Thermo Scientific 9512HPBNWP Instruction Manual
User Guide
High Performance
Ammonia Ion
Selective
Electrode

ROSS and the COIL trade dress are trademarks of Thermo Fisher Scientific Inc.
AQUAfast, Cahn, ionplus, KNIpHE, No Cal, ORION, perpHect, PerpHecT, PerpHecTion,
pHISA, pHuture, Pure Water, Sage, Sensing the Future, SensorLink, ROSS, ROSS
Ultra, Sure-Flow, Titrator PLUS and TURBO2 are registered trademarks of Thermo
Fisher.
1-888-pHAX-ION, A+, All in One, Aplus, AQUAsnap, AssuredAccuracy, AUTO-BAR,
AUTO-CAL, AUTO DISPENSER, Auto-ID, AUTO-LOG, AUTO-READ, AUTO-STIR, Auto-Test,
BOD AutoEZ, Cable-Free, CERTI-CAL, CISA, DataCOLLECT, DataPLUS, digital LogR,
DirectCal, DuraProbe, Environmental Product Authority, Extra Easy/Extra Value,
FAST QC, GAP, GLPcal, GLPcheck, GLPdoc, ISEasy, KAP, LabConnect, LogR, Low
Maintenance Triode, Minimum Stir Requirement, MSR, NISS, One-Touch, One-Touch
Calibration, One-Touch Measurement, Optimum Results, Orion Star, Pentrode,
pHuture MMS, pHuture Pentrode, pHuture Quatrode, pHuture Triode, Quatrode,
QuiKcheK, rf link, ROSS Resolution, SAOB, SMART AVERAGING, Smart CheK, SMART
STABILITY, Stacked, Star Navigator 21, Stat Face, The Enhanced Lab, ThermaSense,
Triode, TRIUMpH, Unbreakable pH, Universal Access are trademarks of Thermo
Fisher.
Guaranteed Success and The Technical Edge are service marks of Thermo Fisher.
PerpHecT meters are protected by U.S. patent 6,168,707.
PerpHecT ROSS are protected by U.S. patent 6,168,707.
ORION Series A meters and 900A printer are protected by U.S. patents 5,198,093,
D334,208 and D346,753.
ionplus electrodes and Optimum Results solutions are protected by
US Patent 5,830,338.
ROSS Ultra electrodes are protected by US patents 6,793,787.
Orion ORP Standard is protected by US Patent 6,350,367.
Orion NoCal electrodes with stabilized potential patent pending.
© 2007 Thermo Fisher Scientific Inc. All rights reserved. All trademarks are the
property of Thermo Fisher Scientific Inc. and its subsidiaries.
The specifications, descriptions, drawings, ordering information and part numbers
within this document are subject to change without notice.
This publication supersedes all previous publications on this subject.

Table of Contents
General Information . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .1
Introduction . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .1
Required Equipment. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 2
Electrode Setup . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .3
Electrode Assembly . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .3
Electrode Preparation with Preassembled
Outer Body and Membrane Cap Assembly . . . . . . . . . . . . . . . . . 4
Electrode Preparation with Loose Membrane . . . . . . . . . . . . . . . 6
Checking Electrode Operation (Slope) . . . . . . . . . . . . . . . . . . . . . 9
Units of Measurement . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .10
Sample Requirements . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 10
Measuring Hints . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 11
Sample Storage . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 12
Electrode Storage. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 12
Analytical Techniques . . . . . . . . . . . . . . . . . . . . . . . . . . . .13
Typical Calibration Cur ve. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 15
Direct Calibration . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 16
Low-Level Measurements By Direct Calibration . . . . . . . . . . . .20
Measuring Ammonia in Solutions that Wet the Membrane . . . . 23
Measuring Organic Nitrogen . . . . . . . . . . . . . . . . . . . . . . . . . . .24
Application Notes . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .24
Known Addition . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 25
Electrode Characteristics . . . . . . . . . . . . . . . . . . . . . . . . .31
Electrode Response . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .31
Temperature Effects . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .32
Interferences . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 32
pH Effects. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .33
Complexation . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .33
Effect of Dissolved Species . . . . . . . . . . . . . . . . . . . . . . . . . . . .33
Membrane Life. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .33
Theory of Operation . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .34
Troubleshooting. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .37
Troubleshooting Checklist. . . . . . . . . . . . . . . . . . . . . . . . . . . . . .37
Troubleshooting Guide . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 40
Assistance . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 42
Warranty. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .42
Ordering Information . . . . . . . . . . . . . . . . . . . . . . . . . . . . .43
Specifications . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .44

General Information
Introduction
The high performance ammonia ion selective electrode (ISE)
allows fast, simple, economical, and accurate measurements
of dissolved ammonia in aqueous solutions. This gas sensing
electrode can also be used to measure the ammonium ion after
conversion to ammonia or to measure organic nitrogen after
Kjeldahl digestion of the sample. Sample color and turbidity
do not affect the measurement and samples do not need to be
distilled. Almost all anions, cations and dissolved species, other
than volatile amines, do not interfere.
