mouse button an information window appears.
1. Buffer
2. SOLUTIONS 1-4
3. Dilution plates
4. Reagent cassette
5. Patient samples
6. Reaction plate
7. Reagent
Sample ID barcode
It is forbidden to use a sample barcode starting with “R“
or “_“. In these cases, problems occur on interface.
Manual entry of consumables and reagents
In case of barcode reading problem, it is possible to
load barcode information by manual entry with the keyboard. Click with the right mouse button on the corresponding area. A specific window for manual entry of
the barcode data will open.
When a consumable is loaded by manual entry, it is
marked by a symbol (not reaction plate) and on
session log.
If during a scan, a barcode is detected in place of
manual entry ID, scan reading has priority, ID consumable detected by scan erases manual entry ID.
This action is indicated in session log.
Interface drawing of tray positioning may differ from
actual tray position. For manual entry make sure that
place ments of reagent kits are done correctly as illustrated below.
Reagent kit placement
Check the kit’s correct position in
reagent tray with kit notch towards to
the transparent window. A kit mispositioning could impact data results.
Caution
Manual identification of consumables or sample
tubes is under user responsibility. Entering incorrect
data may lead to wrong result. This option is to be
used only in case of barcode read
i
ng fault while
waiting for repair of problem cause.
Important User Information
Notch
1
2
3
4
1
2
3
4
1
2
3
4
4
3
2
1
Page 33

B·R·A·H·M·S GmbH
B·R·A·H·M·S KRYPTOR compact PLUS User Manual (Version R08en) Page 24 of 30
Pipetting/ Reading sequence
During pipetting/ reading sequence it is forbidden to
close interface to avoid to lose samples results or to
have bad behavior of results list management.
Clot management
A clot management is available on the system during
aspiration step on sample and dilution. The user is
warn ed of clot presence by two kinds of information.
Case 1:
Clot has been released into the tube, tip has been
washed before continuing the pipetting sequence.
Sample tube with clot is in red status with tests not
pipetted in cancel status, and test with clot problem
with flag “System error: clot detected”.
User intervention should be done on tube (clot removal
or sample tube change) before to launching new tests.
Case 2:
Clot could not be released, tip is blocked. This popup
appears to inform that pipetting sequence is stopped
with carrousel hood open.
Tip cleaning after fatal clot detection
• Open fluidic hood, place tip on Zhome position.
• Clean point of tip.
• Unscrew tubing on tip, liquid must flow out into
wash bowl.
• Disconnect tip electronic board, Caution to not bend
the tip point.
• Pass through a fish line (dia 0.45 mm) into the tip
from the small end towards the tip board.
• Clean by blowing with a neutral gas through the
tube from the small end.
• Connect tip electronic board on specific location.
• Close fluidic hood.
• Close carousel hood.
• Check dot point throught monthly maintenance
“Automatic check dot”.
• Launch a prime.
Help
Some help is available, in three languages (English,
French and German), through button or Help/User
Manual menu.
Page 34

B·R·A·H·M·S GmbH
B·R·A·H·M·S KRYPTOR compact PLUS User Manual (Version R08en) Page 25 of 30
Tube positioning: The tube must be straight and the
bars must visible throughout the window width.
A: Good position
B-C: Bad position, the barcode reader may not be able
to scan the code
Paper quality: A paper having a too high ink absorption will give a blurred result and will decrease the
amount of good readings (this problem will have a
higher impact on barcodes having a low resolution).
Paper brightness: The barcode readers are very sens itive to the light reflection. Even though the barcode
reader is mechanically adjusted in order to limit the
light reflection a too glossy paper may affect the
reading performances of the codes.
Printing quality: The printer quality has an influence
on the reading performances. The best printers for
bar
c
odes are thermal printers (recommended for low
resolution barcodes) but most of the time a laser
printer is sufficient.
C
A: Printing quality is good.
Good contrast. Good resolution (accurate printing).
B: Printing quality is low.
The printer resolution is not good
enough. The contrast is not sufficient.
BA
B
Available symbologies on KRYPTOR instruments:
• Code 128
• Code 39
• Codabar
• Code 2/5 interleaved
• Code UPC/EAN
Other specifications:
• Minimum resolution: 0.21 mm
(size of narrowest bar (black or white bar))
• Minimum ratio = 2.5
(ratio of narrow bars to wide bars)
• Maximum ratio = 3.0
Silence section (blank section before and after the
code): at least 10 times the resolution (a silent section
before and after the code is mandatory for a good reading otherwise the code cannot be recognized).
Barcode positioning: We recommend to stick the
label 5 mm above the bottom of the tube (if the code
is long and the label is stuck too high the barcode reader may have some difficulties to read the bars located
at the top, specially if the resolution is low (thin bars)).
At least a few bars from the barcode must be in the
window formed by the tube holder: the window will
determine the barcode position within the cassette.
The code must not be stuck entirely above the tube
holder because there is a risk of misidentification in
this case (a tube in position 2 may be seen in position
3 for example).
A B
C
A: Barcode position is acceptable but it’s not the best
position.
B: Best barcode position.
C: This barcode position is forbidden. At least a few bars
must be in the tube holder window.
Barcodes Specifications
A
Page 35

