Thermo Fisher TaqPath Instructions Manual

TaqPath™ COVID‑19 HT Kit
INSTRUCTIONS FOR USE
Multiplex real‑time RT‑PCR test intended for the qualitative
detection of nucleic acid from SARS‑CoV‑2 in high‑throughput
workf lows
REF. A50883
Publication Number MAN0024711
Revision A.0
For In Vitro Diagnostic Use.
Life Technologies Corporation |
6055 Sunol Blvd |
Pleasanton, California 94566 USA
For descriptions of symbols on product labels or product documents, go to thermofisher.com/symbols-definition.
Life Technologies Europe B.V.
Kwartsweg 2, 2665 NN Bleiswijk
The Netherlands
The customer is responsible for validation of assays and compliance with regulatory requirements that pertain to their procedures and
uses of the instrument.
The information in this guide is subject to change without notice.
DISCLAIMER: TO THE EXTENT ALLOWED BY LAW, THERMO FISHER SCIENTIFIC INC. AND/OR ITS AFFILIATE(S) WILL NOT BE
LIABLE FOR SPECIAL, INCIDENTAL, INDIRECT, PUNITIVE, MULTIPLE, OR CONSEQUENTIAL DAMAGES IN CONNECTION WITH OR
ARISING FROM THIS DOCUMENT, INCLUDING YOUR USE OF IT.
Revision history: Pub. No. MAN0024711
Revision Date Description
A.0 29 January 2021 New TaqPath™ COVID‑19 HT Kit Instructions for Use.
Important Licensing Information: These products may be covered by one or more Limited Use Label Licenses. By use of these
products, you accept the terms and conditions of all applicable Limited Use Label Licenses.
TRADEMARKS: All trademarks are the property of Thermo Fisher Scientific and its subsidiaries unless otherwise specified. Agilent is
a trademark of Agilent Technologies, Inc.. Axygen is a trademark of Axygen, Inc.. Biotek is a registered trademark and ELx405 is a
trademark of BioTek Instruments, Inc.. Tecan, Fluent, FluentControl, Flexible Channel Arm, and Multichannel Arm are trademarks of
Tecan Group, Ltd. Roche and cobas are registered trademarks of Roche Molecular Systems, Inc.. Quidel is a registered trademark and
Lyra is a trademark of Quidel.
©2021 Thermo Fisher Scientific Inc. All rights reserved.

Contents
■
CHAPTER 1 TaqPath™ COVID‑19 HT Kit product information .................. 5
Intended Use ................................................................... 5
Product description ............................................................. 5
Contents and storage ............................................................ 6
Required materials not supplied ................................................... 7
Assay limitations ................................................................ 7
In‑use reagent stability ........................................................... 8
■
CHAPTER 2 Before you begin ..................................................... 9
Warnings and precautions ........................................................ 9
Sample collection, transport, and storage .......................................... 9
Nucleic acid isolation guidelines ................................................. 10
■
CHAPTER 3 Perform an Amplitude™ Platform Module 1 run .................. 11
Module 1 overview ............................................................. 11
Module 1 required samples, reagents, and consumables ........................... 11
Procedural guidelines ........................................................... 12
Guidelines for controls ..................................................... 12
Guidelines for sample tubes ................................................. 13
Guidelines for sample inactivation ........................................... 13
Before you begin—Module 1 .................................................... 14
Thaw TaqPath™ COVID‑19 HT MS2 Phage Control ................................. 14
Load the Tecan™ Fluent™ 1080 Automation Workstation deck ........................ 14
■
CHAPTER 4 Perform an Amplitude™ Platform Module 2 run .................. 17
Module 2 overview ............................................................. 17
Module 2 required sample input, reagents, and consumables ....................... 17
Before you begin—Module 2 .................................................... 19
Thaw Module 2 reagents ........................................................ 19
Load reagents and consumables on the Tecan™ Fluent™ 780 Automation
Workstation deck ............................................................ 19
TaqPath
™
COVID‑19 HT Kit Instructions for Use
3

Contents
■
CHAPTER 5 Review results (automated workflow) ............................. 21
Automated results process ...................................................... 21
