
VeriFiler™ Express PCR Amplification Kit
USER GUIDE
Catalog Numbers A32014, A32070, A33032
Publication Number 100043588
Revision E
For Research, Forensic, or Paternity Use Only. Not for use in
diagnostic procedures.
Life Technologies Ltd | 7 Kingsland Grange | Woolston, Warrington WA1 4SR | United Kingdom
For descriptions of symbols on product labels or product documents, go to thermofisher.com/symbols-definition.
The information in this guide is subject to change without notice.
DISCLAIMER: TO THE EXTENT ALLOWED BY LAW, THERMO FISHER SCIENTIFIC INC. AND/OR ITS AFFILIATE(S) WILL NOT BE
LIABLE FOR SPECIAL, INCIDENTAL, INDIRECT, PUNITIVE, MULTIPLE, OR CONSEQUENTIAL DAMAGES IN CONNECTION WITH OR
ARISING FROM THIS DOCUMENT, INCLUDING YOUR USE OF IT.
Revision history: Pub. No. 100043588
Revision Date Description
E 8 April 2021 Add “Performance of direct amplification using a 10‑µL PCR reaction
D 18 January 2021 Add the SeqStudio™ Genetic Analyzer. Consolidate sample preparation
C 12 September 2017
B 14 November 2016 Add representative electropherograms for several types of substrate.
A 04 October 2016 New document
volume” on page 116.
for electrophoresis; it is the same for all instruments.
•
Add 4N6FLOQSwabs™ as a validated collection method.
•
Add DNA-dependent artifact VIC110-120 (extra peaks in the
electropherogram).
Important Licensing Information: These products may be covered by one or more Limited Use Label Licenses. By use of these
products, you accept the terms and conditions of all applicable Limited Use Label Licenses.
Trademarks: All trademarks are the property of Thermo Fisher Scientific and its subsidiaries unless otherwise specified.
4N6FLOQSwabs, COPAN, and NUCLEIC-CARD are trademarks of Copan Italia S.P.A., and used by Life Technologies under
their permission. Adobe, Acrobat, and Reader are trademarks of Adobe Systems Incorporated. Agilent is a trademark of Agilent
Technologies, Inc. Bode Buccal DNA Collector is a trademark of Bode Technology Group, Inc. EasiCollect, FTA, and Whatman are
trademarks of Whatman Limited. Harris Micro-Punch is a trademark of Harris, Joel S. TA Shunderson Communications. Robbins
Scientific is a trademark of Molecular Bioproducts, Inc. VWR Scientific is a trademark of VWR International, Inc. Windows and Windows
Vista are trademarks of Microsoft Corporation.
©2021 Thermo Fisher Scientific Inc. All rights reserved.

Contents
■
CHAPTER 1 Product information .................................................. 8
Product description ............................................................. 8
Kit overview ................................................................ 8
Single-source sample types supported ........................................ 9
Substrate examples ......................................................... 9
About the primers ........................................................... 9
Dyes used in the kit ......................................................... 9
Loci amplified by the kit .................................................... 10
Standards and controls that are required ..................................... 11
Allelic ladder profile ........................................................ 12
DNA Control 007 profile .................................................... 12
Contents and storage ........................................................... 13
Required materials not supplied ................................................. 15
Instrument and software compatibility ............................................ 15
Workflow ..................................................................... 17
■
CHAPTER 2 Perform PCR ....................................................... 18
Optimize PCR cycle number (before first use of the kit) ............................. 18
Procedural guidelines Optimize PCR cycle number ............................. 18
Select samples and prepare plates ........................................... 18
Determine optimum PCR conditions ......................................... 19
Before you begin ............................................................... 19
Thaw reagents (before first use of the kit) ..................................... 19
Treated paper substrates: prepare the amplification kit reactions ..................... 20
Sample preparation guidelines: treated paper substrate ........................ 20
Prepare low-TE buer ...................................................... 20
Prepare the reactions: treated paper substrate ................................ 21
Untreated paper substrates: prepare the amplification kit reactions ................... 22
Sample preparation guidelines: untreated paper substrate ...................... 22
Prepare the reactions: untreated paper substrate .............................. 23
Swab substrates: prepare the amplification kit reactions ............................ 24
Sample preparation guidelines: swab substrate ................................ 24
Prepare the sample lysate: room temperature ................................. 25
Prepare the sample lysate: heat protocol ..................................... 25
VeriFiler
™
Express PCR Amplification Kit User Guide
3

Contents
■
Prepare the reactions: swab substrate ........................................ 26
Store the sample lysate ..................................................... 27
Perform PCR .................................................................. 28
CHAPTER 3 Perform electrophoresis ............................................ 29
Allelic ladder requirements for electrophoresis ..................................... 29
Materials required for electrophoresis ............................................. 30
Set up the SeqStudio™ instruments for electrophoresis (before first use of the kit) ...... 30
Electrophoresis software setup .............................................. 30
Perform spectral calibration ................................................. 31
Set up the 3500/3500xL instruments for electrophoresis (before first use of the kit) ..... 32
Electrophoresis software setup .............................................. 32
Obtain and run the HID Updater (v1 and v2 software only) ...................... 33
Perform spectral calibration ................................................. 33
Set up the 3130/3130xl instruments for electrophoresis (before first use of the kit) ...... 34
Electrophoresis software setup .............................................. 34
Obtain and activate 6-dye license ............................................ 34
Perform spectral calibration ................................................. 36
Set up the 3730/3730xl instruments for electrophoresis (before first use of the kit) ...... 36
Electrophoresis software setup .............................................. 36
Obtain and activate the 6-dye license ........................................ 37
Perform spectral calibration ................................................. 39
Prepare samples for electrophoresis .............................................. 39
■
CHAPTER 4 Analyze data with GeneMapper™ ID‑X Software ................ 41
Overview of GeneMapper™ ID‑X Software ......................................... 41
Allelic ladder requirements for data analysis ....................................... 41
File names and versions used in this section ...................................... 42
Set up the GeneMapper™ ID‑X Software for analysis (before first use of the kit) ........ 43
Workflow: Set up GeneMapper™ ID‑X Software ............................... 43
Check panel, bin, and stutter file versions on your computer .................... 43
(If needed) Download newer versions of panel, bin, and stutter files .............. 44
Import panels, bins, and marker stutter ....................................... 44
(Optional) Define custom table or plot settings ................................. 47
Create an analysis method ...................................................... 48
Create an analysis method .................................................. 48
Enter Analysis Method settings .............................................. 49
Create a size standard definition file if needed ..................................... 55
About the GS600_LIZ_ (60– 460) size standard definition file .................... 55
If you use POP-7™ polymer on a 3730 instrument .............................. 55
Create a size standard definition file .......................................... 56
Analyze and edit sample files with GeneMapper™ ID‑X Software ..................... 58
4
VeriFiler™ Express PCR Amplification Kit User Guide

Examine or edit a project ........................................................ 59
For more information on using the GeneMapper™ ID‑X Software ..................... 59
■
CHAPTER 5 Experiments and results ........................................... 60
Importance of validation ........................................................ 60
Experiment conditions .......................................................... 60
Laboratory requirements for internal validation ..................................... 61
Developmental validation ....................................................... 61
SWGDAM guideline 2.2.1 ................................................... 61
SWGDAM guideline 3.9.2 ................................................... 61
PCR components .......................................................... 62
PCR cycle number ......................................................... 63
Thermal cycling temperatures ............................................... 64
Representative data for common sample and substrate types ....................... 65
Accuracy, precision, and reproducibility ........................................... 67