All apparatus and solutions required for measurement, electrode
characteristics and electrode theory are discussed in this user
guide. General analytical procedures, low-level procedures and
a method for measuring ammonia in solutions that wet the
membrane are given.
The High Performance Ammonia Electrode Includes:
• One preassembled outer body and membrane cap- includes
outer body, membrane cap and membrane
• Twenty loose membranes
• Tweezers for handling loose membranes
• Electrode filling solution
• Dispensing cap for electrode filling solution
• User guide
There are two measurement techniques that are recommended
for direct measurements of ammonia samples. The primary
direct measurement technique is for ammonia samples with
concentrations of 1 ppm ammonia as nitrogen and higher. The
low-level measurement technique is for ammonia samples with
concentrations of 1 ppm ammonia as nitrogen and lower. Refer
to the Analytical Techniques section for details.
Technical Support Chemists can be consulted for assistance
and troubleshooting advice. Within the United States call
1.800.225.1480 and outside the United States call 978.232.6000
or fax 978.232.6031. In Europe, the Middle East and Africa,
contact your local authorized dealer. For the most current
contact information, visit www.thermo.com/water.
1High Performance Ammonia Electrode User Guide

Required Equipment
1. Thermo Scientific Orion ISE meter, such as the 4-Star pH/
ISE meter or 5-Star pH/ISE/DO/conductivity meter.
Ammonia electrodes can be used on any ISE meter with a
BNC or U.S. standard connection. The electrodes can also
be used on meters with a variety of inputs when an adapter
cable is used. Visit www.thermo.com/water for details.
2. Thermo Scientific Orion high performance ammonia ion
selective electrode, Cat. No. 9512HPBNWP or 9512HP01.
3. Magnetic stirrer or Orion stirrer probe, Cat. No. 096019.
The Orion stirrer probe can be used with 3-Star, 4-Star and
5-Star benchtop meters.
4. Volumetric flasks, graduated cylinders and beakers.
5. Distilled or deionized water. All water must be ammoniafree. Pass distilled or deionized water through an ionexchange column containing a strong acidic cation exchange
resin, such as Dowex 50W-X8.
6. Ammonia electrode filling solution, Cat. No. 951209.
7. Ammonia calibration standards.
Cat. No. Description
951006 0.1 M ammonia chloride standard
951007 1000 ppm ammonia as nitrogen standard
951207 100 ppm ammonia as nitrogen standard
8. Ionic Strength Adjuster (ISA), which provides a constant
background ionic strength and adjusts the solution pH.
Cat. No. Description
951211 pH-adjusting ISA, for samples that have a
concentration of 1 ppm as nitrogen or higher
951011 Alkaline reagent (low-level ISA), for samples
that have a concentration of 1 ppm as
nitrogen or lower
9. Ammonia electrode storage solution, 100 mL of 1 ppm
standard with 1 mL of alkaline reagent (Cat. No. 951011).
Refer to the
instructions on storage solution preparation.