Interpretation of the results ...................................................... 21
■
APPENDIX A Performance characteristics for Amplitude
™
High‑Throughput COVID-19 Testing Solution ................................... 23
Limit of detection bridging study ................................................. 23
Cross‑contamination study ...................................................... 24
Reactivity (Inclusivity) ........................................................... 25
Amplitude™ High‑Throughput COVID-19 Testing Solution comparator studies ......... 25
■
APPENDIX B Amplitude™ Platform system components ...................... 29
■
Safety ................................................................................. 31
Chemical safety ................................................................ 31
Biological hazard safety ......................................................... 32
■
APPENDIX C Documentation and support ...................................... 33
Customer and technical support ................................................. 33
Limited product warranty ........................................................ 33
4
TaqPath™ COVID‑19 HT Kit Instructions for Use

TaqPath™ COVID‑19 HT Kit product
1
Intended Use
information
Applied Biosystems™ TaqPath™ COVID‑19 HT Kit is the high‑throughput format of the TaqPath
COVID‑19 CE‑IVD RT‑PCR Kit. The kit is intended for use with the MagMAX™ Viral/Pathogen II Nucleic
Acid Isolation Kit, High‑Throughput and is compatible with the Amplitude™ Platform. The kit contains
the reagents and controls for a real‑time reverse transcription polymerase chain reaction (RT‑PCR) test
intended for the qualitative detection of nucleic acid from SARS‑CoV‑2 in upper respiratory specimens
(nasopharyngeal and nasal) from individuals suspected of COVID-19.
Results are for the identification of SARS-CoV-2 RNA. The SARS-CoV-2 RNA is generally detectable
in upper respiratory specimens during the acute phase of infection. Results should not be used as
the sole basis for diagnosis and patient management decisions. Clinical correlation with patient history
and other diagnostic and epidemiological information should be used to evaluate the significance of
the results in order to assist the clinician or epidemiologist to determine patient infection status and
the appropriate treatment regime. Positive results do not rule out bacterial infection or co‑infection
with other viruses. The agent detected may not be the definite cause of disease. Laboratories may be
required to report all positive results to the appropriate Competent Health Authorities. Negative results
do not preclude SARS‑CoV‑2 infection.
Testing with the TaqPath™ COVID‑19 HT Kit is intended for use by qualified and trained clinical
laboratory personnel specifically instructed and trained in the techniques of real‑time PCR and in vitro
diagnostic procedures. The TaqPath™ COVID‑19 HT Kit is intended for use in a qualified laboratory
environment according to the sample type.
™
Product description
The TaqPath™ COVID‑19 HT Kit includes the following components:
•
TaqPath™ COVID‑19 Module 1 MS2 Phage Control—An internal process control for RNA extraction.
•
TaqPath™ COVID‑19 Module 2 Assay Kit—Multiplexed assays that contain three primer/probe
sets specific to 3 dierent SARS-CoV-2 genomic regions (ORF1ab, N gene, and S gene) and
primers/probes for bacteriophage MS2.
•
TaqPath™ COVID‑19 Module 2 IVT RNA Control—An RNA control that contains targets specific to
the SARS-CoV-2 genomic regions targeted by the assays.
•
TaqPath™ COVID‑19 Module 2 Control Dilution Buer
•
TaqPath™ HT Module 2 1-Step Multiplex Master Mix (No ROX)
•
TaqPath™ HT Module 2 Empty 5mL Mixing Tubes
•
HT Water for Dilution
TaqPath™ COVID‑19 HT Kit Instructions for Use
5
Chapter 1 TaqPath™ COVID‑19 HT Kit product information
1
Contents and storage
The TaqPath™ COVID‑19 HT Kit is compatible with the Amplitude™ Platform. As part of the Amplitude
High‑Throughput COVID-19 Testing Solution, the Amplitude™ Platform is used with the following
products to detect SARS-CoV-2 RNA in human upper respiratory specimens.
•
TaqPath™ COVID‑19 HT Kit (Cat. No. A50883)
•
MagMAX™ Viral/Pathogen II Nucleic Acid Isolation Kit, High‑Throughput (Cat. No. A50884)
The results are automatically analyzed with the pre-installed COVID‑19 Interpretive Software Driver
v1.1.0. For more information on the automated results process, see Chapter 5, “Review results
(automated workflow)”.
Contents and storage
The TaqPath™ COVID-19 HT Kit contains sucient reagents for 20,000 reactions.