SWGDAM guideline 3.5 ..................................................... 67
Accuracy observation ...................................................... 67
Precision and size window description ....................................... 69
Precision and size window observation ...................................... 70
Extra peaks in the electropherogram .............................................. 73
Causes of extra peaks ...................................................... 73
Extra peaks: Stutter ........................................................ 73
Extra peaks: Addition of 3' A nucleotide ...................................... 82
Extra peaks: Artifacts ...................................................... 83
Characterization of loci ......................................................... 85
SWGDAM guideline 3.1 ..................................................... 85
Loci in this kit ............................................................. 85
Nature of polymorphisms ................................................... 85
Inheritance ................................................................ 85
Mapping .................................................................. 85
Genetic linkage ............................................................ 86
Species specificity ............................................................. 86
SWGDAM Guideline 3.2 .................................................... 86
Nonhuman studies ......................................................... 87
Sensitivity ..................................................................... 88
SWGDAM guideline 3.3 ..................................................... 88
Sample collection factors that can aect DNA quantity ......................... 88
Eect of DNA quantity on results ............................................ 88
Population data ................................................................ 89
SWGDAM guideline 3.7 ..................................................... 89
Population data overview ................................................... 89
Loci in this kit ............................................................. 89
Population samples used in these studies .................................... 89
Concordance studies ...................................................... 90
Contents
VeriFiler™ Express PCR Amplification Kit User Guide
5

Contents
■
■
Probability of Identity definition .............................................. 90
Probability of identity ....................................................... 90
Probability of paternity exclusion observation ................................ 115
Performance of direct amplification using a 10‑µL PCR reaction volume ............. 116
APPENDIX A Troubleshooting .................................................. 118
APPENDIX B Required materials not supplied ................................ 122
STR kit ...................................................................... 122
Sample preparation required materials ........................................... 122
Treated paper substrate ................................................... 122
Untreated paper substrate ................................................. 123
Swab substrate .......................................................... 123
Thermal cycler required materials ............................................... 124
ProFlex™ PCR System ..................................................... 124
GeneAmp™ PCR System 9700 ............................................. 125
Genetic analyzer required materials ............................................. 125
SeqStudio™ Genetic Analyzer .............................................. 125
3500 Series Genetic Analyzer .............................................. 125
3130 Series Genetic Analyzer .............................................. 126
3730 Series Genetic Analyzer .............................................. 127
Analysis software required materials ............................................. 127
GeneMapper™ ID‑X Software ............................................... 127
Miscellaneous required materials ............................................... 128
Plates and tubes ......................................................... 128
Laboratory supplies ....................................................... 128
■
APPENDIX C Plate layouts ...................................................... 129
Example PCR plate layout ..................................................... 129
Example electrophoresis plate layout ............................................ 130
■
APPENDIX D PCR work areas .................................................. 131
Work area setup and lab design ................................................ 131
PCR setup work area materials ................................................. 131
Amplified DNA work area ...................................................... 132
■
APPENDIX E Safety .............................................................. 133
Chemical safety .............................................................. 134
Biological hazard safety ....................................................... 135
6
VeriFiler™ Express PCR Amplification Kit User Guide

Contents
■
Documentation and support ...................................................... 136
Related documentation ........................................................ 136
Customer and technical support ................................................ 138
Limited product warranty ...................................................... 138
References
Index ..................................................................................... 144
VeriFiler™ Express PCR Amplification Kit User Guide
7

1
Product description .................................................................... 8
■
Contents and storage ................................................................. 13
■
Required materials not supplied ........................................................ 15
■
Instrument and software compatibility ................................................... 15
■
Workflow ............................................................................ 17
■
IMPORTANT! Before using this product, read and understand the information in the “Safety” appendix
in this document.
Product description
Kit overview
Product information
The VeriFiler™ Express PCR Amplification Kit is a 6-dye, short tandem repeat (STR) multiplex assay for
the amplification of single-source human genomic DNA.
The kit is part of a fully integrated and validated forensic DNA workflow backed by best-in-class global
training, service, and support. This kit is optimized for paternity and single-source samples.
The kit amplifies the following loci within the read region of 75–465 nt:
•
23 autosomal STR loci (D3S1358, vWA, D16S539, CSF1PO, TPOX, D8S1179, D21S11, D18S51,
D2S441, D19S433, TH01, FGA, D22S1045, D5S818, D13S317, D7S820, D10S1248, D1S1656,
D12S391, D2S1338, D6S1043, Penta D, Penta E)
•
1 insertion/deletion polymorphic marker on the Y chromosome (Y indel)
•
Amelogenin (sex-determining marker)
The VeriFiler™ Express kit delivers a 25-locus multiplex with the highest discrimination power of
any Thermo Fisher Scientific Human Identification Kit. The highly optimized buer formulation allows
completion of amplification in ~45 minutes.
The VeriFiler™ Express kit uses the same improved process for synthesis and purification of the
amplification primers developed for other next-generation Thermo Fisher Scientific STR chemistries.
The improved amplification primers deliver clean electrophoretic backgrounds that assist interpretation.
8
VeriFiler™ Express PCR Amplification Kit User Guide
Single-source sample types supported
The VeriFiler™ Express kit is optimized to allow direct amplification from the following types of singlesource samples without the need for sample purification:
•
Blood and buccal samples on treated paper substrates.
•
Blood and buccal samples collected on untreated paper substrates and treated with Prep‑n‑Go
Buer.
•
Buccal samples collected on swab substrates and treated with Prep‑n‑Go™ Buer.
Substrate examples
•
Treated paper: COPAN NUCLEIC-CARD™ system or Whatman FTA™ cards
•
Untreated paper: Bode Buccal DNA Collector™ or 903 paper
•
Swab: COPAN 4N6FLOQSwabs™ reference collection devices or cotton swabs
Note: Our testing does not include blood samples on swab substrates. This sample type is not
typically used for the collection of reference samples.
Chapter 1 Product information
Product description
1
™
About the primers
The VeriFiler™ Express kit primers are manufactured using the same synthesis and purification
improvements as the primers in the NGM SElect™, the Sinofiler™, and the Identifiler™ Plus kits. These
improvements enhance the assay signal‑to‑noise ratio and simplify the interpretation of results.
The VeriFiler™ Express kit includes the same primers as the GlobalFiler™ Express PCR Amplification Kit
with the following primer additions and modifications:
•
Addition of new primers for Penta D, Penta E, and D6S1043.
•
Addition of 1 new SNP-specific primer for D19S433 loci to address a SNP in the primer binding
site.
Non-nucleotide linkers are used in primer synthesis for the following loci: D19S433, vWA, CSF1PO,
D2S441, TH01, FGA, and D12S391. For these primers, non-nucleotide linkers are placed between the
primers and the fluorescent dye during oligonucleotide synthesis (Butler 2005, Grossman et al., 1994).
Non-nucleotide linkers enable reproducible positioning of the alleles to facilitate interlocus spacing. The
combination of a 6-dye fluorescent system and the use of non-nucleotide linkers allows simultaneous
amplification and ecient separation of all 25 markers during automated DNA fragment analysis.
