Page 1

PROTOCOL Sanger sequencing
Protocol for Sanger sequencing of the SARS-CoV-2
spike (S) gene
SARS-CoV-2 infections continue to be a challenge across
the globe. Part of the challenge, often seen with viruses, is
that the nucleic acid genome quickly mutates, producing
new strain lineages. These new lineages may spread more
quickly, cause either milder or more severe disease, may
have decreased susceptibility to therapeutic agents, and
may evade vaccine-induced immunity. Importantly, they
can also have the ability to evade detection by sequencebased diagnostic tests, complicating epidemiological
monitoring. While the SARS-CoV-2 mutation rate is thought
to be lower than those of other RNA viruses, the sheer
number of infections raises the chances that novel strain
lineages will appear in circulation [1]. Recently, two new
lineages that appear to have increased infectivity have
been identified [2,3]. Interestingly, both of these lineages
have many new mutations in the SARS-CoV-2 spike (S)
gene. Because mutations in the S gene have the potential
to aect interactions with the angiotensin-converting
enzyme 2 (ACE2) receptor [4], it is important to monitor
S gene sequences for new mutations.
We therefore developed a protocol for analyzing the entire
S gene by Sanger sequencing. The primer sequences
used here are based on those published by the Centers
for Disease Control and Prevention (CDC) [5]. Briefly,
cDNA synthesis is performed on a sample containing
viral RNA. Next, the cDNA is used in specific regions
of target amplification using tailed primers that cover
the S gene. For this, the Applied Biosystems™ BigDye™
Direct Cycle Sequencing Kit and M13 sequence–tagged
primer sets are used. The amplified sequences are then
subjected to cycle sequencing using either M13-forward or
M13-reverse primers provided in the BigDye Direct Cycle
Sequencing Kit. Unincorporated nucleotides and primers
are next removed using the Applied Biosystems™ BigDye
XTerminator™ Purification Kit, and the sequences are read
by standard capillary electrophoresis (CE). The sequences
obtained can be read by any sequencing program, such as
SeqA or Geneious™ software, and compared with known or
expected SARS-CoV-2 sequences (Figure 1).
Some of the sequences generated by this method will
produce CE traces that may be dicult to interpret.
To determine whether a sequencing trace was useful,
we employed quality control metrics generated by
Applied Biosystems™ Sequence Scanner Software v2.0.
These metrics include trace score (average of basecaller
quality values for bases in the clear range), contiguous
read length (CRL), and QV20+ (total number of bases in
the entire trace that have a basecaller quality value of ≥20).
Guidelines for using these metrics for QC and analysis
of results are given at the end of the protocol. However,
standard analysis of sequencing traces is often sucient to
determine whether a novel sequence is present.
RNA
purification
Figure 1. Workflow detection of new SARS-CoV-2 lineages using Sanger sequencing.
RNA is purified from samples using standard techniques. cDNA is synthesized from the RNA, and
specific M13 sequence–tagged amplicons are generated by PCR. The amplicons are sequenced
in the forward and reverse directions using universal M13 primers and the BigDye Direct Cycle
Sequencing Kit. The sequencing reactions are cleaned using the BigDye XTerminator kit and
subjected to CE. The resulting sequencing traces can be analyzed and compared to reference
SARS-CoV-2 sequences to determine if the lineages are present.
For Research Use Only. Not for use in diagnostic procedures.
Amplicon
generation
Cycle
sequencing
Capillary
electrophoresis
Analysis
Page 2

IMPORTANT: This protocol is very sensitive; therefore, utmost care must be taken to prepare the stock solutions and
set up the amplification reactions in an amplicon-free environment.
1. Materials needed
1.1 Equipment
Product Supplier Cat. No.
Veriti 96-Well Fast Thermal Cycler, ProFlex 96-Well PCR System,
or similar thermal cycler
MicroMixer E-36 for 96-well plates Taitec 0027765-000
Single-channel and multichannel micropipettes of various sizes
capable of pipetting volumes from 1.00 µL to 1,000.0 µL
Cold block or ice MLS Any
Plate centrifuge MLS Any
Microcentrifuge or mini centrifuge MLS Any
Vortex mixer MLS Any
1.2 Reagents, kits, and consumables
Thermo Fisher Scientific 4375305 or 4484075
MLS Any
Product Supplier Cat. No.
