Thermo Fisher Scientific 42885 User Manual
Certificate of Analysis
Product No.: 42885
Multi-Element Plasma Standard Solution 4
Matrix: Diluted HNO3 Lot No.: 217274
Expiry Date: June 30, 2021
Intended Use: This solution is intended for use as a certified reference material for inductively coupled plasma optical emission
spectroscopy (ICP-OES), inductively coupled plasma ma ss spectrometry (ICP-MS), or alternative techniques, such as flame or furnace
atomic absorption spectr oscopy (A A or GFA A).
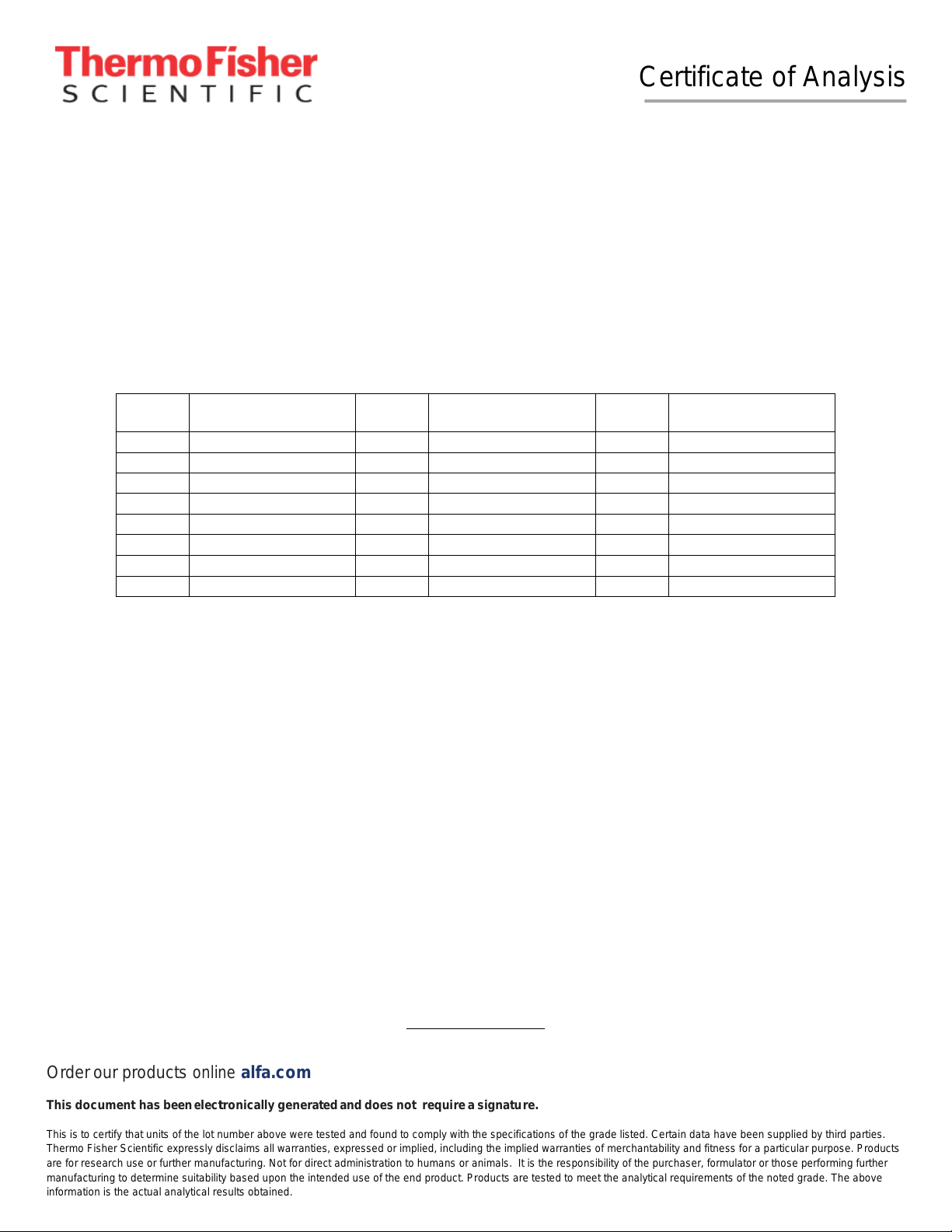
Element
Ag 1001 ± 5 µg/g Cr 1000 ± 5 µg/g Mn 1000 ± 5 µg/g
Al 1000 ± 5 µg/g Cu 1001 ± 5 µg/g Na 1000 ± 5 µg/g
B 1000 ± 5 µg/g Fe 1000 ± 5 µg/g Ni 1000 ± 5 µg/g
Ba 999.9 ± 5.0 µg/g Ga 1000 ± 5 µg/g Pb 999.9 ± 5.0 µg/g
Bi 1000 ± 5 µg/g In 1001 ± 5 µg/g Sr 1000 ± 5 µg/g
Ca 1001 ± 5 µg/g K 999.9 ± 5.0 µg/g Tl 1000 ± 5 µg/g
Cd 1000 ± 5 µg/g Li 1000 ± 5 µg/g Zn 999.6 ± 5.0 µg/g
Co 1001 ± 5 µg/g Mg 999.9 ± 5.0 µg/g
Certification & Traceability: This CRM was manufactured and certified under an ISO 9001, ISO/IEC 17025, and ISO Guide 34
quality management system. This CRM was prepared to the certifi ed concentrations shown above by gravimetric methods using singleelement concentrates that were certified using the “High Performa nce ICP-OES” protocol de veloped by NI ST and are directly tr aceable
to the NIST SRMs listed below. This solution was stabilized using high purity nitric acid (HNO
ohm deionized water. The balances used in the preparation of this CRM are calibrated regular ly with traceability t o NIST. All volumetric
dilutions are performed in Class A calibrated glassware. The certified concentrations were determined based upon gravimetric
procedures. Secondary verification of the certified concentration s was performed using ICP-OES t hat was calibrated and/or refer enced
against NIST SR Ms: 3151, 3101a, 310 7, 3104a, 3106, 3109a, 3108 , 3113, 3112a, 3114, 3126a, 3119a, 3124a, 3141a, 3129a , 3131a,
3132, 3152a, 3136, 3128, 3153a, 3158 and 3168a. The uncertainty associated with each certified concentration represents the
expanded uncertainty at the 95% confidence level using a coverage factor of k=2.