Dyes used in the kit
Dye Color Label
™
6‑FAM
™
VIC
™
NED
™
TAZ
Blue Samples, allelic ladders, and controls
Green
Yellow
Red
VeriFiler™ Express PCR Amplification Kit User Guide
9
Chapter 1 Product information
1
Product description
(continued)
Dye Color Label
™
SID
™
LIZ
Orange GeneScan™ 600 LIZ™ Size Standard v2.0
Loci amplified by the kit
Table 1 VeriFiler™ Express kit loci and alleles
Samples, allelic ladders, and controlsPurple
Locus
designation
D3S1358 3p21.31 9, 10, 11, 12, 13, 14, 15, 16, 17, 18, 19, 20 6-FAM
vWA 12p13.31 11,12, 13, 14, 15, 16, 17, 18, 19, 20, 21, 22, 23,
Chromosome
location
Alleles included in Allelic Ladder Dye label
™
DNA Control
007
15, 16
14, 16
24
D16S539 16q24.1 5, 8, 9, 10, 11, 12,13, 14, 15 9, 10
CSF1PO 5q33.3-34 6, 7, 8, 9, 10, 11, 12, 13, 14, 15 11, 12
TPOX 2p23-2per 5, 6, 7, 8, 9, 10, 11, 12, 13, 14, 15 8, 8
Y indel Yq11.221 1, 2 VIC
Amelogenin X: p22.1-22.3
X, Y X, Y
™
Y: p11.2
D8S1179 8q24.13 5, 6, 7, 8, 9 10, 11, 12, 13, 14, 15, 16, 17, 18, 19 12, 13
D21S11 21q11.2-q21 24, 24.2, 25, 26, 27, 28, 28.2, 29, 29.2, 30, 30.2,
28, 31
31, 31.2, 32, 32.2, 33, 33.2, 34, 34.2, 35, 35.2,
36, 37, 38
D18S51 18q21.33 7, 9, 10, 10.2, 11, 12, 13, 13.2, 14, 14.2, 15, 16,
12, 15
17, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27
2
Penta E 15q26.2 5, 6, 7, 8, 9, 10, 11, 12, 13, 14, 15, 16, 17, 18,
19, 20, 21, 22, 23, 24, 25, 26
D2S441 2p14 8, 9, 10, 11, 11.3, 12, 13, 14, 15, 16, 17 NED
™
D19S433 19q12 6, 7, 8, 9, 10, 11, 12, 12.2, 13, 13.2, 14, 14.2, 15,
15.2, 16, 16.2, 17, 17.2, 18.2, 19.2
TH01 11p15.5 4, 5, 6, 7, 8, 9, 9.3, 10, 11, 13.3 7, 9.3
FGA 4q28 13, 14, 15, 16, 17, 18, 19, 20, 21, 22, 23, 24, 25,
26, 26.2, 27, 28, 29, 30, 30.2, 31.2, 32.2, 33.2,
42.2, 43.2, 44.2, 45.2, 46.2, 47.2, 48.2, 50.2,
51.2
D22S1045 22q12.3 8, 9, 10, 11, 12, 13, 14, 15, 16, 17, 18, 19 TAZ
10
VeriFiler™ Express PCR Amplification Kit User Guide
™
7, 12
14, 15
14, 15
24, 26
11, 16
Table 1 VeriFiler Express kit loci and alleles (continued)
Chapter 1 Product information
Product description
1
Locus
designation
D5S818 TAZ
D13S317 13q22-31 5, 6, 7, 8, 9, 10, 11, 12, 13, 14, 15, 16 11, 11
D7S820 7q11.21-22 6, 7, 8, 9, 10, 11, 12, 13, 14, 15 7, 12
D6S1043 6q16.1 9, 10, 11, 12, 13, 14, 15, 16, 17, 18, 19, 20, 21,
D10S1248 10q26.3 8, 9, 10, 11, 12, 13, 14, 15, 16, 17, 18, 19 SID
D1S1656 1q42.2 9, 10, 11, 12, 13, 14, 14.3, 15, 15.3, 16, 16.3, 17,
D12S391 12p13.2 14, 15, 16, 17, 18, 19, 19.3, 20, 21, 22, 23, 24,
D2S1338 2q35 11, 12, 13, 14, 15, 16, 17, 18, 19, 20, 21, 22, 23,
Penta D 21q22.3 2.2, 3.2, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14, 15, 16,
Chromosome
location
5q21-31 7, 8, 9, 10, 11, 12, 13, 14, 15, 16, 17, 18 11, 11
22, 23, 24, 25
17.3, 18.3, 19.3, 20.3
25, 26, 27
24, 25, 26, 27, 28
17
Alleles included in Allelic Ladder Dye label
™
™
DNA Control
007
12, 14
12, 15
13, 16
18, 19
20, 23
11, 12
Standards and controls that are required
For the VeriFiler™ Express kit, the panel of standards needed for PCR amplification, PCR product sizing,
and genotyping are:
•
DNA Control 007—A positive control for evaluating the eciency of the amplification step and STR
genotyping using the VeriFiler™ Express Allelic Ladder. DNA Control 007 is present in the kit. See
“DNA Control 007 profile” on page 12.
•