B·R·A·H·M·S GmbH
B·R·A·H·M·S KRYPTOR compact PLUS User Manual (Version R08en) Page 26 of 30
Troubleshooting Guide
Result Window Messages
FLAG NO. RESULT WINDOW MESSAGE WHAT TO DO
0 “System Warning: Low Laser Power”
• Call the Hot Line
1 “System Error: Reaction Plate position”
• Request a reader initialization in Daily Maintenance or restart the system
completely.
• Unload the reaction plate, check that the reaction plate was correctly
loaded and load a new reaction plate.
• If the problem persists, call the Hot Line.
2 “System Warning: Reagent Cooler out
of Range”
• Adjust the room temperature within the specification (between 18 - 30°C).
• Check if a reagent cassette fan is out of order.
• If the problem persists, call the Hot Line.
3 “System Warning: Incubator Out
of Range”
• Stop the B·R·A·H·M·S KRYPTOR compact PLUS and wait a few minutes
before switching on the instrument.
• Adjust the room temperature within the specification (between 18 - 30°C).
• If the problem persists, call the Hot Line.
4 “System Error: Missed Reading”
• Call the Hot Line.
6 “Error: Insufficient Sample Volume”
Check if the trouble occurs on one or several samples tubes, cup or
calibrator vials.
If it concerns one tube:
• check the liquid level in the sample cup, the sample tube or the calibrator vial.
• check the tube positioning in the carrousel.
If it concerns several tubes:
• Call the Hot Line.
7 “System Error: Insufficient Reagent
Volume”
• Check the liquid level in reagent bottles.
• Call the Hot Line.
9 “System Warning: Late T0/TM”
• If the problem occurs more than once in a series, shut down, and then
restart the instrument after 1 or 2 minutes. If the problem persists,
call the Hot Line.
• If the problem occurs sporadically while a significant work list is loaded
on B·R·A·H·M·S KRYPTOR compact PLUS, the problem is not relevant.
It is then advisable to relaunch the sample displaying this message.
11 “System Warning: Late TE”
• If the problem occurs more than once in a series, shut down, and then
restart the instrument after 1 or 2 minutes. If the problem persists, launch
an intervention. If the problem persists, call the Hot Line.
• If the problem occurs sporadically while a significant work list is loaded
on B·R·A·H·M·S KRYPTOR compact PLUS, the problem is not relevant.
It is then advisable to relaunch the sample displaying this message.
12 “System Error: Pipetting”
• Check obstacle in the path (Cap on sample tube…).
Remark: In that case, the carousel hood is open and the run is interrupted.
• Check the liquid level in vial.
Remark: In that case, test is in pending status. If following popup appears
<Error: tip level sense baseline. Contact technical support>, the carousel
hood is open and the run is interrupted.
13 “System Error: Clot Detected”
• Clean the tip (fish line, Secure tip cleaning from the monthly maintenance).
• Check the sample and relaunch the test.
• If the trouble persists, call the Hot Line.
14 “System Error: Pipetting in Sample area”
• Check obstacle in the path (Cap…).
• Check the sample tube positioning.
15 “System Error: Pipetting in Dilution area”
• Check the dilution plate positioning.
16 “System Error: Pipetting in Reagent area”
• Check obstacle in the path (reagent area lid, aluminium foil…).
• Check the reagent kit positioning.
17 “System Error: Pipetting in Wash area”
• Check if the SOLUTION bottles are present, open and if sufficient liquid
remains.
• Check if there is any obstruction on the path.
• If the problem persists, call the Hot Line.
18 “System Error: Pipetting in Reaction area”
• Check if there is any obstruction in the path.
• If the problem persists, call the Hot Line.
Page 36

B·R·A·H·M·S GmbH
B·R·A·H·M·S KRYPTOR compact PLUS User Manual (Version R08en) Page 27 of 30
FLAG NO. RESULT WINDOW MESSAGE WHAT TO DO
19 “System Warning: Heated Tip
Out of Range”
• Prime the system to remove bubbles.
• If the problem persists, call the Hot Line.
20 “System Error: Interrupted”
• Reboot the system.
• If the problem persists, call the Hot Line.
21 “System Warning: Not Performed”
• Start testing or delete it from the work list.
24 “Check Results”
• The software is working properly. If the “Flag report” option in the combined
analytes is checked, any flag on the individual tests A1 and A2 generates a “Check
results” message on the combined result which makes it only acceptable manually.
25 “Math Error”
• Call the Hot Line.
26 “Reflex Test Launched”
• It is not a problem, this is an information only.
29 “Clot detected or insufficient
sample volume”
Message occurs when a clot is detected too close to sample’s zmax.
The current test will be flagged in red.
• Clean the tip (fish line, Secure tip cleaning from the monthly maintenance).
• Check the sample and relaunch the test.
• If the trouble persists, call the Hot Line.
30 “Data Error: Unknown Error”
• Call the Hot Line.
31 “Data Error: Ratio”
• Call the Hot Line.
32 “Data Error: Response”
• Call the Hot Line.
33 “Data Warning: Abnormal”
• Prime the system to remove bubbles.
• Check the samples for bubbles.
• Check sample and reaction plate for dust.
• Check if there are any bubbles in the reaction plate.
• If the problem persists, call the Hot Line.
34 “Data Warning: Out of Range”
• Automatic dilution is started depending on the Preferences configuration in the
Administration menu.
35 “Data Warning: Detection Limit”
• Result lower than the lowest detectable concentration.
36 “Data Error: Above max. Range”
• Relaunch the test manually with a small dilution factor (1/2 or 1/5).
38 “Data Warning: Calibrator Warning”
• Start a calibration not later than the following day.
39 “Data Warning: Below Normal”
• Information that sample is below the cut off.
40 “Data Warning: Above Normal”
• Information that sample is above the cut off.
42 “Concentration not Consistent
with Dilution Used”
• Relaunch the test with another dilution. Use a lower dilution factor.
43 “Sample Pipetting Problem -
Check Sample”
• Check the sample for bubbles or foam and relaunch the test.
44 “Data Warning: Abnormal Kinetics”
• Prime the system and relaunch the test.
• Check the samples for bubbles.
• Check sample and reaction plate for dust.
• Check if there are any bubbles in the reaction plate.
• If the problem persists, call the Hot Line.
45 “_Data Warning: Second Response
Used”
• Information only when logon as Service.
46 “_Data Warning: Short Lived
Fluorescence”
• Information only when logon as Service.
48 “Data Warning: Control out of
2 SD range”
• Repeat measurement with a new aliquot of the control.
• Recalibrate the reagent.
• Use a fresh reagent box and reconstitute a fresh control vial.
51 “Inconsistent incubation time”
• Check in the result list the time of “end of test” and “start of test”. If the real
incubation time = theoretical incubation time + <4 minutes => the result can be
accepted. If not check if system is disconnected.
• Close the KRYPTOR compact PLUS program or restart the computer.
52 “System Error: Missed flashes”
• Call the Hot Line.
69 “Pre-incubating”
• Preincubation test, awaiting Phase II incubation.
70 “System Error: Pre-incubation has
failed”
• Pipetting problem during the Phase II.
• Reconstitution is required during the pre-incubation.
• No reagent unit on board at the end of the pre-incubation.
• The carrousel hood stills open at the end of the pre-incubation.
• Kit discrepancy because the diluent is empty (Insufficient reagent volume).
Page 37