Electrode Storage section for detailed
2 High Performance Ammonia Electrode User Guide

Electrode Setup
Electrode Assembly
Note: A new electrode is shipped without a membrane. You
must install a preassembled outer body and membrane cap
assembly or a new membrane before using the electrode.
Note: A new electrode is shipped dry. You must soak the inner
body of the electrode in the electrode filling solution for at least
two hours before using the electrode. For best results, soak the
inner body overnight in electrode filling solution.
Assemble the electrode according to the instructions listed in
the Electrode Preparation with a Preassembled Outer Body
and Membrane Cap Assembly or Electrode Preparation with
Loose Membranes section. Avoid excessive handling of the
membrane during assembly; this may affect the hydrophobic
properties of the membrane and cause a shortened membrane
life. Use the tweezers provided. A membrane will last from one
week to several months, depending on usage.
3High Performance Ammonia Electrode User Guide
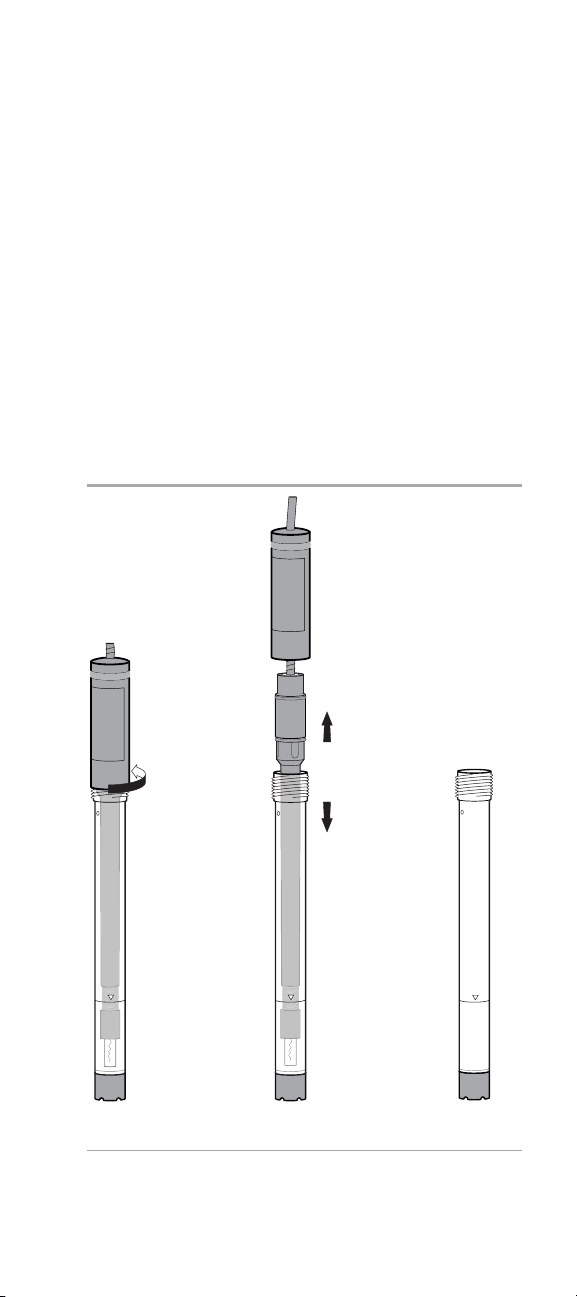
Electrode Preparation with a Preassembled Outer
fill fill
fill
Body and Membrane Cap Assembly
1. Hold the electrode vertically and unscrew the electrode cap
from the electrode body. See Figure 1.
2. Carefully remove the inner body from the outer body
assembly. See Figure 2. Dispose of any electrode filling
solution that is in the outer body.
Note: A new electrode is shipped dry. You must soak the
inner body of the electrode in the electrode filling solution
for at least two hours before using the electrode. For best
results, soak the inner body overnight in electrode filling
solution.