GeneScan™ 600 LIZ™ Size Standard v2.0—Used for obtaining sizing results. This standard, which
has been evaluated as an internal size standard, yields precise sizing results for PCR products.
Order the GeneScan™ 600 LIZ™ Size Standard v2.0 (Cat. No. 4408399) separately.
•
VeriFiler™ Express Allelic Ladder—Developed for accurate characterization of the alleles
amplified by the kit. The Allelic Ladder is present in the kit and allows automatic genotyping of
most of the reported alleles for the loci in the kit. See “Allelic ladder profile” on page 12.
VeriFiler™ Express PCR Amplification Kit User Guide
11
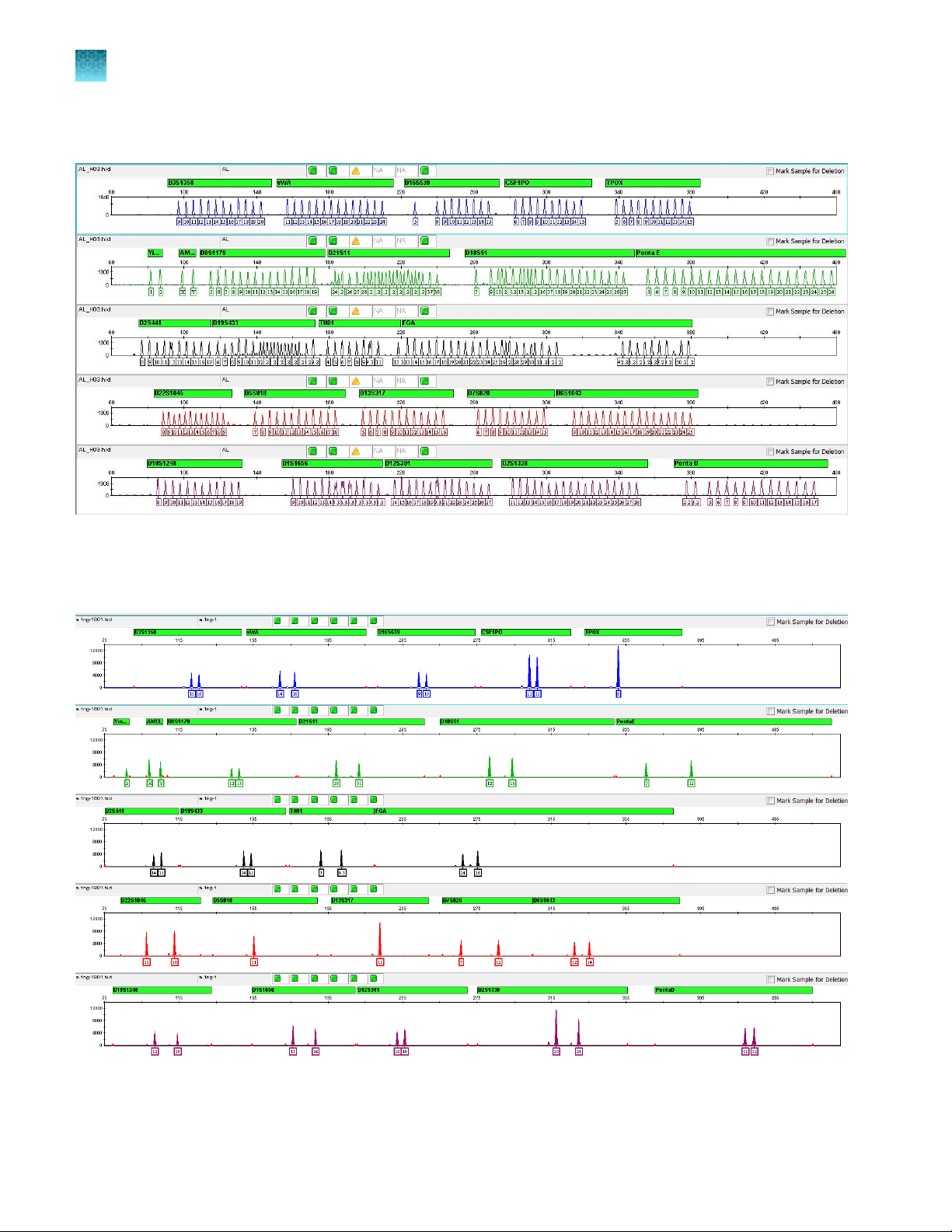
Chapter 1
1
Product description
Product information
Allelic ladder profile
Figure 1 GeneMapper™ ID‑X Software plot of the VeriFiler™ Express Allelic Ladder
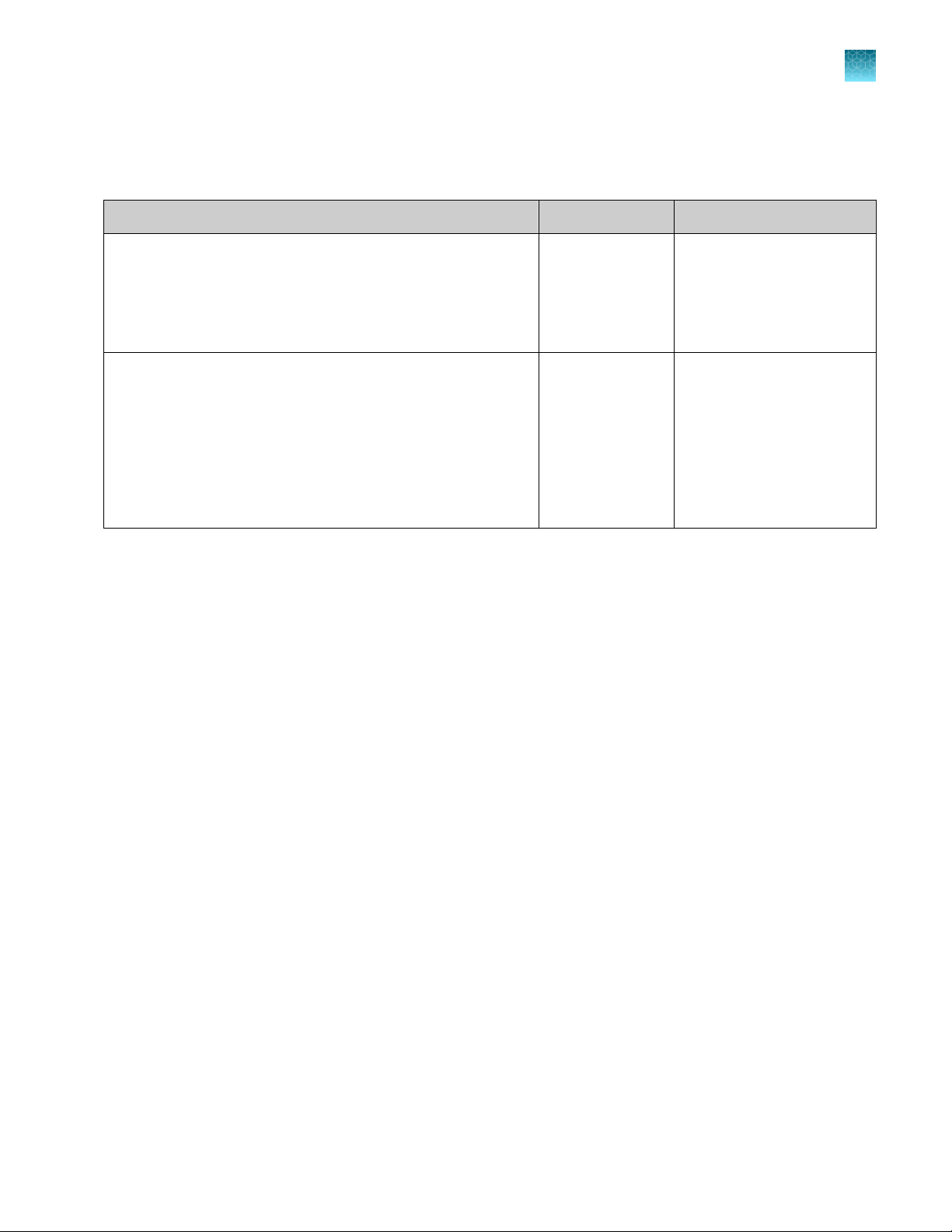
DNA Control 007 profile
Figure 2 DNA Control 007 (2 ng) amplified at 27 PCR cycles with the VeriFiler™ Express kit and analyzed on an
Applied Biosystems™ 3500xL Genetic Analyzer (Y-axis scale 0 to 14,000 RFU).
12
VeriFiler™ Express PCR Amplification Kit User Guide
Chapter 1 Product information
Contents and storage
Contents and storage
Table 2 VeriFiler™ Express PCR Amplification Kit (Cat. No. A32014; 200 reactions)
Contents Amount Storage
1
VeriFiler™ Express Master Mix
Contains MgCl2, dATP, dGTP, dCTP, and dTTP, bovine serum
albumin, enzyme, and 0.05% sodium azide in buer and salt.
VeriFiler™ Express Primer Set
Contains locus-specific 6-FAM™, VIC™, NED™,TAZ™, and SID
dye-labeled and unlabeled primers in buer. The primers
amplify the STR loci D3S1358, vWA, D16S539, CSF1PO,TPOX,
D8S1179, D21S11, D18S51, Penta E, D2S441, D19S433,
TH01, FGA, D22S1045, D5S818, D13S317, D7S820, D6S1043,
D10S1248, D1S1656, D12S391, D2S1338, Penta D, and the
sex-determining markers, Y indel and Amelogenin.
™
2 tubes, 1.0 mL –25°C to –15°C on receipt.
2°C to 8°C after first use, for
up to 6 months or up to the
expiration date stated on the
kit (whichever comes first).
2 tubes, 1.0 mL –25°C to –15°C on receipt.
2°C to 8°C after first use, for
up to 6 months or up to the
expiration date stated on the
kit (whichever comes first).
Store protected from light.
VeriFiler™ Express PCR Amplification Kit User Guide
13
Chapter 1 Product information
1
Contents and storage
Table 2 VeriFiler Express PCR Amplification Kit (Cat. No. A32014; 200 reactions) (continued)
Contents Amount Storage
VeriFiler™ Express Allelic Ladder
Contains the following amplified alleles:
•
6-FAM™ dye (blue): D3S1358 9–20; vWA 11–24; D16S539
5, 8–15; CSF1PO 6–15; TPOX 5–15.
•
VIC™ dye (green): Y indel 1, 2; Amelogenin X, Y; D8S1179
5-19; D21S11 24, 24.2, 25–28, 28.2, 29, 29.2, 30, 30.2, 31,
31.2, 32, 32.2, 33, 33.2, 34, 34.2, 35, 35.2, 36–38; D18S51
7, 9, 10, 10.2, 11–13, 13.2, 14, 14.2, 15–27; DYS391 7–13;
Penta E 5–26.
•
NED™ dye (yellow): D2S441 8–11, 11.3, 12–17; D19S433
6–12, 12.2, 13, 13.2, 14, 14.2, 15, 15.2, 16, 16.2, 17, 17.2,
18.2, 19.2; TH01 4–9, 9.3, 10, 11, 13.3; FGA 13–26, 26.2,
27–30, 30.2, 31.2, 32.2, 33.2, 42.2, 43.2, 44.2, 45.2, 46.2,
47.2, 48.2, 50.2, 51.2.
•
TAZ™ dye (red): D22S1045 8–19; D5S818 7–18; D13S317
5–16; D7S820 6–15; D6S1043 9–25.
•
SID™ dye (purple): D10S1248 8–19; D1S1656 9–14, 14.3,
15, 15.3, 16, 16.3, 17, 17.3, 18.3, 19.3, 20.3; D12S391 14–
19, 19.3, 20–27; D2S1338 11–28; Penta D 2.2, 3.2, 5–17.