B·R·A·H·M·S GmbH
B·R·A·H·M·S KRYPTOR compact PLUS User Manual (Version R08en) Page 28 of 30
Degraded Mode
When one of the following sensors has a malfunction
the local hotline can disable it to finalize the ongoing
measurement protocol. The sensors listed are installed
to warrant safe routine use. As the sensor malfunctions
do not affect the patient result they can be disabled. In
these exceptional cases the user has to follow the
precautions described below. This allows use of the
instrument until service engineer intervention.
After the local hotline has disabled a sensor, the
window “Securities disabled” appears on the interface.
When you click on the window, detail of sensors
dis abled is shown.
Specific conditions to use the instrument
Caution
The user must be careful when using the instrument
because the securities are off and some specific
conditions are mandatory. The user must follow the
advice given.
Fluidic Hood: The tip and the carousel may move
while the fluidic hood is open. The user must be vigilant
at each requested action on the interface (starting a
pipett
ing sequence, a carrousel scan, maintenances,
etc ...).
Lower Barcode Reader: Sample tubes and reagent
kits must be identified by manual entry. Refer to
„Important User informations“ for precautions.
Upper Barcode Reader: Sample cassettes are not
detected. Their positions are imposed on the carrou sel
in positions 4 and 5. The dilution plates and
SOLU TIONS 1-4 must be identified manually. Refer to
the “Important User Information“ for precautions.
Con sumables status (liquid level, dilution wells avail able,...) will be managed as usual.
Plexi Door: The Plexi door is the clear flap which is
lowered when loading a new reaction plate. The software no longer identifies if it is open or closed. Check
that the plexi door is closed before loading the reaction
plate to prevent damage to the reaction plate carriage.
Reader Front Door: This door is used to load the
reaction plate and the software no longer identifies if it
is open or closed. Check that the door is closed after
loading the reaction plate to avoid allowing light into
the reaction area and maintaining temperature control
of the incubator.
Silica Window: This is the Reader Head Window and
the heating is disabled. There are no special precau tions to be applied in addition to daily maintenance.
Clot Detection: The clot detection for samples is
dis abled, check samples before placing them on the
carousel.
Waste Status: The liquid level in the Waste bottle is
no longer managed as well as in the waste collector
placed below the washbowl. The status of the waste
is in green colour on the screen. Check waste level
before starting a pipetting sequence. Check that no
liquid comes out underneath the instrument.
IRDA Communication: IRDA is used to inform the
system about temperature of the reagent cassettes.
The temperature is still controlled accurately but it is
not reported to the system which leads to display of
the alarm “System Warning: reagent cooler out of
range” on all test results. The temperature status of
the reagent cassette appears in red on the screen.
When IRDA communication is OFF, the position of
the reagent cassette is imposed at location 1 of the
carousel. The identification of the reagent cassette is
R1. Reagent kits are always detected through the
lower bar code reader.
Page 38

Page 39

© 2018 Thermo Fisher Scientific Inc. All rights re serve d. All trademarks are the prope rty of Thermo Fisher Scientific and
its subsi diarie s unless otherw ise spec ified. Win dows is a registered trademark of Microsof t Corporation in the United
States and other countries. KRYPTOR and TR ACE are registered trademarks of CIS bio inte rnation al, licensed for use
by B·R·A·H·M·S, a part of Thermo Fisher Scientific. CEZANNE SAS ho ld exclusive rights in the B·R·A·H·M·S KRYPTOR
compact PLUS software.
Thermo Fisher Scientific products a re distributed worldwide; not all intende d uses and applications mentioned in this
printing are registered in every country.
106228.8
Find out more at thermoscientific.com/kryptor
+49 (0)3302 883 0
+49 (0)3302 883 100 fax
info.brahms@thermofisher.com
www.thermoscientific.com/brahms
Thermo Fisher Scientific
B·R·A·H·M·S GmbH
Neuendorfstr. 25
16761 Hennigsdorf
Germany
Clinical Diagnostics
B·R·A·H·M·S GmbH
Neuendorfstr. 25
16761 Hennigsdorf
Germany
+49-3302-883-0
+49-3302-883-100 fax
info.brahms@thermofisher.com
www.thermoscientific.com/brahms
www.thermoscientific.com/copeptin
www.thermoscientific.com/proadrenomedullin
www.thermoscientific.com/procalcitonin
www.thermoscientific.com/kryptor
International Product Support
CEZANNE SAS
Allée Graham Bell
Parc Scientifique Georges Besse
30035 N îmes Ce dex 01
France
+33-466-365-246
+33-466-365-261 fax
productsupport.brahms.frnim@thermofisher.com
Date: 21.02.2018
This version supersede s all earlier User Manuals.
Soft ware 7.06.00
Page 40

Page 41

Kimby Barton, Interim Director, Medical Devices Bureau/Directrice intérimaire, Bureau des matériels médicaux
_________________________________________________________
* AMENDED *
* MODIFIÉE *
No d'homologation:
96541
Licence Number:
Première date de délivrance:
2016/02/12
First Issue Date:
2017/01/16
Application Number:
Numéro de la demande:
263153
116875
Manufacturer ID:
Identificateur du fabricant:
Date de modification:Amended Date:
La présente homologation est délivrée en vertu
de l'article 36 du Règlement sur les instruments
médicaux pour l'instrument médical suivant:
This Licence is issued in accordance with the
Medical Devices Regulations, Section 36,
for the following medical device:
Device Class/Classe de l'instrument: 2
16761
GERMANY
HENNIGSDORF
NEUENDORFSTRASSE 25
Manufacturer Name & Address/Nom du fabricant & adresse
BRAHMS GMBH
ADDITION OF DEVICES
Reason for Amendment/Raison de la modification
BRAHMS KRYPTOR
Licence Name/Nom de l'homologation:
Licence Type/Type d'homologation:
System / Système
Therapeutic Products Directorate
Medical Devices Bureau
Direction des produits thérapeutiques
Bureau des matériels médicaux
96541
LN/NH:
Santé Health
Canada Canada
Homologation d'un instrument médicalMedical Device Licence
Page 42