Figure 1 Figure 2 Figure 3
4 High Performance Ammonia Electrode User Guide
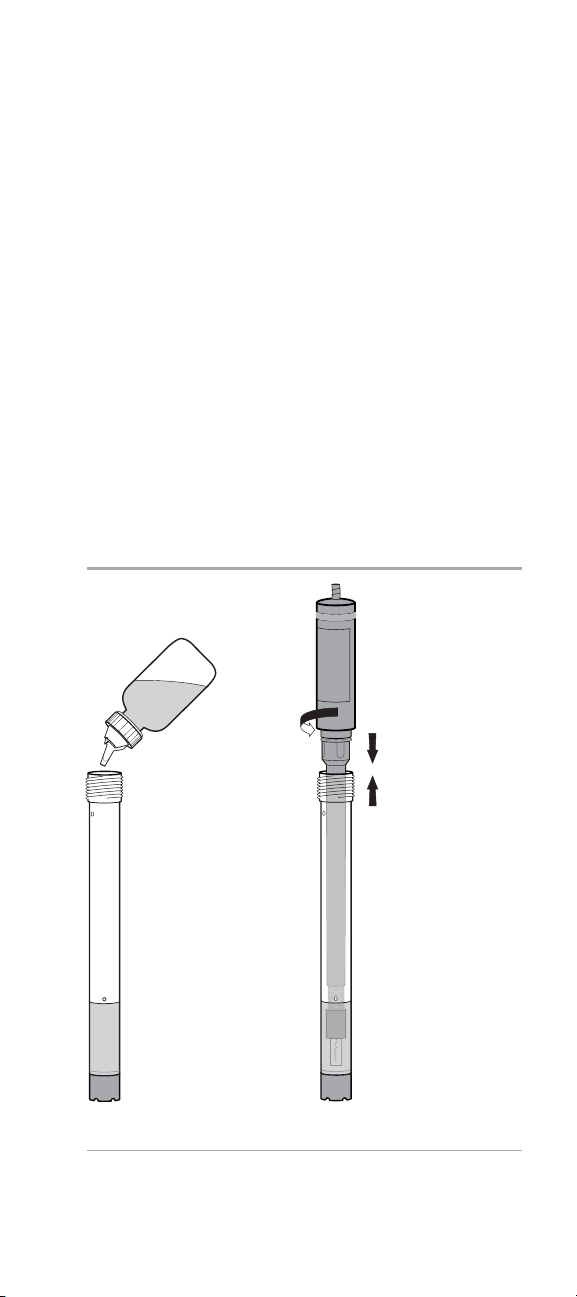
3. Select a new preassembled outer body and membrane cap
fill
filli
n
g
soluti
o
n
fill
assembly and verify that the membrane is not wrinkled or
torn. See Figure 3.
4. Fill the preassembled outer body and membrane cap to the
fill line with electrode filling solution. See Figure 4.
5. Insert the inner body into the preassembled outer body
and membrane cap. Ensure that the inner body is fully
inserted in the top of the outer body and then screw on the
electrode cap. See Figure 5.
6. Gently tap the side of the electrode to remove air bubbles.
7. Soak the electrode in the ammonia electrode storage
solution for at least 15 minutes before use. Prepare the
storage solution by combining 100 mL of 1 ppm ammonia
standard with 1 mL of alkaline reagent, Cat. No. 951011.