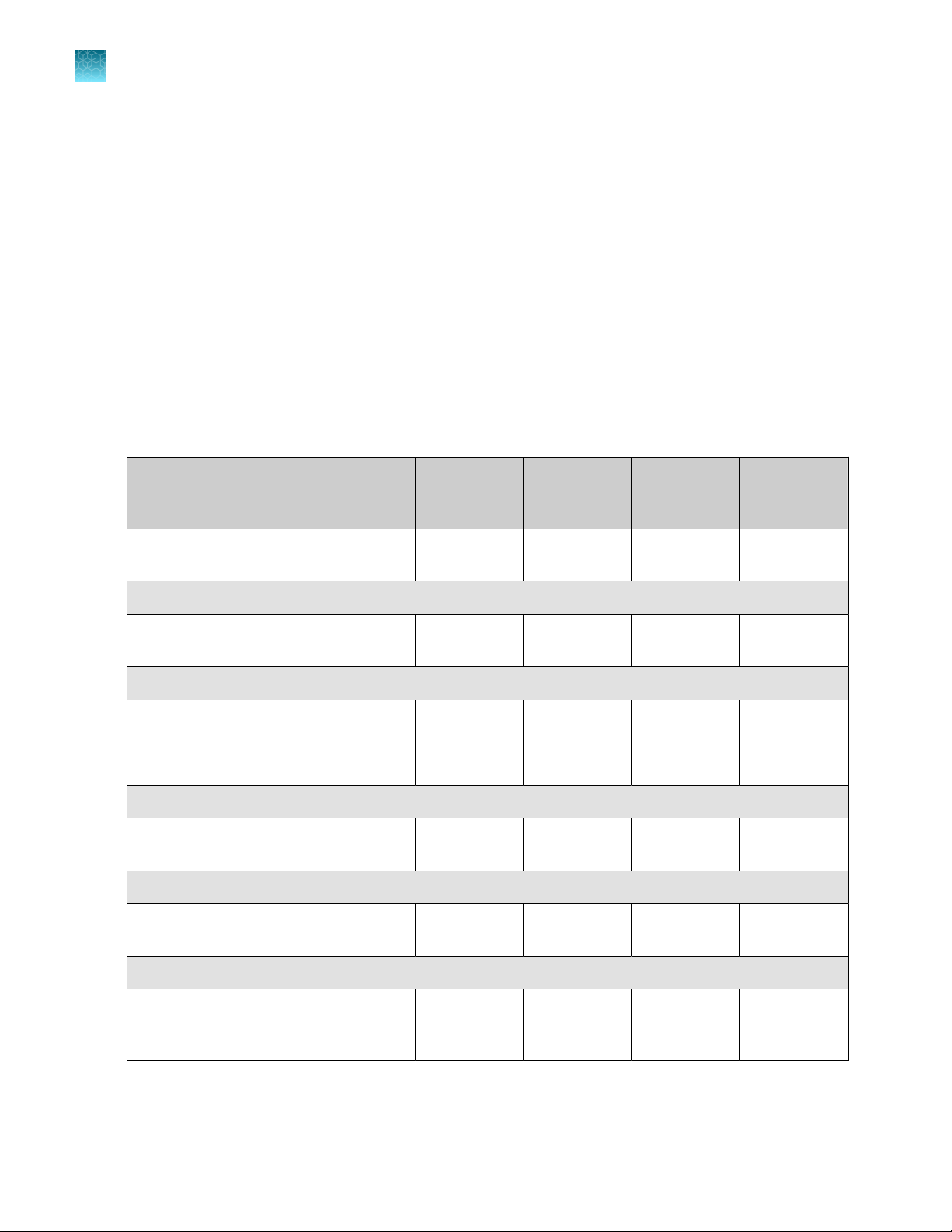
Table 1 TaqPath™ COVID-19 HT Kit (REF. A50883)
Amplitude
Platform
module
Module 1 and
Module 2
™
Component REF. Quantity Amount Storage
HT Water for Dilution R05846F1 3 3 × 20 mL 15°C to 25°C
™
[1]
TaqPath™ COVID‑19 Module 1 MS2 Phage Control (Cat. No. A50749)
Module 1
TaqPath™ COVID‑19 Module 2 Assay Kit (Cat. No. A50750)
Module 2
TaqPath™ COVID‑19 Module 2 IVT RNA Control (Cat. No. 966128)
Module 2
TaqPath™ COVID‑19 Module 2 Control Dilution Buer (Cat. No. A50751)
Module 2
TaqPath™ HT Module 2 1-Step Multiplex Master Mix (No ROX) (Cat. No. A50772)
Module 2
TaqPath™ COVID‑19 HT
MS2 Phage Control
TaqPath™ COVID‑19 HT
qPCR Assay