Components/Parts/Accessories/Devices for this Licence
Les composantes, parties, accessoires et instruments médicaux pour cette homologation
BRAHMS KRYPTOR COMPACT PLUS
Device Identifier / Identificateur de l'instrument
(Model/Catalog Detail/No de modèle/Catalogue):
Device ID/No de l'instrument: 813302
106172
BRAHMS PCT SENSITIVE KRYPTOR
Device Identifier / Identificateur de l'instrument
(Model/Catalog Detail/No de modèle/Catalogue):
Device ID/No de l'instrument: 813303
825.050
BRAHMS PCT SENSITIVE KRYPTOR CAL
Device Identifier / Identificateur de l'instrument
(Model/Catalog Detail/No de modèle/Catalogue):
Device ID/No de l'instrument: 813304
82591
BRAHMS PCT SENSITIVE KRYPTOR QC
Device Identifier / Identificateur de l'instrument
(Model/Catalog Detail/No de modèle/Catalogue):
Device ID/No de l'instrument: 813305
82592
BRAHMS CGA II KRYPTOR
Device Identifier / Identificateur de l'instrument
(Model/Catalog Detail/No de modèle/Catalogue):
Device ID/No de l'instrument: 815670
839.050
BRAHMS CGA II KRYPTOR CAL
Device Identifier / Identificateur de l'instrument
(Model/Catalog Detail/No de modèle/Catalogue):
Device ID/No de l'instrument: 815671
LN/NH: 96541
Santé Health
Canada Canada
Therapeutic Products Directorate
Medical Devices Bureau
Direction des produits thérapeutiques
Bureau des matériels médicaux
Application Number:
Numéro de la demande:
263153
Page 2
Manufacturer ID:
Identificateur du fabricant:
116875
Page 43

83991
BRAHMS CGA II KRYPTOR QC
Device Identifier / Identificateur de l'instrument
(Model/Catalog Detail/No de modèle/Catalogue):
Device ID/No de l'instrument: 815672
83992
BRAHMS ANTI-TGN KRYPTOR
Device Identifier / Identificateur de l'instrument
(Model/Catalog Detail/No de modèle/Catalogue):
Device ID/No de l'instrument: 823274
830.075
BRAHMS ANTI-TGN KRYPTOR CAL
Device Identifier / Identificateur de l'instrument
(Model/Catalog Detail/No de modèle/Catalogue):
Device ID/No de l'instrument: 823275
83091
BRAHMS ANTI-TGN/-TPON KRYPTOR QC
Device Identifier / Identificateur de l'instrument
(Model/Catalog Detail/No de modèle/Catalogue):
Device ID/No de l'instrument: 823276
85392
BRAHMS ANTI-TPON KRYPTOR
Device Identifier / Identificateur de l'instrument
(Model/Catalog Detail/No de modèle/Catalogue):
Device ID/No de l'instrument: 823277
852.075
BRAHMS ANTI-TPON KRYPTOR CAL
Device Identifier / Identificateur de l'instrument
(Model/Catalog Detail/No de modèle/Catalogue):
Device ID/No de l'instrument: 823278
85291
LN/NH: 96541
Santé Health
Canada Canada
Therapeutic Products Directorate
Medical Devices Bureau
Direction des produits thérapeutiques
Bureau des matériels médicaux
Application Number:
Numéro de la demande:
263153
Page 3
Manufacturer ID:
Identificateur du fabricant:
116875
Page 44

BRAHMS HTG SENSITIVE KRYPTOR
Device Identifier / Identificateur de l'instrument
(Model/Catalog Detail/No de modèle/Catalogue):
Device ID/No de l'instrument: 823279
832.075
BRAHMS HTG SENSITIVE KRYPTOR CAL
Device Identifier / Identificateur de l'instrument
(Model/Catalog Detail/No de modèle/Catalogue):
Device ID/No de l'instrument: 823280
83291
BRAHMS HTG SENSITIVE KRYPTOR QC
Device Identifier / Identificateur de l'instrument
(Model/Catalog Detail/No de modèle/Catalogue):
Device ID/No de l'instrument: 823281
83292
BRAHMS TRAK HUMAN KRYPTOR
Device Identifier / Identificateur de l'instrument
(Model/Catalog Detail/No de modèle/Catalogue):
Device ID/No de l'instrument: 823282
801.050
BRAHMS TRAK HUMAN KRYPTOR CAL
Device Identifier / Identificateur de l'instrument
(Model/Catalog Detail/No de modèle/Catalogue):
Device ID/No de l'instrument: 823283
80191
BRAHMS TRAK HUMAN KRYPTOR QC
Device Identifier / Identificateur de l'instrument
(Model/Catalog Detail/No de modèle/Catalogue):
Device ID/No de l'instrument: 823284
80192
LN/NH: 96541
Santé Health
Canada Canada
Therapeutic Products Directorate
Medical Devices Bureau
Direction des produits thérapeutiques
Bureau des matériels médicaux
Application Number:
Numéro de la demande:
263153
Page 4
Manufacturer ID:
Identificateur du fabricant:
116875
Page 45