DNA Control 007
Contains 2.0 ng/μL human male genomic DNA in 0.05% sodium
azide and buer.
The profile of this male DNA is D3S1358 15,16; vWA 14,
16; D16S539 9, 10; CSF1PO 11,12; TPOX 8; D8S1179 12,
13; D21S11 28, 31; D18S51 12, 15; Penta E 7, 12; D2S441
14,15; D19S433 14,15; TH01 7, 9.3; FGA 24, 26; D22S1045 11,
16; D5S818 11; D13S317 11; D7S820 7,12; D6S1043 12, 14;
D10S1248 12, 15; D1S1656 13, 16; D12S391 18, 19; D2S1338
20, 23; Penta D 11, 12.
1 tube, 0.05 mL –25°C to –15°C on receipt.
2°C to 8°C after first use, up
to the expiration date stated
on the kit.
Store protected from light.
IMPORTANT! The allelic
ladder contains PCR
products. Do not amplify.
To avoid contamination,
store the allelic ladder
separate from the other
kit components and
unamplified DNA.
1 tube, 0.05 mL –25°C to –15°C on receipt.
2°C to 8°C after first use, up
to the expiration date stated
on the kit.
14
VeriFiler™ Express PCR Amplification Kit User Guide
Required materials not supplied
See Appendix B, “Required materials not supplied”.
Instrument and software compatibility
Catalog numbers that appear as links open the web pages for those products.
Chapter 1 Product information
Required materials not supplied
1
Instrument
type
Thermal
cyclers
Genetic
analyzers
Validated models
•
ProFlex™ 96‑well PCR System (Cat. No. 4484075)
•
GeneAmp™ PCR System 9700, 96-Well Silver (Cat. No. N8050001)
•
GeneAmp™ PCR System 9700, 96-Well Gold-Plated (Cat. No. 4314878)
IMPORTANT! VeriFiler
Veriti™ 96‑Well Thermal Cycler (Cat. No. 4479071)
·
Veriti™ Fast 96‑Well Thermal Cycler (Cat. No. 4375305)
·
GeneAmp™ PCR System 9700, 96-Well Aluminum (Cat. No. 4314879)
·
[1]
SeqStudio™ Genetic Analyzer
•
SeqStudio™ Data Collection Software v1.2
•
SeqStudio™ Data Collection Software v1.2.1
3500/3500xL Genetic Analyzer
•
3500 Series Data Collection Software 1 (Windows™ Vista operating system) and HID Updater
3500 Data Collection Software v2 (Cat. No. 4480670)
•
3500 Series Data Collection Software 2 (Windows™ 7 operating system) and HID Updater
3500 Data Collection Software v2 (Cat. No. 4480670)
•
3500 Series Data Collection Software 3 (Windows™ 7 operating system)
•
3500 Series Data Collection Software 3.1 (Windows™ 7 operating system)
•
3500 Series Data Collection Software 4 (Windows™ 10 operating system)
•
3500 Series HID Data Collection Software v4.0.1 (Windows™ 10 operating system)
™
Express kit is NOT validated for use with:
3130/3130xl Genetic Analyzer
•
3130 Series Data Collection Software 4 (Windows™ 7 operating system)
•
3130/3730 Data Collection 4 6-Dye Module v1
VeriFiler™ Express PCR Amplification Kit User Guide
15
Chapter 1 Product information
1
Instrument and software compatibility
(continued)
Instrument
type
Genetic
analyzers
[1]
•
3730 Series Data Collection Software 4 (Windows™ 7 operating system)
•
3730 Series Data Collection Software 4 6-Dye Module v1
•
3730xl Data Collection Software 5 (Windows™ 10 operating system)
3730/3730xl DNA Analyzer
Validated models
Note: For information on using the 3730xl DNA Analyzer, see the 3730xl Data
Collection Software 5 for HID User Bulletin: New Features and Developmental Validation
(Pub. No. MAN0019461).
Analysis
software
[1]
We conducted validation studies using the SeqStudio™, 3130xl, 3500, 3500xL, and 3730xl instruments. For validation information on the
SeqStudio™ instrument, see the SeqStudio™ Genetic Analyzer for HID Instrument and Software v1.2.1 User Bulletin—New Features and
Developmental Validation (Pub. No. 100086084). For validation information on the 3730xl instrument, see the 3730xl Data Collection Software 5
for HID User Bulletin: New Features and Developmental Validation (Pub. No. MAN0019461).
GeneMapper™ ID‑X Software v1.4 or later
Windows™ XP, Windows™ 7, or Windows™ 10 operating system
16
VeriFiler™ Express PCR Amplification Kit User Guide

Workflow
Chapter 1 Product information
Workflow
1
Perform PCR on treated or untreated paper
substrates
“Prepare the reactions: treated paper substrate” on
page 21 or
“Prepare the reactions: untreated paper substrate” on
page 23
▼ ▼
Obtain punch with Harris Manual Punch or BSD
Semi-Automated Dried Sample Punch Instrument
▼
Untreated paper only: Process with Prep‑n‑Go
Buer
▼ ▼
Process with VeriFiler™ Express kit Process with VeriFiler™ Express kit
▼ ▼
Amplify with the GeneAmp™ PCR System 9700 or ProFlex™ 96‑well PCR System
™
Perform PCR on swab substrates
“Prepare the reactions: swab substrate” on page 26
Lyse in Prep‑n‑Go™ Buer
▼
Perform electrophoresis
“Set up the SeqStudio™ instruments for electrophoresis (before first use of the kit)” on page 30 or
“Set up the 3500/3500xL instruments for electrophoresis (before first use of the kit)” on page 32 or
“Set up the 3130/3130xl instruments for electrophoresis (before first use of the kit)” on page 34 or
“Set up the 3730/3730xl instruments for electrophoresis (before first use of the kit)” on page 36
▼
“Prepare samples for electrophoresis” on page 39
▼
Analyze data
“Set up the GeneMapper™ ID‑X Software for analysis (before first use of the kit)” on page 43
“Create an analysis method” on page 48
“Create a size standard definition file” on page 56
“Analyze and edit sample files with GeneMapper™ ID‑X Software” on page 58
“Examine or edit a project” on page 59
VeriFiler™ Express PCR Amplification Kit User Guide
17

Perform PCR
2
Optimize PCR cycle number (before first use of the kit) .................................... 18
■
Before you begin ..................................................................... 19
■
Treated paper substrates: prepare the amplification kit reactions ........................... 20
■
Untreated paper substrates: prepare the amplification kit reactions ......................... 22
■
Swab substrates: prepare the amplification kit reactions ................................... 24
■
Perform PCR ......................................................................... 28
■
Optimize PCR cycle number (before first use of the kit)
Before using the VeriFiler™ Express kit for the first time, perform a single initial sensitivity experiment
to determine the appropriate cycle number to use during internal validation studies and operational use
of the kit. This experiment accounts for instrument‑to‑instrument and sample‑to‑sample variations. If
you are processing multiple sample type and substrate combinations (for example, buccal samples on
treated paper and buccal samples on swabs), perform separate sensitivity experiments for each sample
type and substrate to be used for testing.
Procedural guidelines Optimize PCR cycle number
•
Use 19 samples so that you can complete electrophoresis using a single 96‑well plate. This
minimizes the impact of run‑to‑run variation on the results. Additional samples may be used for
a more comprehensive sensitivity experiment. Examples of PCR and electrophoresis plate layouts
are provided on page “Example PCR plate layout” on page 129.
•
To maximize result quality, prepare and amplify Plate 1, then repeat for Plates 2, 3, and 4. Do not
prepare all 4 plates before amplification.
•
To minimize the eect of instrument‑to‑instrument variation, use the same thermal cycler to amplify
all 4 plates.
Select samples and prepare plates
1.