Figure 4 Figure 5
5High Performance Ammonia Electrode User Guide
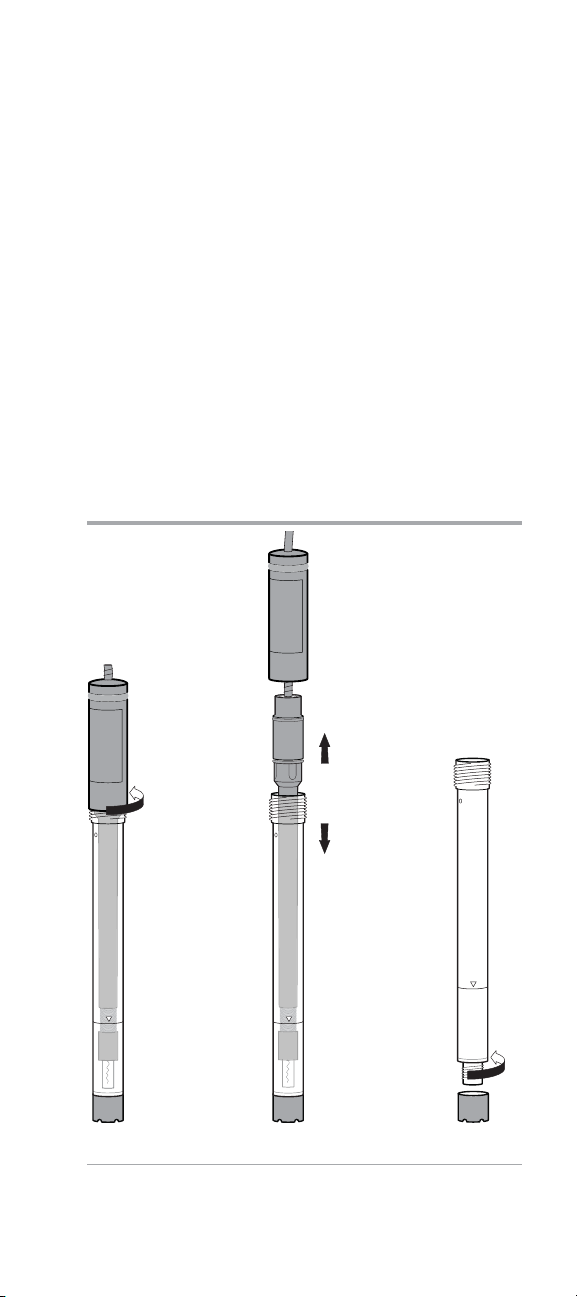
Electrode Preparation with Loose Membrane
fill fill
fill
1. Hold the electrode vertically and unscrew the electrode cap
from the electrode body. See Figure 6.
2. Carefully remove the inner body from the outer body
assembly. See Figure 7. Dispose of any electrode filling
solution that is in the outer body.
Note: A new electrode is shipped dry. You must soak the
inner body of the electrode in the electrode filling solution
for at least two hours before using the electrode. For best
results, soak the inner body overnight in electrode filling
solution.
3. Unscrew the membrane cap from the outer body. See
Figure 8. Remove the membrane from the membrane cap
if a membrane was previously installed.
Figure 6 Figure 7 Figure 8
6 High Performance Ammonia Electrode User Guide
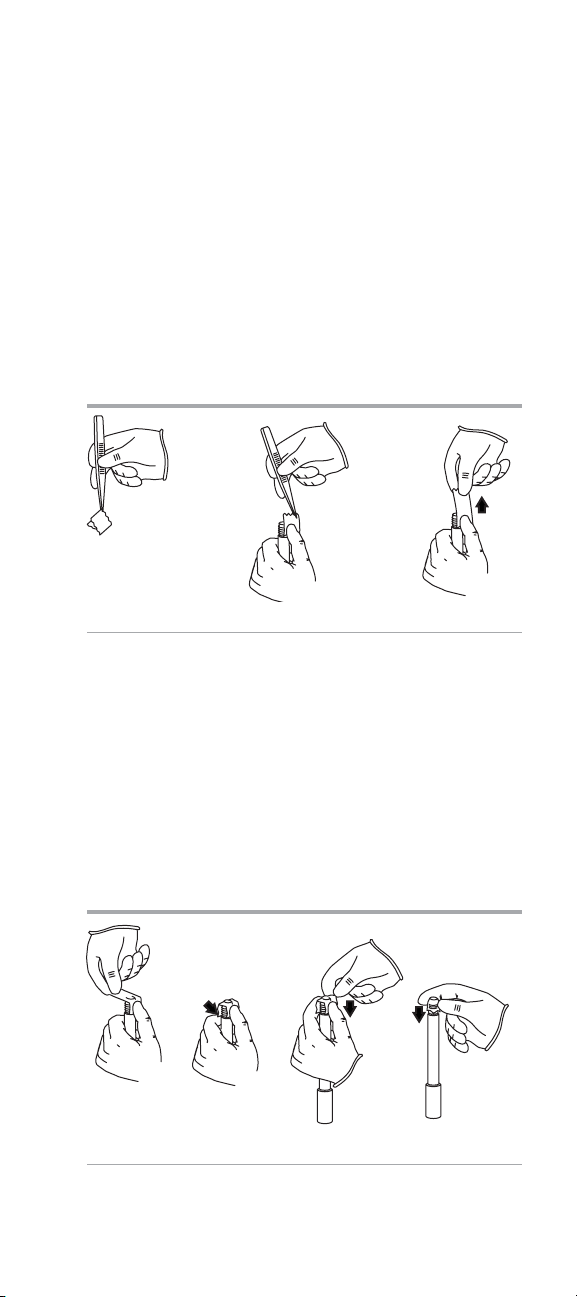
4. Wear gloves and use tweezers to carefully grasp the
corner of the white membrane from between wax paper
separators. See Figure 9.