2‑mL mixing tubes
TaqPath™ COVID‑19 HT
IVT RNA Control
TaqPath™ HT Control
Dilution Buer
TaqPath™ 1-Step
Multiplex Master Mix (No
ROX)
[2]
100099158 3 6 × 10 mL
100099159 3 6 × 1,600 μL
4339337 3 6 tubes 15°C to 30°C
91‑6128 3 3 × 25 µL ≤–70°C
100099160 3 3 × 750 µL
100032397F4 3 2 × 10 mL
–30°C to
–10°C
–30°C to
–10°C
–30°C to –
10°C
–30°C to –
10°C
6
TaqPath™ COVID‑19 HT Kit Instructions for Use
Chapter 1
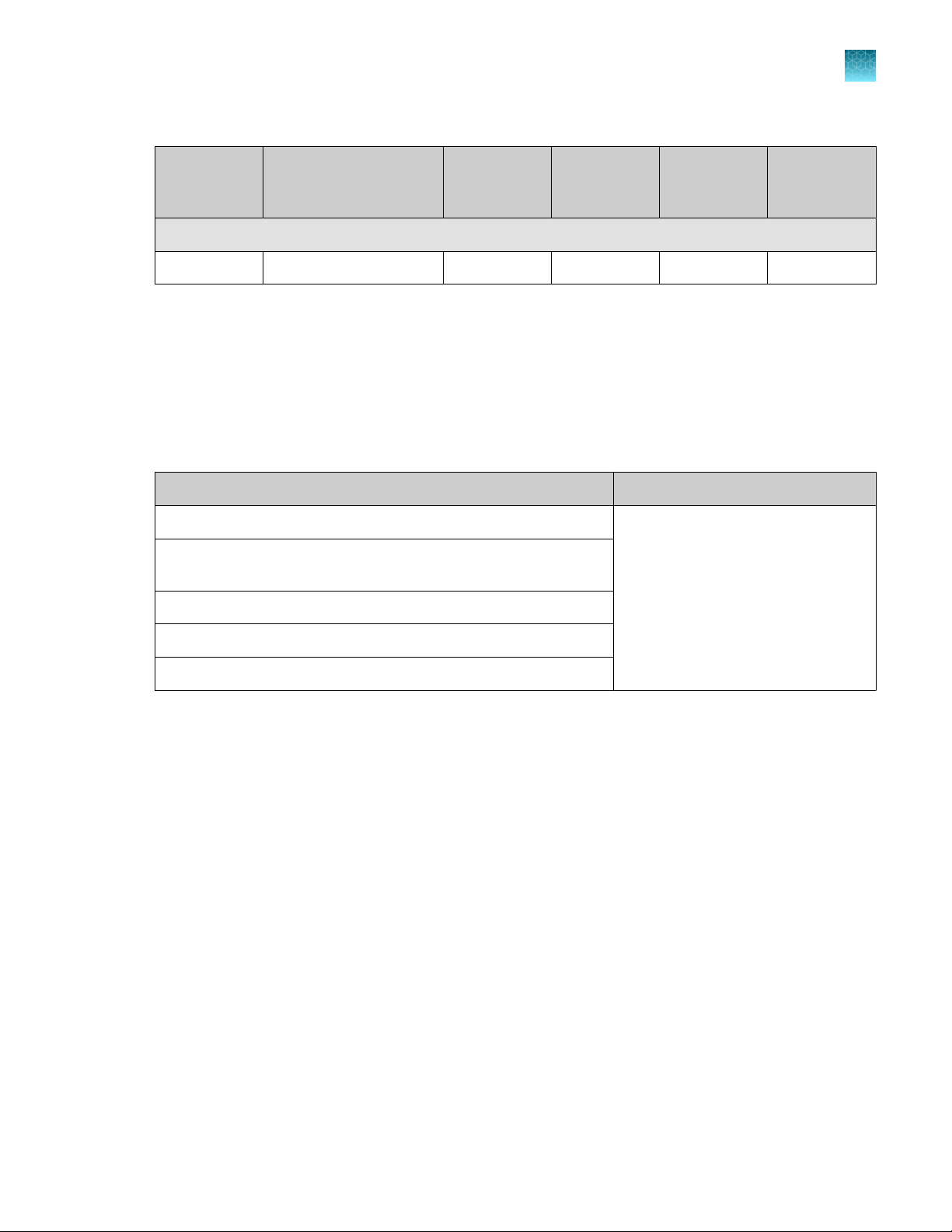
Table 1 TaqPath COVID-19 HT Kit (REF. A50883) (continued)
Amplitude
Platform
module
TaqPath™ HT Module 2 Empty 5mL Mixing Tubes (Cat. No. A50893)
Module 2 5‑mL mixing tubes — 3 25 tubes 15°C to 30°C
[1]
See label for expiration date.