Select 19 of each sample+substrate type. Ensure that the selected samples represent a "typical"
range of samples analyzed in your laboratory.
2.
Prepare the samples and the reactions as described in the appropriate protocols later in this
chapter. Prepare sucient PCR reagents to complete amplification of three replicate plates.
3.
Create the first of 4 identical PCR plates (see page “Example PCR plate layout” on page 129 for a
suggested plate layout).
18
VeriFiler™ Express PCR Amplification Kit User Guide
Chapter 2 Perform PCR
Before you begin
4.
Amplify each plate using a dierent cycle number to determine the optimum conditions for use in
your laboratory.
Suggested cycle numbers for dierent sample type and substrate combinations are listed in the
following table.
2
Sample type
Blood 25, 26, 27, 28 cycles 25, 26, 27, 28 cycles N/A
Buccal 25, 26, 27, 28 cycles 25, 26, 27, 28 cycles 25, 26, 27. 28 cycles
Treated paper Untreated paper Swab
Determine optimum PCR conditions
1.
Run the PCR products on the appropriate CE platform using the recommended protocol that is
described in Chapter 3, “Perform electrophoresis”.
2.
Based on the results of the sensitivity study, select the appropriate PCR cycle number for future
experiments.
Our studies indicate the optimum PCR cycle number should generate profiles with the following
heterozygote peak heights, with no instances of allelic dropout and minimal occurrence of o‑scale
allele peaks:
Instrument
3500 Series 3,000–12,000 RFU
3130 Series 1,000–3,000 RFU
Substrate
Heterozygous peak height
3730 Series 3,000–12,000 RFU
SeqStudio™ Genetic Analyzer 3,000–12,000 RFU
When amplifying single‑source, unpurified samples, you will see greater sample‑to‑sample variation in
peak height than you see with purified samples. Careful optimization of the cycle number helps to
minimize this variation.
Before you begin
Thaw reagents (before first use of the kit)
Thaw the Master Mix and Primer Set.
IMPORTANT!
amplified DNA, allelic ladder, and size standard from light when not in use.
IMPORTANT! Thawing is required only during first use of the kit. After first use, reagents are stored at
2°C to 8°C and do not require subsequent thawing. Do not refreeze the reagents.
The fluorescent dyes attached to the primers are light-sensitive. Protect the primer set,
VeriFiler™ Express PCR Amplification Kit User Guide
19

Chapter 2 Perform PCR
2
Treated paper substrates: prepare the amplification kit reactions
Treated paper substrates: prepare the amplification kit
reactions
Sample preparation guidelines: treated paper substrate
•
Do not add water to the wells on the reaction plate before adding the punches. If you observe
static issues with the paper discs, you can prepare and dispense the 25-µL reaction mix into the
wells of the reaction plate before adding the punches.
Alternatively, dispense 3 µL of low-TE Buer into each sample and negative amplification control
well (not the positive amplification control wells) before adding the punches.
•
Make the punch as close as possible to the center of the sample to ensure optimum peak intensity.
Increasing the size of the punch may cause inhibition during PCR amplification.
•
For manual punching: Place the tip of a 1.2 mm Harris Micro-Punch on the card, hold the barrel of
the Harris Micro-Punch (do not touch the plunger), gently press and twist 1/4-turn, then eject the
punch in to the appropriate well on the reaction plate.
•
For automated punching: See the User Guide of your automated or semiautomated disc punch
instrument for proper guidance.
Prepare low-TE buer
For optimal results, we recommend using low-TE buer for sample preparation. Prepare it as described
in this procedure or buy it from Teknova (Cat. No. T0223).
1.
Mix together:
•
10 mL of 1 M Tris-HCl, pH 8.0
•
0.2 mL of 0.5 M EDTA, pH 8.0
•
990 mL glass-distilled or deionized water
Note: Adjust the volumes accordingly for specific needs.
2.
Aliquot, then autoclave the solutions.
3.
Store the aliquots at room temperature.
20
VeriFiler™ Express PCR Amplification Kit User Guide
Treated paper substrates: prepare the amplification kit reactions
Prepare the reactions: treated paper substrate
IMPORTANT! The fluorescent dyes attached to the primers are light-sensitive. Protect the primer set,
amplified DNA, allelic ladder, and size standard from light when not in use.
If this is the first time you are using the kit, follow the instructions in “Thaw reagents (before first use of
the kit)” on page 19 before proceeding.
1.
Add samples to the MicroAmp™ Optical 96‑well Reaction Plate:
To these wells ... Add...
Negative control 1.2 mm blank disc
Test samples 1.2 mm sample disc
Chapter 2
Perform PCR
2
Positive control
IMPORTANT! Do not add a blank disc
to the positive control well.
Note: The volumes of positive control are suggested amounts and can be adjusted if peak heights
are too high or too low for your optimized cycle number.
2.
Vortex the Master Mix and Primer Set for 3 seconds. Before opening the tubes or bottles, remove
droplets from the caps by centrifuging the tubes briefly or tapping the bottles on the bench.
3.
Pipet the required volumes of components into an appropriately sized polypropylene tube.
Reaction component
Master Mix 10.0 μL
Primer Set 10.0 μL
Low-TE buer 5.0 μL
Note: Include volume for additional reactions to provide excess volume for the loss that occurs
during reagent transfers.
For 25 and 26 cycles 3 μL of Control DNA 007
For 27 cycles 2 μL of Control DNA 007
For 28 cycles 1 μL of Control DNA 007
Volume per reaction
IMPORTANT! This kit is optimized for a 25‑µL PCR reaction volume to overcome the PCR
inhibition that is expected when amplifying unpurified samples. Using a lower PCR reaction
volume may reduce the ability of the kit chemistry to generate full STR profiles. See page 116
for performance testing results using a 10‑µL PCR reaction volume.
4.
Vortex the reaction mix for 3 seconds, then centrifuge briefly.
5.
Dispense 25 µL of the reaction mix into each reaction well of a MicroAmp™ Optical 96-Well
Reaction Plate.
VeriFiler™ Express PCR Amplification Kit User Guide
21

1
Chapter 2 Perform PCR
2
Untreated paper substrates: prepare the amplification kit reactions
6.
Seal the plate with MicroAmp™ Clear Adhesive Film (Cat. No. 4306311) or MicroAmp™ Optical
Adhesive Film (Cat. No. 4311971).
IMPORTANT! We recommend adhesive film for plate sealing to provide a consistent seal across
all wells and prevent evaporation. Do not use caps, which may not provide a consistent seal across
all wells.
IMPORTANT! If you are using the GeneAmp
block and adhesive clear film instead of caps to seal the plate wells, place a MicroAmp™ Optical
Film Compression Pad (Cat. No. 4312639) on top of the plate to prevent evaporation during
thermal cycling. Other validated thermal cyclers do not require a compression pad.
7.
Centrifuge the plate at 3,000 rpm for about 20 seconds in a tabletop centrifuge with plate holders.
8.
Amplify the samples as described in Chapter 2, “Perform PCR”.
IMPORTANT! This kit is not validated for use with the GeneAmp
aluminum 96-well block. Use of this thermal cycling platform may adversely aect performance of
this kit.
™
PCR System 9700 with silver or gold-plated silver
™
PCR System 9700 with the
Untreated paper substrates: prepare the amplification kit
reactions
Sample preparation guidelines: untreated paper substrate
•
Make a 1.2 mm punch as close as possible to the center of the sample to ensure optimum peak
intensity. Increasing the size of the punch may cause inhibition during PCR amplification.
•
If you are using a Bode Buccal DNA Collector™, make
a 1.2 mm punch as close as possible to the tip of
the DNA collector to ensure optimum peak intensity.
A larger punch may cause inhibition during PCR
amplification.
•
For manual punching: Place the tip of a 1.2 mm Harris
Micro-Punch on the card, hold the barrel of the Harris
Micro-Punch (do not touch the plunger), gently press
and twist 1/4-turn, then eject the punch in to the
appropriate well on the reaction plate.