Page 46

B'R'A'H'M'S
Aktiengesellschaft
510(k)
Premarket
Notification
B'R'A'H'M'S
PCT
sensitive
KRYPTOR
Test
System
Section
5.0,
510(k)
Summary
Page
1
of
6
510(k)
SUMMARYZo
General
Information
MAR
3
1
2008
Submitted
by:
B.R-A.H.M.S
Aktiengesellschafi
Neuendorfstrasse
25
D-
16761
Henningsdorf
near
Berlin
Germany
Contact
Person:
Jonas
Leichtner
BR-A.H.M.S
USA,
Inc.
2568A
Riva
Road,
Suite
207
Annapolis,
MD
21401
Phone:
(410)
897
9960
Fax:
(410)
972
2452
Email:
i.leichtnera)brahms-usa.com
Date
Prepared:
February
14,
2008
Device
Name
Trade
Name:
B.R.A.H.M.S
PCT
sensitive
KRYPTOR®
Test
System
Common
Name:
Inflammatory
Response
Marker
Classification
Name:
Antigen,
Inflammatory
Response
Marker,
Sepsis,
21
CFR
866.3210
Predicate
Device
Manufacturer
Product
Name
510(k)
No.
BR.A.H.M.S
Aktiengesellschaft
B-R.A.H.M.S
PCT
LIA
K040887
Device
Description
The
BR.A.H-M-S
PCT
sensitive
KRYPTOR®
assay
is
a
homogeneous
sandwich
immunoassay
for
detection
of
PCT
in
human
serum
or
plasma.
The
B-R.A.H.M.S
KRYPTOR®
analyzer
is a fully
automated
system.
The
B.R.A.H-M'S
KRYPTOR®
analyzer
is
a
closed
system
and
can
only
operate
utilizing
special
reagents
provided
by
B.R.A-H-M.S
Aktiengesellschaft.
The
measuring
principle
is
based
on
Time-
Resolved
Amplified
Cryptate
Emission
(TRACE®)
technology,
which
measures
the
signal
that
is
emitted
from
an
immunocomplex
with
time
delay.
Page 47

B'R'A'H'M'S
Aktiengesellschaft
510(k)
Premarket
Notification
B'R'A'H'M'S
PCT
sensitive
KRYPTOR
®
Test
System
Section
5.0,
510(k)
Summary
Page
2
of
6
The
basis
of
the
TRACE®
technology
is
a
non-radiative
energy
transfer
from
a
donor
[a
cage-like
structure
with
a
europium
ion
in
the
center
(cryptate)]
to
an
acceptor
(XL
665).
The
proximity
of
donor
(cryptate)
and
acceptor
(XL
665)
in
a
formed
immunocomplex
and
the
spectral
overlap
between
donor
emission
and
acceptor
absorption
spectra
on
the
one
hand
intensifies
the
fluorescent
signal
and
on
the
other
hand
extends
the
life
span
of
the
acceptor
signal,
allowing
for
the
measurement
of
temporally
delayed
fluorescence.
After
the
sample
to
be
measured
has
been
excited
with
a
nitrogen
laser
at
337
nm, the
donor
(cryptate)
emits
a
long-life
fluorescent
signal
in
the
milli-second
range
at
620
nm,
while
the
acceptor
(XL
665)
generates
a
short-life
signal
in the
range
of
nano-
seconds
at
665
nm.
When
both
components
are
bound
in
an
immunocomplex,
both
the
signal
amplification
and
the
prolonged
life
span
of
the
acceptor
signal
occur
at
665
nm,
and
the life
is
in
the
microsecond
range.
This
delayed
acceptor
signal
is
proportional
to
the
concentration
of
the analyte
to
be
measured.
The
specific
fluorescence
which
is
proportional
to
the
antigen
concentration
is
obtained
through
a
double
selection:
spectral
(separation
depending
on
wave-length)
and
temporal
(time
resolved
measurement).
This
enables
an
exclusive
measurement
of
the signal
emitted
by
the
immunological
complex
and
the
ratio
between
the
two
wave-lengths
(665/620)
allows
a
real-time
correction
of
the
variations
in
optic
transmission
from
the
medium.
The
contents
of
the
BR.A.H.M.S
PCT
sensitive
KRYPTOR
assay
are:
Quantity
for
50
Reagent
deemntos
Content
determinations
Cryptate
1
bottle
Cryptate
conjugate,
cryptate
labeled,
Conjugate
lyophilized
anti-PCT
antibody
(polyclonal,
sheep),
3.2
ml
after
reconstitution
with
KRYPTOR®
Solution
1
and
KRYPTOR®
Solution
2
XL665
1
bottle
XL665
conjugate,
XL665
labeled,
Conjugate
lyophilized
anti-PCT
antibody
(monoclonal,
mouse),
3.95
ml
after
reconstitution
with
KRYPTOR®
Solution
1
and
KRYPTOR®
Solution
2
Diluent
1
bottle
Defibrinated
human
plasma,
for
diluting
samples
above
50
ng/ml,
ready
to
use
Controls,
Calibrator,
and
Consumables
are
provided
separate
from
the
reagent
unit.
Page 48