[2]
Empty plastic tubes used for the serial dilution of the TaqPath™ COVID‑19 Module 2 IVT RNA Control in the TaqPath™ COVID‑19
Module 2 Control Dilution Buffer.
™
Component REF. Quantity Amount Storage
Required materials not supplied
Unless otherwise indicated, all materials are available through thermofisher.com. "MLS" indicates that
the material is available from fisherscientific.com or another major laboratory supplier.
Item Source
TaqPath™ COVID‑19 HT Kit product information
Required materials not supplied
1
[1]
Amplitude™ Platform Module 1 and Module 2 equipment
MagMAX™ Viral/Pathogen II Nucleic Acid Isolation Kit,
High‑Throughput
80% Ethanol
Silicone oil
Amplitude™ High‑Throughput Consumable Package 3 Plastics
[1]
See also “Module 1 required samples, reagents, and consumables” on page 11 and “Module 2 required sample input, reagents,
and consumables” on page 17.
Assay limitations
•
The TaqPath™ COVID‑19 HT Kit performance was established using nasal and nasopharyngeal
swab samples only. Specimen types other than nasal and nasopharyngeal swab should not be
tested with this assay.
•
Samples must be collected, transported, and stored using appropriate procedures and conditions.
Improper collection, transport, or storage of specimens may hinder the ability of the assay to detect
the target sequences.
•
Chemical inactivation and other known clinically relevant interfering substances and techniques
can lead to inappropriate primary samples and interfere with test results.
•
The Amplitude™ Platform workflow must be performed according to the specified methods
described in the user guide for the system.
•
The quality of the biological samples is essential for the quality of the results generated with this kit.
See the user guide for the Amplitude
Platform (Pub. No. MAN0019842)
™
[1]
TaqPath™ COVID‑19 HT Kit Instructions for Use
7
Chapter 1 TaqPath™ COVID‑19 HT Kit product information
1
In‑use reagent stability
•
False-negative results may arise from:
–
Improper sample collection
–
Degradation of the SARS-CoV-2 RNA during shipping/storage
–
Specimen collection after SARS-CoV-2 RNA can no longer be found in the specimen matrix
–
Using unauthorized extraction or assay reagents
–
The presence of RT-PCR inhibitors
–
Mutation in the SARS-CoV-2 virus
–
Failure to follow instructions for use
•
False-positive results may arise from:
–
Cross contamination during specimen handling or preparation
–
Cross contamination between patient samples
–
Specimen mix-up
–
RNA contamination during product handling
•
The impacts of vaccines, antiviral therapeutics, antibiotics, chemotherapeutic or
immunosuppressant drugs have not been evaluated. The TaqPath™ COVID‑19 HT Kit cannot rule
out diseases caused by other bacterial or viral pathogens.
•
Negative results do not preclude infection with SARS-CoV-2 virus and should not be the sole basis
of a patient management decision.
•
Laboratories are required to report all test results to the appropriate public health authorities.