Note: Do not touch the center of the membrane.
5. Hold the outer body oriented with the smaller diameter
threads at the top. See Figure 10.
6. Align the serrated edge of the membrane against the
threaded shoulder and hold the membrane with your
thumb. See Figure 11.
Figure 9 Figure 10 Figure 11
7. With the other hand, gently place the membrane across the
opening. See Figure 12.
8. Then place the membrane down to align the other edge
with the opposite shoulder. See Figure 13.
9. While holding each edge on both sides, gently place each
smooth side of the membrane out and down over the
threads and ensure that the membrane surface is smooth
and without wrinkles. See Figure 14.
10. Smooth any loose material, taking care not to touch center
of membrane. See Figure 15.
Figure 12 Figure 13 Figure 14 Figure 15
7High Performance Ammonia Electrode User Guide
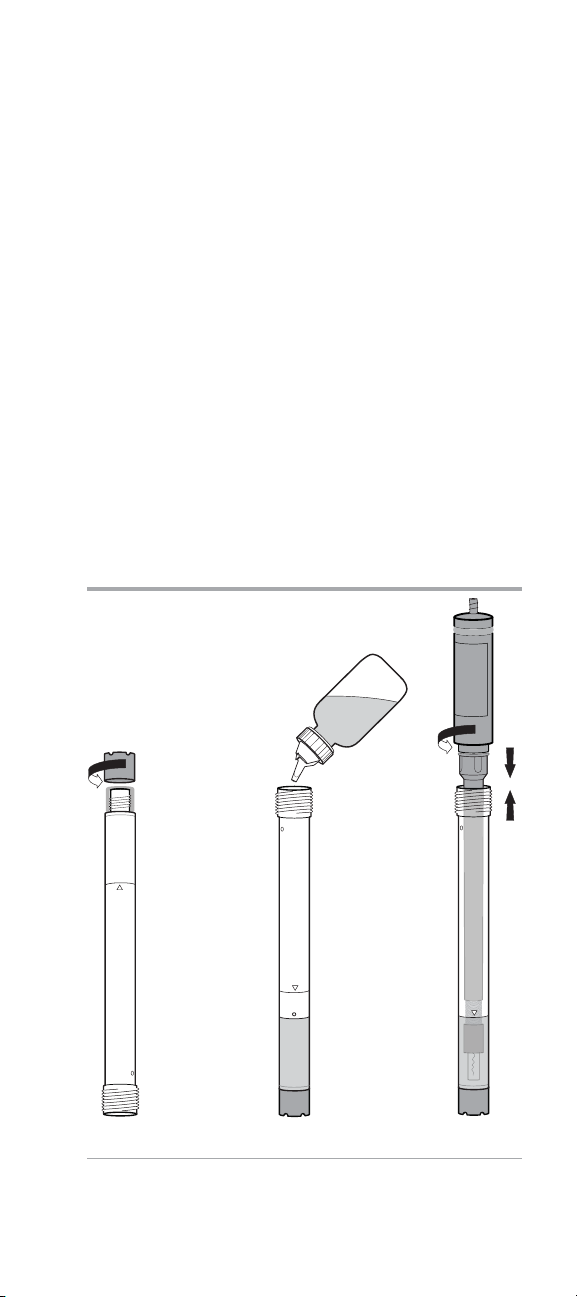
Note: Do not stretch the membrane.