•
For automated punching: See the User Guide of your
automated or semiautomated disc punch instrument for
proper guidance.
1
Location of punch with a Bode Buccal
DNA Collector
™
22
VeriFiler™ Express PCR Amplification Kit User Guide
Untreated paper substrates: prepare the amplification kit reactions
Prepare the reactions: untreated paper substrate
IMPORTANT! The fluorescent dyes attached to the primers are light-sensitive. Protect the primer set,
amplified DNA, allelic ladder, and size standard from light when not in use.
If this is the first time you are using the kit, follow the instructions in “Thaw reagents (before first use of
the kit)” on page 19 before proceeding.
1.
Add Prep‑n‑Go™ Buer (Cat. No. 4467079) to the MicroAmp™ Optical 96-Well Reaction Plate:
To these wells ... Add...
Negative control 5 μL of Prep‑n‑Go™ Buer
Test samples 5 μL of Prep‑n‑Go™ Buer
Positive control For 25 and 26 cycles 2 μL of Prep‑n‑Go™ Buer
For 27 cycles 3 μL of Prep‑n‑Go™ Buer
For 28 cycles 4 μL of Prep‑n‑Go™ Buer
Chapter 2 Perform PCR
2
2.
Add samples to the reaction plate:
To these wells ...
Negative control 1.2 mm blank disc
Test samples 1.2 mm sample disc
Positive control
IMPORTANT! Do not add a blank disc
to the positive control well.
Note: The volumes of positive control are suggested amounts and may be adjusted if peak
heights are too high or too low for your optimized cycle number.
3.
Centrifuge the plate to ensure that the punches are immersed in the Prep‑n‑Go™ Buer.
4.
Vortex the Master Mix and Primer Set for 3 seconds. Before opening the tubes or bottles, remove
droplets from the caps by centrifuging the tubes briefly or tapping the bottles on the bench.
5.
Pipet the required volumes of components into an appropriately sized polypropylene tube.
Reaction component
Master Mix 10.0 μL
For 25 and 26 cycles 3 μL of Control DNA 007
For 27 cycles 2 μL of Control DNA 007
For 28 cycles 1 μL of Control DNA 007
Add...
Volume per reaction
Primer Set 10.0 μL
VeriFiler™ Express PCR Amplification Kit User Guide
23

Chapter 2
2
Swab substrates: prepare the amplification kit reactions
Perform PCR
Note: Include volume for additional reactions to provide excess volume for the loss that occurs
during reagent transfers.
IMPORTANT! This kit is optimized for a 25‑µL PCR reaction volume to overcome the PCR
inhibition that is expected when amplifying unpurified samples. Using a lower PCR reaction
volume may reduce the ability of the kit chemistry to generate full STR profiles. See page 116
for performance testing results using a 10‑µL PCR reaction volume.
6.
Vortex the reaction mix for 3 seconds, then centrifuge briefly.
7.
Dispense 20 µL of the reaction mix into each reaction well of a MicroAmp™ Optical 96-Well
Reaction Plate.
The final volume in each well is 25 µL (reaction mix plus Prep‑n‑Go™ Buer and sample or positive
control).
8.
Seal the plate with MicroAmp™ Clear Adhesive Film (Cat. No. 4306311) or MicroAmp™ Optical
Adhesive Film (Cat. No. 4311971).
IMPORTANT! We recommend adhesive film for plate sealing to provide a consistent seal across
all wells and prevent evaporation. Do not use caps, which may not provide a consistent seal across
all wells.
IMPORTANT! If you are using the GeneAmp
block and adhesive clear film instead of caps to seal the plate wells, place a MicroAmp™ Optical
Film Compression Pad (Cat. No. 4312639) on top of the plate to prevent evaporation during
thermal cycling. Other validated thermal cyclers do not require a compression pad.
9.
Centrifuge the plate at 3,000 rpm for about 20 seconds in a tabletop centrifuge with plate holders.
10.
Amplify the samples as described in Chapter 2, “Perform PCR”.
IMPORTANT! This kit is not validated for use with the GeneAmp
aluminum 96-well block. Use of this thermal cycling platform may adversely aect performance of
this kit.
™
PCR System 9700 with silver or gold-plated silver
™
PCR System 9700 with the
Swab substrates: prepare the amplification kit reactions
Sample preparation guidelines: swab substrate
•
Detach each buccal swab head from the swab shaft before lysis.
•
If you are using the heated lysis protocol, perform lysis in either of the following formats:
–
1.5-mL tubes with a heat block (VWR™ Scientific Select dry heat block or similar)
–
PrepFiler™ 96-Well Processing Plates (Cat. No. A47010)
–
Robbins Scientific™ Model 400 Hybridization Incubator or similar
24
VeriFiler™ Express PCR Amplification Kit User Guide

Swab substrates: prepare the amplification kit reactions
–
Agilent™ Benchtop Rack for 200 μL Tubes/V Bottom Plates (metal) or similar (Cat. No. 410094)
IMPORTANT! Do not use a plastic plate adaptor.
•
For optimum performance, lyse the entire swab. If you need to preserve the sample, use half of the
lysate prepared from the entire swab.
Prepare the sample lysate: room temperature
This protocol may improve the performance for challenging or aged samples.
1.
Add 400 µL Prep‑n‑Go™ Buer (Cat. No. 4471406) to 1.5-mL tubes or the appropriate wells of a
PrepFiler™ 96-Well Processing Plate (Cat. No. A47010).
2.
Into each tube or well, put the entire head of each swab, then let stand for 20 minutes at room
temperature (20°C to 25°C) to lyse the sample.
3.
After 20 minutes, transfer the sample lysate out of the sample plate into tubes or plates for storage,
then discard the deep‑well plate containing the swab heads.
Note: To minimize the risk of contamination, do not remove the swab heads from the sample
lysate plate before transferring the lysate.
Chapter 2
Perform PCR
2
4.
Go to “Prepare the reactions: swab substrate” on page 26 or “Store the sample lysate” on
page 27.
Prepare the sample lysate: heat protocol
This protocol may improve the performance of challenging or aged samples.
1.
Preheat the heat block to 90°C, or preheat the oven with a metal plate adaptor to 99°C.
2.
Add 400 µL of Prep‑n‑Go™ Buer (for buccal swabs, Cat. No. 4471406) to 1.5-mL tubes or to the
appropriate wells of a PrepFiler™ 96-Well Processing Plate (Cat. No. A47010).
3.
Into each tube or well, put the entire head of each swab. If you are using tubes, cap the tubes.
4.
Let the tubes or plate stand for 20 minutes in the preheated heat block or oven to lyse the sample.
5.
After 20 minutes, remove the tubes or plate from the heat block or oven, then let the lysate stand
at room temperature for ≥15 minutes to cool the lysate (for accurate pipetting).
6.
Transfer the sample lysate to tubes or plates for storage. Discard the 1.5‑mL tubes or PrepFiler
96-Well Processing Plate containing the swab heads.
Note: To minimize the risk of contamination, do not remove the swab heads from the sample
lysate plate before transferring the lysate.
™
7.
Go to “Prepare the reactions: swab substrate” on page 26 or “Store the sample lysate” on
page 27.
VeriFiler™ Express PCR Amplification Kit User Guide
25
Chapter 2
2
Swab substrates: prepare the amplification kit reactions
Perform PCR
Prepare the reactions: swab substrate
IMPORTANT! The fluorescent dyes attached to the primers are light-sensitive. Protect the primer set,
amplified DNA, allelic ladder, and size standard from light when not in use.
If this is the first time you are using the kit, follow the instructions in “Thaw reagents (before first use of
the kit)” on page 19 before proceeding.
1.
Vortex the Master Mix and Primer Set for 3 seconds. Before opening the tubes or bottles, remove
droplets from the caps by centrifuging the tubes briefly or tapping the bottles on the bench.
2.