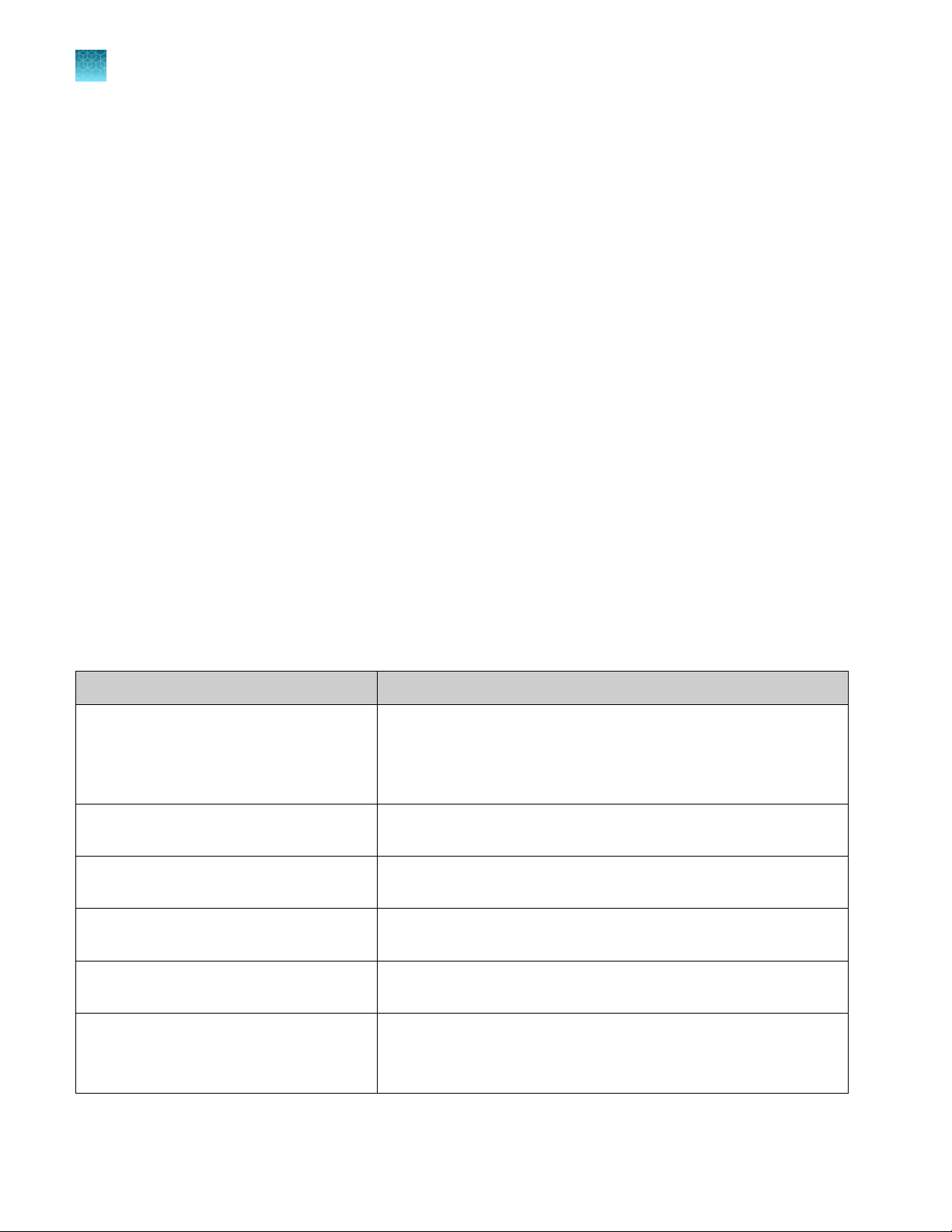
In‑use reagent stability
Reagent
TaqPath™ COVID‑19 HT MS2 Phage Control
TaqPath™ COVID‑19 HT qPCR Assay
TaqPath™ COVID‑19 HT IVT RNA Control
TaqPath™ HT Control Dilution Buer
TaqPath™ 1-Step Multiplex Master Mix (No
ROX)
Purified sample RNA
Stability information
Once thawed and placed in the Tecan™ Fluent™ 1080 Automation
Workstation reagent carrier (15°C to 30°C), use within 24 hours or
store capped at –30°C to –10°C.
Perform a maximum of 5 freeze-thaw cycles.
Once placed in the Tecan™ Fluent™ 780 Automation Workstation
reagent carrier (9°C), the assay is stable for 24 hours.
Once placed in the Tecan™ Fluent™ 780 Automation Workstation
reagent carrier (9°C), the control is stable for 48 hours.
Once placed in the Tecan™ Fluent™ 780 Automation Workstation
reagent carrier (9°C), the dilution buer is stable for 24 hours.