SuperScript IV VILO Master Mix Thermo Fisher Scientific 1175 6 55 00
Nuclease-Free Water Thermo Fisher Scientific AM9937 or equivalent
BigDye Direct Cycle Sequencing Kit Thermo Fisher Scientific 4458688 or equivalent
BigDye XTerminator Purification Kit Thermo Fisher Scientific 4376486 or equivalent
MicroAmp Fast Optical 96-Well Reaction Plate with Barcode, 0.1 mL Thermo Fisher Scientific 4346906 or 4366932
MicroAmp Optical Adhesive Film Thermo Fisher Scientific
Nonstick, RNase-Free Microcentrifuge Tubes, 1.5 mL Thermo Fisher Scientific AM12450 or equivalent
5 mL tube, PCR clean MLS Any
Sterilized aerosol barrier (filter) pipette tips MLS Any
4311971, 4313663, or
4360954
Page 3
1.3 Prime r s
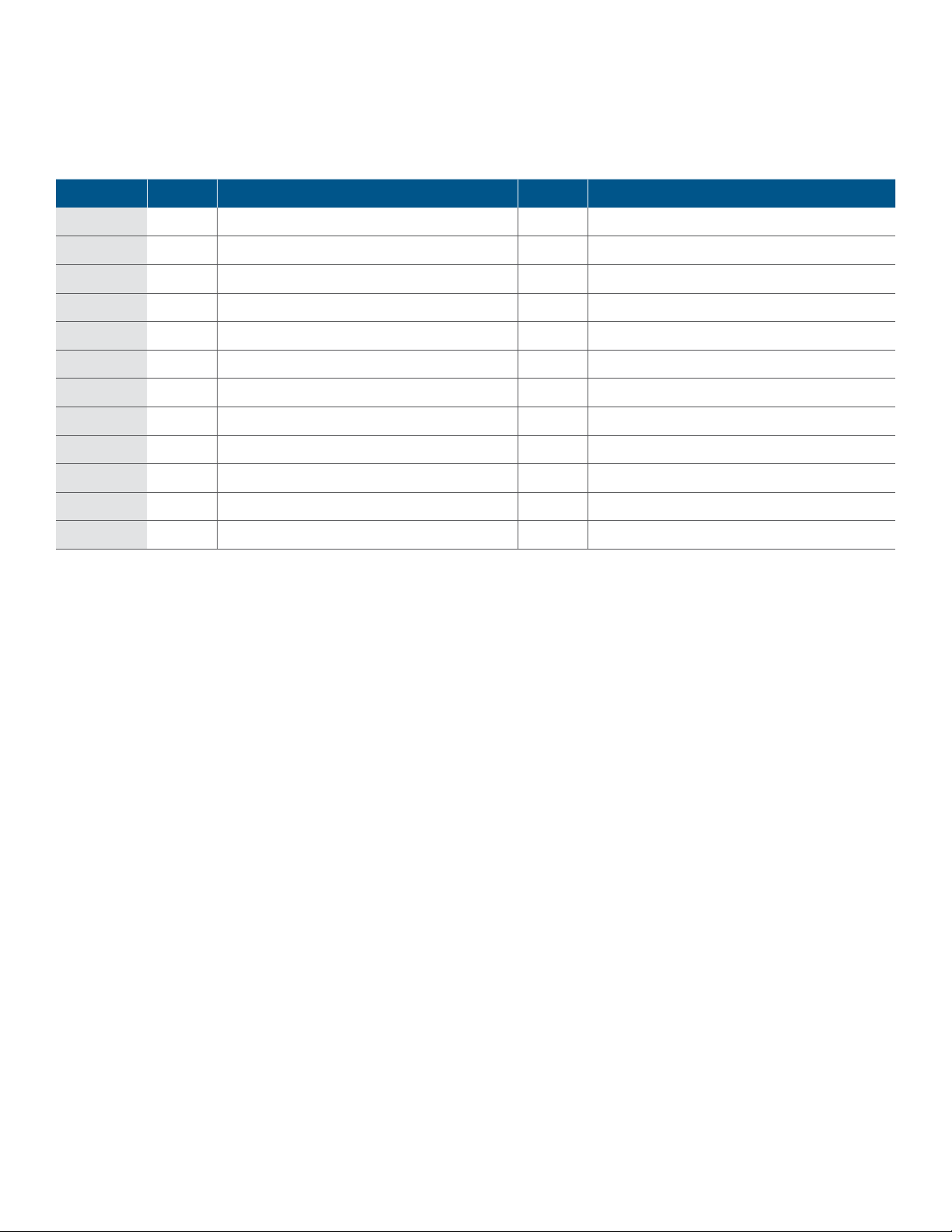
• Primer sequences are given in Table 1.
Table 1. Sequences of M13-tagged primers for analyzing the S gene. A subset of primer pairs that focus on specific regions of the S gene can be
chosen according to researchers’ needs; the complete list is provided in here. The M13 sequence tags are highlighted in red.
Coordinates*
20990-21562
21421-21916
21775-22345
22203-22697
22563-23128
22986-23519
23379-23876
23737-24231
24095-24623
24493-25003
24858-25369
25214-25790
* Based on N C_0 45512.2 coordinates.