B'R'A'H'M'S
Aktiengesellschaft
510(k)
Premarket
Notification
B.R.A.H'M'S
PCT
sensitive
KRYPTOR®
Test
System
Section
5.0,
510(k)
Summary
Page
3
of
6
Intended
Use
The
B.R-A'H'M'S
PCT
sensitive
KRYPTOR®
is
an
immunofluorescent
assay
used
to
determine
the
concentration
of
PCT
(procalcitonin)
in
human
serum
and
plasma.
The
B.R.A.H.M.S
PCT
sensitive
KRYPTOR®
is
intended
for
use
in
conjunction
with
other
laboratory
findings
and
clinical
assessments
to
aid
in
the
risk
assessment
of
critically
ill
patients
on
their
first
day
of
ICU
admission
for
progression
to
severe
sepsis
and
septic
shock.
Technological
Comparison
The
B.R.A.H-M.S
PCT
sensitive
KRYPTOR®
and the
B.R.A.H.M.S
PCT
LIA
have
similar
indications
for
use,
are
quantitative
assays,
and
are
used
to
determine
the
concentration
of
PCT
in
human
serum
and
plasma
The
devices
utilize
different
technologies
and
instruments
to
obtain
the
results.
TRACE®
technology
(immunofluorescence)
is
used
with
the
B.R.A.H-M-S
KRYPTOR®
analyzer
and
chemiluminescence
is
used with
a
luminometer
for
BRA-H'M'S
PCT
LIA.
Each
device
uses
2
antibodies,
one
of
which
is
the
same
for
both
devices.
The
monoclonal
antibody
used
in
both
devices
binds
to
Katacalcin.
For
the
second
antibody,
the
B-R'A'H'M'S
PCT sensitive
KRYPTOR®
uses
a
polyclonal
antibody,
which
is
directed
against
Calcitonin.
The
B.R.A-H-M.S
PCT
LIA
uses
a
monoclonal
antibody
directed
against Calcitonin
instead
of a polyclonal
antibody.
Although
the antibodies
directed
against
Calcitonin
come
from
different
origin
they
bind
to
the
same
epitopes.
Therefore
they
will
bind
to the
same
PCT
molecule.
Performance
Summary
Precision
and
Reproducibility
Based
on
CLSI testing,
the
analytical
sensitivity
was
determined
to
be
0.02
ng/ml
and
the
functional
assay
sensitivity
(FAS)
was
determined
to
be
0.06
ng/ml.
In
addition,
the
total
precision
ranges
from
3.2 -13.4
%
CV
and
the
within
run
precision
ranges
from
1.0
-
13.6 % CV.
High
Dose Hook
Effect
The
B.R.AoH.M.S
PCT
sensitive
KRYPTOR®
assay
is
homogenous,
and
does
not
require
separation
or
washing
steps.
It
is
thus
possible
to
obtain
data
without
interrupting
the
immunological
reaction.
High
concentration
samples
(>
50
ng/ml)
are
detected
in
the
first
few
seconds
of
incubation
and
may
be
diluted
by
the appropriate
dilution
factor,
then
re-assayed
automatically.
This
process
allows
for
sample
measurements
greater
than
50
ng/ml
up
to
5000
ng/ml.
Page 49

B'R'A'H'M'S
Aktiengesellschaft
510(k)
Premarket
Notification
B.R.AH'M'S
PCT
sensitive
KRYPTOR®
Test
System
Section
5.0,
510(k)
Summary
Page
4
of
6
Interference
and
Cross
Reactivity
Based
on
CLSI
testing,
the
substances
evaluated
with
the
B.R.A.H.M.S
PCT
sensitive
KRYPTOR®
assay
were
found
not
to
affect
the
test
performance
at
concentrations
reasonably
and
consistently
found
in
clinical
situations.
The
substances
included
the
following
*
bilirubin,
*
hemoglobin,
*
triglycerides,
*
albumin,
•
substances
that
share
amino
acid
sequences
with
procalcitonin,
*
drugs
which
are
typically
used
for
septic
patients
in
intensive
care
units,
and
*
drugs
which
may
be
commonly
used
in
subjects
at
greater
risk
of
developing
community
acquired
pneumonia
than
the
general
population,
such
as
in
asthma
and/or
COPD
patients.
Method
Comparison
Summary
The
B.R.A.H.M.S
PCT
sensitive
KRYPTOR®
and the
B.R.A.H.M.S
PCT
LIA
both
detect
procalcitonin
(PCT)
in
human
serum
or
plasma.
A
correlation
study
was
performed
in
accordance
with
CLSI
guideline
EP9-A,
"Method
Comparison
and
Bias
Estimation
Using
Patient
Samples"
between
the
B-R'A'H'M'S
PCT
sensitive
KRYPTOR®
assay
and
the
B.R.A.H-M-S
PCT
LIA
assay.
There
were
184
samples
from
three
(3)
sites,
which
had
B.R-A.H.M.S
PCT
LIA
measurements
of
0.3
ng/ml
(the
functional
assay
sensitivity
of
13BR.A.H.MS
PCT
LIA)
or
higher
and/or
B.R.A.H-M.S
PCT
sensitive
KRYPTOR®
measurements
of
0.06
ng/ml
(the
functional
assay
sensitivity
of
B-R'A'H'M'S
PCT
sensitive
KRYPTOR®)
or
higher.
Passing-Bablock
analysis
shows
a
nearly
perfect
correlation
of
the
B-R-A.H.M.S
PCT
sensitive
KRYPTOR®
assay
and
B.R.A-H.M.S
PCT
LIA
assay,
as
demonstrated
in
the
correlation
graph
below.
Page 50