Instructions for Use: We recommend that the solution be thoroughly m ixed by repeated shak ing or swirling of the bottle imm ediately
prior to use. To achieve the highest accuracy the analyst shou ld: (1) use only pre-cleaned containers and transferw are, (2) not pipette
directly from the CRM’s original container, (3) use a minimum su b-samp le size of 50 0µL, (4) ma ke dilution s using calib r ated b alances or
certified volumetric clas s A flasks and pipettes, (5) dilu te with the same matrix as the original CRM, and (6) never pour used product
back into the original container. The solution should be kept tightly capped and stored under normal laboratory conditions. Do not freeze,
heat, or expose to direct sunlight. Minimize exposure to moisture or high humidity.
Order our products
This document has been
This is to certify that units of the lot number above were tested and found to comply with the specifications of the grade listed. Certain data have been supplied by third parties.
Thermo Fisher Scientific expressly disclaims all warranties, expressed or implied, including the implied warranties of merchantability and fitness for a particular purpose. Products
are for research use or further manufacturing. Not for direct administration to humans or animals. It is the responsibility of the purchaser, formulator or those performing further
manufacturing to determine suitability based upon the intended use of the end product. Products are tested to meet the analytical requirements of the noted grade. The above
information is the actual analytical results obtained.
Period of Validity: Alfa Aesar guarantees the accuracy of this Spe cpure® solution until the expiry date shown above, pr ovided the
instructions for use are followed. During the period of validity, the purchaser will be notified if this product is recalled due to any
significant changes in the stability of the solution.
online
electronically generated and does not require a signature.
Certified Concentration
& Uncertainty
alfa.com
Element
Certified Concentration
______1/26/2021______
Certification Date
& Uncertainty
Density: 1.084 g/mL @ 20°C
Element
) and diluted with filtered (0.22µm), 18 M-
3
Certified Concentration
& Uncertainty
Page 1 of 2

Certificate of Analysis
Order our products
This document has been
This is to certify that units of the lot number above were tested and found to comply with the specifications of the grade listed. Certain data have been supplied by third parties.
Thermo Fisher Scientific expressly disclaims all warranties, expressed or implied, including the implied warranties of merchantability and fitness for a particular purpose. Products
are for research use or further manufacturing. Not for direct administration to humans or animals. It is the responsibility of the purchaser, formulator or those performing further
manufacturing to determine suitability based upon the intended use of the end product. Products are tested to meet the analytical requirements of the noted grade. The above
information is the actual analytical results obtained.
Hazard Information: Refer to the Safety Data Sheet (SDS).
Homogeneity: This solution was determined to be homogeneous by procedures consistent with the requirements of ISO Guide 34 and
ISO Guide 35. Replicate samples of the finished solution were analyzed to confirm its homogeneity, in accordance with QSP 6-13
Assessment of Homogeneity and Stability. To ensure h omogeneity, users should not take a smaller sub-sample than specified in the
Instructions for Use, as d oing so will i nvalida te t he ce rtifie d val ues and unc ert aintie s.
Further Information: Please contact Alfa Aesar for further informatio n about th is CRM.
Quality Certifications: This CRM was prepared under a quality management system that is:
• Registered to ISO 9001 – Quality Management Sys tems – Requireme nts (TUV USA Cert. No. 12-1280)
• Accredited to ISO Guide 34 – General Requirem ents for the Competence of Reference Material Prod ucers (A2LA Cert. No.
2848.02)
o ISO Guide 34 references a dditi onal requi reme nts sp ecifie d in IS O Guide 31 a nd IS O Guid e 35
• Accredited ISO/IEC 17025 – Gener al Requirements for the Competen ce of Testing and Calibration Labor atories (A2LA Cert.
No. 2848.01)
online
alfa.com
Page 2 of 2
electronically generated and does not require a signature.
 Loading...
Loading...