fill
fil
l
ing
so
l
ut
io
n
fill
fill
fill
11. Screw the membrane cap onto the outer body, being
careful not to touch membrane. See Figure 16. Screw the
membrane cap on half way and wrap any loose membrane
material onto the threads and under the cap. Make sure the
cap is fully screwed on.
12. Fill the outer body to the fill line with electrode filling
solution. See Figure 17.
13. Insert the inner body into the outer body. Ensure that the
inner body is fully inserted in the top of the outer body and
then screw on the electrode cap. See Figure 18.
14. Gently tap the side of the electrode to remove air bubbles.
15. Soak the electrode in the ammonia electrode storage
solution for at least 15 minutes before use. Prepare the
storage solution by combining 100 mL of 1 ppm ammonia
standard with 1 mL of alkaline reagent, Cat. No. 951011.
Figure 16 Figure 17 Figure 18
8 High Performance Ammonia Electrode User Guide

Checking Electrode Operation (Slope)
This procedure measures electrode slope. Slope is defined
as the change in millivolts obser ved with every tenfold change
in concentration. Obtaining the slope value provides the best
means for checking electrode operation. These are general
instructions that can be used with most meters to check the
electrode operation. See the meter user guide for more specific
information.
1. If the electrode has been stored dr y, prepare the electrode
as described in the Electrode Preparation section.
2. Connect the electrode to the meter.
3. Place 100 mL of distilled water into a 150 mL beaker. Add
2 mL of pH-adjusting ISA, Cat. No. 951211. Stir the solution
thoroughly. Set the meter to the mV mode.
4. Rinse the electrode by immersing it in distilled water, blot
it dry and place it into the solution prepared in step 3. To
prevent air entrapment on the membrane surface, be sure
to use an electrode holder that keeps the electrode at a
20˚ angle. If air bubbles appear on the sensing membrane,
gently tap the electrode to remove the bubbles.
5. Select either a 0.1 M ammonium chloride or 1000 ppm
ammonia as nitrogen standard. Pipette 1 mL of the
standard into the beaker and stir the solution thoroughly.
When a stable reading is displayed, record the electrode
potential in millivolts.
6. Pipette 10 mL of the same standard into the same beaker.
Stir the solution thoroughly. When a stable reading is
displayed, record the electrode potential in millivolts.
7. There should be a 54 to 60 mV difference between the
two millivolt readings when the solution temperature is
between 20-25 ˚C. If the millivolt potential is not within this
range, refer to the Troubleshooting section.
Note: If working with ammonia samples with concentrations
of 1 ppm as nitrogen and lower, refer to the Low-Level
Measurements by Direct Calibration section for a low-level
checking electrode operation procedure.
9High Performance Ammonia Electrode User Guide

Units of Measurement
Ammonia can be measured in units of moles per liter (M), parts
per million as ammonia, parts per million as nitrogen or any
other convenient unit. See Table 1.
Table 1
Concentration Unit Conversion Factors
moles/liter (M) ppm as nitrogen (N) ppm as ammonia (NH3)
-4
10
1.40 1.70
10-3 14.0 17.0
-2
10
-1
10
140 170
1400 1700
Sample Requirements
Samples must be aqueous; they must not contain organic
solvents. Contact Technical Support for recommendations on
the use of the electrode in unusual applications. Samples and
standards should be at the same temperature. A 1 ˚C difference
in temperature will give rise to about 2% measurement error.