B'R'A'H'M'S
Aktiengesellschafi
510(k)
Premarket
Notification
B'R.A-H'M'S
PCT
sensitive
KRYPTOR®
Test
System
Section
5.0,
510(k)
Summary
Page
5
of
6
300
250
0~~~~~~~~~~~~~~~~~~~
¢-'
>-200-
250
-
-
0
-~~~~~~~~~~~~
>
1
00
./
-
//-
·
~ ~ ~ ~ ~ ~ ~
~ ~ ~
I
~~~~~~o
50-
y=
0.95x
+
0.03
r~0.98
0~~~~~~~~~
Oe~~~https://manualmachine.com/
100
100
150
200
250
300
B.RAoH. M.$
PCT
LIA
Interpretation
of
Results
The
B.R.A.H-M.S
PCT
sensitive
KRYPTOR
®
is
intended
to
aid
in
the
risk
assessment
of
critically
ill
patients
on
their
first
day
of
ICU
admission
for
progression
to
severe
sepsis
and
septic
shock.
SIRS,
Sepsis,
Severe
Sepsis,
and
Septic
Shock
were
categorized
according
to
the
criteria
of
the
consensus
conference
of
the
American
College
of
Chest
Physicians/Society
of
Critical
Care
Medicine.
PCT
should
always
be
interpreted
in
the
clinical
context
of
the
patient.
Therefore,
clinicians
should
use
the
PCT
results
in
conjunction
with
other
laboratory
findings
and
clinical
signs
of
the
patient.
Data
support
the
following
interpretative
risk
assessment
criteria:
PCT
> 2 ng/ml
PCT
levels
above
2.0
ng/ml
on
the
first
day
of
ICU
admission
represent
a
high
risk
for
progression
to
severe
sepsis
and/or
septic
shock.
PCT
<
0.5
ng/ml
PCT
levels
below
0.5
ng/ml
on
the:
first
day
of
ICU
admission
represent
a
low
risk
for
progression
to
severe
sepsis
and/or
septic
shock.
Page 51

B.R.A'H'M'S
Aktiengesellschaft
5
10(k)
Premarket
Notification
B.R.A.H'M'S
PCT
sensitive
KRYPTOR®
Test
System
Section
5.0,
510(k)
Summary
Page
6
of
6
Note:
PCT
levels
below
0.5
ng/ml
do
not
exclude
an
infection,
because
localized
infections
(without
systemic
signs)
may
also
be
associated
with
such
low levels.
If
the
PCT
measurement
is
done
very
early
after
the
systemic
infection
process
has
started
(usually
<
6
hours),
these
values
may
still
be
low.
As
various
non-infectious
conditions
are
known
to
induce
PCT
as
well,
PCT
levels
between
0.5
ng/ml
and
2.0
ng/ml
should
be
reviewed
carefully
to
take
into
account
the
specific
clinical
background
and
condition(s)
of
the
individual
patient.
Expected
Values
In
normal
subjects,
PCT
concentrations
are
<
0.1
ng/ml.
In
a
population
of
151
subjects,
146
had
a
PCT
value
<
0.1
ng/ml.
Specimen
Collection
and
Handling
Serum
or
plasma
may
be
used.
B-R-A.HM.S
recommends
the
use
of
only
one
matrix,
i.e.,
use
the
same
material
(either
serum
or
plasma
[EDTA
or
heparin])
throughout
the
patient's
clinical
course.
It
is
recommended
that
citrate
plasma
not
be
used,
since
concentrations
were
underestimated
with
citrate
plasma.
CLSI
guidelines
should
be
followed
for
collecting,
transporting,
and
processing
patient
samples.
The
sample
volume
needed
for
an
assay
is
50
PI.
Testing
demonstrated
that
there
is
no
difference
between
the
use
of
glass
and
plastic
collecting
tube
types
and
that filling
volume
has
no
impact
on
the
result.
In
any
case,
the
results
of
the
BR.A.H.M.S
PCT
sensitive
KRYPTOR®
assay
should
be
evaluated
in
context
of
all
laboratory
findings
and
the
total
clinical
status
of
the
patient.
In
cases
where
the
laboratory
results
do
not
agree
with
the
clinical
picture
or
history,
additional
tests
should
be
performed.
Samples
may
be
stored
up
to
5
days
at
:2
-
80C.
Samples
may
be
frozen
(-20
0
C)
and
thawed
four
times.
Conclusions
The
BR.A.H.M.S
PCT
sensitive
KRYPTOR®
Test
System
is
substantially
equivalent
to
legally
marketed
Inflammatory
Response
Markers.
Page 52

DEPARTMENT
OF
HEALTH
&
HUMAN
SERVICES
Public
Health
Service
Food
and
Drug
Administration
2098
Gaither
Road
Rockville
MD
20850
Mr.
Jonas
Leichtner
Director
of
Marketing
BR.A.H.M.S
Aktiengelsellschaft
2568A
Riva
Road,
Suite
207
Annapolis,
MD
21401
Re:
K070310
Trade/Device
Name:
BR.A.H.M-S
PCT
sensitive
KRYPTOR®
Test
System
Regulation
Number:
21
CFR
866.3210
Regulation
Name:
Antigen,
Inflammatory
Response
Marker,
Sepsis
Regulatory
Class:
Class
II
Product
Code:
NTM
Dated:
February
20,
2008
Received:
February
20,
2008
Dear
Mr.
Leichtner:
We
have
reviewed
your
Section
5
10(k)
premarket
notification
of
intent
to
market
the
device
referenced
above
and
have
determined
the
device
is
substantially
equivalent
(for
the
indications
for use
stated
in
the
enclosure)
to
legally
marketed
predicate
devices
marketed
in
interstate
commerce
prior
to
May
28,
1976,
the
enactment
date
of
the
Medical
Device
Amendments,
or
to
devices
that
have
been
reclassified
in
accordance
with
the
provisions
of
the
Federal
Food,
Drug,
and
Cosmetic
Act
(Act)
that
do
not
require
approval
of
a
premarket
approval
application
(PMA).
You
may,
therefore,
market
the
device,
subject
to
the
general
controls
provisions
of
the
Act.
The
general
controls
provisions
of
the
Act
include
requirements
for
annual
registration,
listing
of
devices,
good
manufacturing
practice,
labeling,
and
prohibitions
against
misbranding
and
adulteration.
If
your
device
is
classified
(see
above)
into
either
class
II
(Special
Controls)
or class
III
(PMA),
it
may
be
subject
to
such
additional
controls.
Existing
major
regulations
affecting
your
device
can
be
found
in
Title
21,
Code
of
Federal
Regulations
(CFR),
Parts
800
to
895.
In
addition,
FDA
may
publish
further
announcements
concerning
your
device
in
the
Federal
Register.
Please
be
advised
that
FDA's
issuance
of
a
substantial
equivalence
determination
does
not
mean
that
FDA
has
made
a
determination
that
your
device
complies
with
other
requirements
of
the
Act
or
any
Federal
statutes
and
regulations
administered
by
other
Federal
agencies.
You
must
comply
with
all
the
Act's
requirements,
including,
but
not
limited
to:
registration
and
listing
(21
CFR
Part
807);
labeling
(21
CFR
Parts
801
and
809);
and
good
manufacturing
practice
requirements
as
set
forth
in
the
quality
systems
(QS)
regulation
(21
CFR
Part
820).
This
letter
will
allow
you
to
begin
marketing
your
device
as
described
in
your
Section
510(k)
premarket
notification.
The
FDA
finding
of
substantial
equivalence
of
your
Page 53

Page
2
-
This
letter
will
allow
you
to
begin
marketing
your
device
as
described
in
your
Section
5
10(k)