Pipet the required volumes of components into an appropriately sized polypropylene tube.
Reaction component
Master Mix 10.0 μL
Primer Set 10.0 μL
Low-TE buer 3.0 μL
Note: Include volume for additional reactions to provide excess volume for the loss that occurs
during reagent transfers.
Volume per reaction
IMPORTANT! This kit is optimized for a 25‑µL PCR reaction volume to overcome the PCR
inhibition that is expected when amplifying unpurified samples. Using a lower PCR reaction
volume may reduce the ability of the kit chemistry to generate full STR profiles. See page 116
for performance testing results using a 10‑µL PCR reaction volume.
3.
Vortex the reaction mix for 3 seconds, then centrifuge briefly.
4.
Dispense 23 μL of the reaction mix into each reaction well of a MicroAmp™ Optical 96-Well
Reaction Plate.
The final volume in each well is 25 μL (reaction mix plus Prep‑n‑Go™ Buer or sample lysate).
5.
Add samples to the MicroAmp™ Optical 96-Well Reaction Plate:
26
To these wells ...
Test samples 2 μL of sample lysate
Positive control For 25 and 26 cycles 3 μL of Control DNA
For 27 cycles 2 μL of Control DNA
For 28 cycles 1 μL of Control DNA
Note: The volumes of positive control are suggested amounts and may be adjusted if peak
heights are too high or too low for your optimized cycle number.
VeriFiler™ Express PCR Amplification Kit User Guide
Add...
007
007
007

Chapter 2 Perform PCR
Swab substrates: prepare the amplification kit reactions
6.
Seal the plate with MicroAmp™ Clear Adhesive Film (Cat. No. 4306311) or MicroAmp™ Optical
Adhesive Film (Cat. No. 4311971).
IMPORTANT! We recommend adhesive film for plate sealing to provide a consistent seal across
all wells and prevent evaporation. Do not use caps, which may not provide a consistent seal across
all wells.
2
IMPORTANT! If you are using the GeneAmp
block and adhesive clear film instead of caps to seal the plate wells, place a MicroAmp™ Optical
Film Compression Pad (Cat. No. 4312639) on top of the plate to prevent evaporation during
thermal cycling. Other validated thermal cyclers do not require a compression pad.
7.
Vortex the reaction mix at medium speed for 3 seconds.
8.
Centrifuge the plate at 3,000 rpm for about 20 seconds in a tabletop centrifuge with plate holders.
9.
Amplify the samples as described in Chapter 2, “Perform PCR”.
IMPORTANT! This kit is not validated for use with the GeneAmp
aluminum 96-well block or the Veriti™ Thermal Cyclers. Use of these thermal cycling platforms may
adversely aect performance of this kit.
Store the sample lysate
1.
Cap the sample lysate storage tubes or seal the sample lysate storage plate with MicroAmp™ Clear
Adhesive Film.
2.
Store the sample lysate as needed.
Storage time
™
PCR System 9700 with silver or gold-plated silver
™
PCR System 9700 with the
Storage temperature
<2 weeks 2°C to 8°C
>2 weeks –25°C to –15°C
Note: The eects of multiple freeze/thaw cycles on the lysate have not been fully evaluated.
Therefore, multiple freeze/thaw cycles are not recommended.
VeriFiler™ Express PCR Amplification Kit User Guide
27
Chapter 2 Perform PCR
2
Perform PCR
Perform PCR
IMPORTANT! This kit is validated for use with the validated thermal cyclers listed in “Instrument and
software compatibility” on page 15
1.
Program the thermal cycling conditions.
IMPORTANT! If you are using the GeneAmp
If you are using the ProFlex™ 96‑well PCR System, select the GeneAmp™ PCR System 9700
simulation mode.
™
PCR System 9700, select the Max ramping mode.
Initial
incubation
step
HOLD CYCLE HOLD HOLD
95°C,
1 minute
[1]
See “Optimize PCR cycle number (before first use of the kit)” on page 18.
[2]
The infinity (∞) setting allows an unlimited hold time.
2.
Load the plate into the thermal cycler, close the heated cover, then start the run.
Denature Anneal Extend
94°C,
3 seconds
Optimum cycle number
59°C,
16 seconds
[1]
65°C,
29 seconds
Final
extension
60°C,
5 minutes
Final hold
4°C,
up to 24 hours
IMPORTANT! If you are using adhesive clear film instead of caps to seal the plate wells, be sure
to place a MicroAmp™ Optical Film Compression Pad (Cat. No. 4312639) on top of the plate to
prevent evaporation during thermal cycling. The ProFlex™ 96‑well PCR System does not require a
compression pad.
3.
When the run is complete, store the amplified DNA.
If you are storing the DNA...
<2 weeks 2°C to 8°C
>2 weeks –25°C to –15°C
Then place at...
[2]
28
IMPORTANT! Protect the amplified DNA from light.
VeriFiler™ Express PCR Amplification Kit User Guide
Perform electrophoresis
3
Allelic ladder requirements for electrophoresis ........................................... 29
■
Materials required for electrophoresis ................................................... 30
■
Set up the SeqStudio™ instruments for electrophoresis (before first use of the kit) ............ 30
■
Set up the 3500/3500xL instruments for electrophoresis (before first use of the kit) ........... 32
■
Set up the 3130/3130xl instruments for electrophoresis (before first use of the kit) ............ 34
■
Set up the 3730/3730xl instruments for electrophoresis (before first use of the kit) ............ 36
■
Prepare samples for electrophoresis .................................................... 39
■
Allelic ladder requirements for electrophoresis
To accurately genotype samples, you must run an allelic ladder with the samples.
Instrument
3500 1 per 3 injections 8 samples 23 samples + 1 allelic ladder
3500xL 1 per injection 24 samples 23 samples + 1 allelic ladder
3130 1 per 4 injections 4 samples 15 samples + 1 allelic ladder
3130xl 1 per injection 16 samples 15 samples + 1 allelic ladder
3730/3730xl,
48‑capillary
SeqStudio
IMPORTANT! Variation in laboratory temperature can cause changes in fragment migration speed and
sizing variation between runs. Follow the guidelines in the preceding table, which should account for
normal variation in run speed. Perform internal validation studies to verify the required allelic ladder
injection frequency, to ensure accurate genotyping of all samples in your laboratory environment.
It is critical to genotype using an allelic ladder run under the same conditions as the samples. Size
values obtained for the same sample can dier between instrument platforms, because of dierent
polymer matrices and electrophoretic conditions.
™
Number of allelic
ladders to run
3 per injection 48 samples 15 samples + 1 allelic ladder
1 per 6 injections 4 samples 23 samples + 1 allelic ladder
One injection
equals
Number of samples per allelic ladder(s)
VeriFiler™ Express PCR Amplification Kit User Guide
29
Chapter 3 Perform electrophoresis
3
Materials required for electrophoresis
Materials required for electrophoresis
Appendix B, “Required materials not supplied” lists the required materials that are not supplied with this
kit.
IMPORTANT! The fluorescent dyes attached to the primers are light-sensitive. Protect the primer set,
amplified DNA, allelic ladder, and size standard from light when not in use.
Set up the SeqStudio™ instruments for electrophoresis
(before first use of the kit)
Electrophoresis software setup
The following table lists the data collection software and the run modules that you can use to analyze
PCR products generated by this kit. For details on the procedures, see the documents listed in
“Documentation and support” on page 136.
Genetic
Analyzer
SeqStudio
Data Collection
Software
™
SeqStudio
Data Collection
Software v1.2.1
Additional software Run modules and conditions
™
None
•
Run Module: HIDAnalysis
•
Injection Conditions: 1.2 kV/10 sec
•
Run Conditions: 11 kV/1,120 sec
•
Dye Set J6
•
Kit: VeriFiler™ Express (to enable marker-to-marker
pull-up reduction feature)
30
VeriFiler™ Express PCR Amplification Kit User Guide
 Loading...
Loading...