In all analytical procedures, ISA must be added to all samples
and standards immediately before measurement. After
addition of the ISA, all solutions should fall within a pH 11 to
14 range (solutions should be blue in this range when using
the pH-adjusting ISA, Cat. No. 951211) and have a total level
of dissolved species below 1 M. If the total level of dissolved
species is above 1 M, see the
section.
Effect of Dissolved Species
10 High Performance Ammonia Electrode User Guide

Measuring Hints
• Store samples according to the Sample Storage section.
• Use beakers that minimize the ratio of surface area to
volume.
• Keep beakers containing standards and samples covered
between measurements.
• If working with samples that have a concentration of 1 ppm
as nitrogen or higher, add 2 mL of pH-adjusting ISA (Cat.
No. 951211) to every 100 mL of sample or standard. Once
the ISA is added, a blue color should be obser ved.
• For samples that have a concentration of 1 ppm as nitrogen
or lower, add 1 mL of alkaline reagent (Cat. No. 951011) to
every 100 mL of standard or sample.
• ISA should be added immediately before a measurement is
made to prevent ammonia loss.
• Between measurements, rinse the electrode by immersing
it in distilled water.
• Be sure samples, standards and the electrode are at the
same temperature.
• Samples and standards should be stirred using a magnetic
stirrer or stirrer probe. Some magnetic stirrers generate
enough heat to change solution temperature. Place a piece
of insulating material such as cork or styrofoam between
the beaker and the stirring plate to minimize this effect.
• Stir all standards and samples at a moderate, uniform rate.
• Verify calibration every two hours by placing the electrode
in a fresh aliquot of the middle or high concentration
standard used for calibration. If the value has changed,
recalibrate the electrode.
• Always use fresh standards for calibration.
• After immersion in solution, check the electrode for any air
bubbles on the membrane surface and remove bubbles by
gently tapping the electrode.
• If electrode response is slow, replace the membrane and
soak the electrode for at least 15 minutes in the ammonia
electrode storage solution. Refer to the Electrode
Preparation section.
11High Performance Ammonia Electrode User Guide

Sample Storage
If possible, alkaline samples should be measured immediately.
The rate of ammonia loss at room temperature from a stirred
100 mL basic solution in a 100 mL beaker is about 50% in six
hours. If samples must be stored, make them slightly acidic
(pH 6) by adding 0.5 mL of 1 M HCl to each liter of sample, and
place samples in tightly capped vessels. Make stored samples
basic by adding ISA immediately before measurement.
For information on EPA regulations on ammonia sample storage,
visit the Applications and Technical Resources section of our
website at www.thermo.com/water.
Electrode Storage
Note: The membrane must not be allowed to dr y out. If the
membrane is left out of solution, replace the membrane. Refer
to the Electrode Preparation section.
Storage between measurements– Store the electrode in
the ammonia electrode storage solution. Prepare the storage
solution by combining 100 mL of 1 ppm standard with 1 mL of
alkaline reagent (Cat. No. 951011).
To make the storage solution using the 0.1 M ammonia chloride
standard, pipette 0.07 mL of the standard and 1 mL of the
alkaline reagent into a 100 mL volumetric flask and dilute to the
mark on the flask with distilled water.
To make the storage solution using the 1000 ppm ammonia as
nitrogen standard, pipette 0.1 mL of the standard and 1 mL of
the alkaline reagent into a 100 mL volumetric flask and dilute to
the mark on the flask with distilled water.
To make the storage solution using the 100 ppm ammonia as
nitrogen standard, pipette 1 mL of the standard and 1 mL of the
alkaline reagent into a 100 mL volumetric flask and dilute to the
mark on the flask with distilled water.
Overnight storage and storage up to one week– Store the
electrode in the electrode filling solution, Cat. No. 951209.
Storage over one week– Disassemble the electrode and rinse
all the components with distilled water. Dry and reassemble
the electrode without electrode filling solution and without a
membrane. When preparing the electrode for use, follow the
procedures in the Electrode Preparation section.
12 High Performance Ammonia Electrode User Guide