premarket
notification.
The
FDA
finding
of
substantial
equivalence
of
your
device
to
a
legally
marketed
predicate
device
results
in
a
classification
for
your
device
and
thus,
permits
your
device
to
proceed
to
the
market.
If
you
desire
specific
advice
for
your
device
on
our
labeling
regulation
(21
CFR
Part
801),
please
contact
the
Office
of
In
Vitro
Diagnostic
Device
Evaluation
and
Safety
at
240-276-0450.
Also,
please
note
the
regulation
entitled,
"Misbranding
by
reference
to
premarket
notification"
(21CFR
Part
807.97).
For
questions
regarding
postmarket
surveillance,
please
contact
CDRH's
Office
of
Surveillance
and
Biometric's
(OSB's)
Division
of
Postmarket
Surveillance
at
240-276-3474.
For
questions
regarding
the
reporting
of
device
adverse:
events
(Medical
Device
Reporting
(MDR)),
please
contact
the
Division
of
Surveillance
Systems
at
240-276-3464.
You
may
obtain
other
general
information
on
your
responsibilities
under
the
Act
from
the
Division
of
Small
Manufacturers,
International
and
Consumer
Assistance
at
its
toll-free
number
(800)
638-2041
or
(240)
276-3150
or
at
its
Internet
address
http://www.fda.gov/cdrh/industrV/support/index.html.
Sincerely
yours,
Sally
A.
Hojvat,
M.Sc.,
Ph.D.
Director
Division
of
Microbiology
Devices
Office
of
In
Vitro
Diagnostic
Device
Evaluation
and
Safety
Center
for
Devices
and
Radiological
Health
Enclosure
Page 54

INDICATIONS
FOR
USE
510(k)
Number
(if
known):
K070310
Device
Name:
B.R.A.H.M.S
PCT
sensitive
KRYPTOR
®
Test
System
Sponsor
Name:
B-R.A.H.M.S
Aktiengesellschaft
Indications
for
Use:
The
B.R.A.H.M.S
PCT
sensitive
KRYPTOR®
is
designed
for
automated
detection
of
PCT
(procalcitonin)
in
human
serum
or
plasma
(EDTA,
heparin)
samples
by
the
immunofluorescent
B.R.A.H.M.S
PCT
sensitive
KRYPTOR®
assay.
The
B.R.A.H.M.S
PCT
sensitive
KRYPTOR®
is
intended
for
use
in
conjunction
with
other
laboratory
findings
and
clinical
assessments
to
aid
in
the
risk
assessment
of
critically
ill
patients
on
their
first
day
of
ICU
admission
for
progression
to
severe
sepsis
and
septic
shock.
Prescription
Use
[
And/Or
Over-The-Counter
Use
[_
(21
CFR
801
Subpart
D)
(21
CFR
807
Subpart
C)
Do
Not
Write
Below
This
Line-
Continue
on
Another
Page
if
Needed
Concurrence
of
CDRH,
Office
of
In
Vitro
Diagnostic
Devices
(OIVD)
Diasin
Sign-O
Office
of
In
Vitro
Diagnostic
Device
Evaluation
and
Safety
SIA~k
v
Page 55

Page 56

Australian Register of Therapeutic Goods Certificate
Issued to
Microgenics Diagnostics Pty Ltd
for approval to supply
Instrument/analyser IVDs
ARTG Identifier 190292 Class 1
ARTG Start date 7/10/2011
Product Category: Medical Device Included - IVD Class 1
GMDN CT943
GMDN Term Instrument/analyser IVDs
Intended Purpose Instruments / Analysers used as an in vitro diagnostic medical device
(IVD) for the purposes of processing, examining and/or providing
information about a clinical specimen.
Manufacturer Details Address Certificate number(s)
Brahms GmbH Neuendorfstrasse 25
Hennigsdorf, , 16761
Germany
ARTG Standard Conditions
The above Medical Device Included - IVD Class 1 has been entered on the Register subject to the
following conditions:
· The automatic conditions applicable to the inclusion of all kinds of medical devices in the Register are
as specified in section 41FN of the Therapeutic Goods Act 1989.
· The standard conditions that are imposed under section 41FO of the Therapeutic Goods Act 1989
when kinds of medical devices are included in the Register are as set out in the following paragraphs.
· For a medical device included in the Register under Chapter 4 and imported into Australia, the Sponsor
must ensure that information about the Sponsor is provided in such a way as to allow the sponsor to be
identified.
· Each sponsor shall retain records of the distribution of all of the sponsor's medical devices included in
the Register under Chapter 4. In the case of records relating to a Class AIMD medical device, Class III
medical device, or Class IIb medical device that is an implantable medical device, the distribution
records shall be retained for a minimum period of 10 years. In the case of records relating to any other
device, the distribution records shall be retained for a minimum period of 5 years.
· The sponsor of a medical device included in the Register under Chapter 4 shall keep an up to date log
of information of the kind specified in Regulation 5.8.
· A sponsor shall ensure that a medical device within their control is stored and transported in
accordance with the instructions and information provided by the manufacturer.
· It is a condition of inclusion in the ARTG that the sponsor of a medical device that is a Class 4 IVD
provides three consecutive annual reports to the Head of the Office of Devices Authorisation,
Therapeutic Goods Administration following inclusion of the device in the ARTG. (as specified in 5.8 of
the regulations) Annual reports are due on 1 October each year. Reports should be for the period 1 July
to 30 June. The first report following the date of inclusion in the ARTG must be for a period of at least
six months but no longer than 18 months. Subsequent reports are to be provided on 1 October for a
further 2 years. The annual report must include all complaints received by the manufacturer relating to
problems with the use of the device that have been received by them over the year.
· Goods which would require an application audit under Regulation 5.3 if subject to a separate
application for entry in the Register cannot be included under this ARTG entry until a request to vary the
entry has been submitted and approved by the TGA.
Products covered by this Entry
1. Instrument/analyser IVDs
Page 57

This entry: does not contain System(s)/Procedure Pack(s)
Product Specific Conditions
No specific conditions have been recorded against this entry.
Therapeutic Goods Administration
PO Box 100, Woden ACT 2606 Australia
Phone: 1800 020 653
Email: info@tga.gov.au
ARTG Identifier: 190292
ARTG Start Date: 7/10/2011
Page 58

Page 59

Page 60

 Loading...
Loading...