Once placed in the Tecan™ Fluent™ 780 Automation Workstation
reagent carrier (9°C), the master mix is stable for 48 hours.
Once extracted, purified sample RNA is stable at 4°C for 48 hours.
For long term storage, store according to the guidelines established
by your laboratory.
8
TaqPath™ COVID‑19 HT Kit Instructions for Use
2
Warnings and precautions
The system and assay should only be used in a qualified laboratory environment according to the
sample type by qualified and trained sta to avoid cross-contamination and the risk of erroneous
results. For information about operator roles and requirements, see the user guide for your system.
•
Before using this product, read and understand the information provided in this guide and the user
guide for your system.
•
Read and understand the Safety Data Sheets (SDSs) provided by the chemical manufacturer before
you store, handle, or work with any chemicals or hazardous materials. To obtain SDSs, see the
"Documentation and Support" section in this document.
•
Samples and controls should always be treated as if infectious and/or biohazardous in accordance
with safe laboratory procedures.
•
Follow necessary precautions when handling specimens. Use personal protective equipment (PPE)
consistent with current guidelines for the handling of potentially infectious samples.
•
Always use pipette tips with aerosol barriers. Tips that are used must be sterile and free from
DNases and RNases.
•
Do not eat, drink, smoke, or apply cosmetic products in the work areas.
•
Modifications to assay reagents, assay protocol, or instrumentation are not permitted.
•
Reagents must be stored and handled as specified in “Contents and storage” on page 6.
•
Do not use system consumables or reagents after the indicated expiry date.
•
Dispose of waste in compliance with local regulations.
Before you begin
Sample collection, transport, and storage
CAUTION! Handle all samples and controls as if they are capable of transmitting infectious agents.
•
Patient samples must be collected according to appropriate laboratory guidelines. The Amplitude
Platform can be used with patient samples collected via nasopharyngeal or nasal swab.
•
Store samples at 2–8°C for up to 72 hours after collection. If a delay in testing is expected, store
samples at ≤-70°C.
TaqPath™ COVID‑19 HT Kit Instructions for Use
™
9

Chapter 2 Before you begin
2
Nucleic acid isolation guidelines
Nucleic acid isolation guidelines
For information on isolation and purification of viral nucleic acid from human respiratory specimens,
see the MagMAX™ Viral/Pathogen II Nucleic Acid Isolation Kit, High‑Throughput Instructions for Use
(Pub. No. MAN0019941).
10
TaqPath™ COVID‑19 HT Kit Instructions for Use
Perform an Amplitude™ Platform
3
Module 1 overview
Module 1 run
Module 1 operation is controlled by the Tecan™ FluentControl™ Software, via the Tecan™ Fluent™ 1080
Automation Workstation touchscreen. For more information, see the user guide for the Amplitude
Platform (Pub. No. MAN0019842).
During Module 1 operation:
•
Technicians load prepared sample tube runners, reagents, and consumables on the Tecan
Fluent™ 1080 Automation Workstation deck and start runs.
•
The instrument prepares 4 sample extraction plates containing the following components:
–
200 µL of sample from the sample tubes
–
MagMAX™ MVPII HT Proteinase K
–
TaqPath™ COVID‑19 HT MS2 Phage Control
–
Binding Bead Mix
–
Silicone oil
•
The Tecan™ Fluent™ 1080 Automation Workstation scans the barcodes on sample tubes and
sample extraction plates, then registers the information with the SampleManager LIMS™ Software
for sample tracking.
™
Module 1 required samples, reagents, and consumables
For a complete list of required samples, reagents, and consumables, see the user guide for the
Amplitude™ Platform (Pub. No. MAN0019842).
™
Required samples, reagents, and consumables
Sample input
Barcoded sample tubes in sample carrier racks 376 samples per run
Tips and Plates
KingFisher™ Deep‑Well 96 Plate with Barcode (sample extraction plates) 4 plates per run
1,000 µL Flexible Channel Arm™ (FCA) Disposable Tips Rack 15 racks per 3 runs
200 µL Flexible Channel Arm™ (FCA) Disposable Tips Rack 3 racks per 6 runs
TaqPath™ COVID‑19 HT Kit Instructions for Use
Usage
(Reload frequency)
11
 Loading...
Loading...