Forward
primer name Forward primer sequence
SC 2M 1-5 4_
LE F T_ M13
SC2M1-55_
LE F T_ M13
SC 2M 1-5 6_
LE F T_ M13
SC 2M1-5 7_
LE F T_ M13
SC 2M 1-5 8_
LE F T_ M13
SC2M1-59_
LE F T_ M13
SC2M1-60_
LE F T_ M13
SC 2M 1-6 1_
LE F T_ M13
SC 2M 1-6 2b _
LE F T_ M13
SC 2M 1-6 3_
LE F T_ M13
SC2M1-64_
LE F T_ M13
SC2M1-65_
LE F T_ M13
TGTAAA ACGACGGCCAGTTGATTGGTG ATTGTGCAACTGTACA
TGTAAA ACGACGGCCAGTAGGGGTACTGCTGTTATGTCTTTAAA
TGTAAA ACGACGGCCAGTTGGGACCAATGGTACTAAGAGGT
TGTAAA ACGACGGCCAGTGTGATCTCCCTCAGGGT TTT TCG
TGTAAA ACGACGGCCAGTACTTGTGCCCT TTTGGTGAAGT
TGTAAA ACGACGGCCAGTCCGGTAGCACACCTTGTAATGG
TGTAAA ACGACGGCCAGTACCAGGTTGCTGTTCTTTATCAGG
TGTAAA ACGACGGCCAGTAATTCTACCAGTGTCTATGACCAAGAC
TGTAAA ACGACGGCCAGTTGCAGATGCTGGCTTCATCA
TGTAAA ACGACGGCCAGTAAATGATATCCTTTCACGTCTTGACAAA
TGTAAA ACGACGGCCAGTGCACACACTGGT TTGTAACACAA
TGTAAA ACGACGGCCAGTTAGGTTTTATAGCTGGCTTGATTGC
Reverse
primer name Reverse primer sequence
SC 2M 1-5 4_
RI GHT_M 13
SC2M1-55_
RI GHT_M 13
SC 2M 1-5 6_
RI GHT_M 13
SC 2M1-5 7_
RI GHT_M 13
SC 2M 1-5 8_
RI GHT_M 13
SC2M1-59_
RI GHT_M 13
SC2M1-60_
RI GHT_M 13
SC 2M 1-6 1_
RI GHT_M 13
SC 2M 1-6 2b _
RI GHT_M 13
SC 2M 1-6 3_
RI GHT_M 13
SC2M1-64_
RI GHT_M 13
SC2M1-65_
RI GHT_M 13
• Primers can be ordered from our custom oligo ordering web page
(https://www.thermofisher.com/order/custom-standard-oligo).
CAGGAAACAGCTATGACCTGTTCGTTTAGTTGTTAACAAGAACATCA
CAGGAAACAGCTATGACCAAGTAGGGACTGGGTCTTCGAA
CAGGAAACAGCTATGACCACCAGCTGTCCAACCTGAAGAA
CAGGAAACAGCTATGACCACTTAAAAGTGGAAAATGATGCGGAA
CAGGAAACAGCTATGACCTGCTGGTGCATGTAGAAGTTCA
CAGGAAACAGCTATGACCCCCCTATTAAACAGCCTGCACG
CAGGAAACAGCTATGACCCAGCTATTCCAGTTAAAGCACGGT
CAGGAAACAGCTATGACCGCACCAAAGGTCCAACCAGAAG
CAGGAAACAGCTATGACCCACAC TCTGACATT TTAGTAGCAGC
CAGGAAACAGCTATGACCTGAGTCTAATTCAGGTTGCAA AGGA
CAGGAAACAGCTATGACCTTTGACTCCTTTG AGCACTGGC
CAGGAAACAGCTATGACCCATTTCCAGCAAAGCCAAAGCC
– 25 nmol of dried and desalted primers can be ordered, but order can be scaled up as needed.
• Resuspend dried oligos to final concentration of 100 µM with TE buer.
1.4. Amplification mixes of primers
• Prepare the target-specific amplification primer mixes:
– Label clean microcentrifuge tubes for each primer pair (e.g., SC2M1-54, SC2M1-55, etc.).
Add 492 µL of TE buer to each tube.
– Add 4 µL each of both the left and right oligos of a pair to the appropriate tube (i.e.,
SC2M1-54_LEFT_M13 and SC2M1-54_RIGHT_M13 in one tube, SC2M1-55_LEFT_M13
and SC2M1-55_RIGHT_M13 to the next, etc.).
– These will be the 10X sequencing amplification primer mixes, with each oligo at 0.8 µM, that will
be used in step 3.1–3.2.
Page 4

2. cDNA synthesis
2.1. For each sample, combine:
Final volume
Reagent
5X SuperScript IV VILO Master Mix 10 µL
Sample 1–15 µL
Water To final 50 µL
50 µL
2.2. Vortex for 2–3 seconds, then centrifuge briefly (5–10 seconds) at 1,000 x g.
Note: Sample input volume can be adjusted for sensitivity. For example, up to 15 µL of a sample that
is expected to have low titer may be used.
2.3. Reverse transcription
2.3.1. Program a thermal cycler with the following profile:
Stage/step
Polymerase
Annealing
Temperature 25°C 50°C 80°C 4°C
Time 10 min 15 m in 10 m in Indefinitely
extension
Polymerase
inactivation HoldParameter
2.3.2. Put samples in the thermal cycler and run the program.
Note: Samples can be held at 4°C or on ice for up to 8 hours; for longer storage, freeze at –20°C.
Page 5

3. PCR amplifications of targets
3.1. For each sample, a forward and reverse reaction will be run. The initial PCR amplification,
therefore, requires two identical reactions to be set up. An example 96-well plate setup for four
samples is shown below:
1 2 3 4 5 6 7 8 9 10 11 12
A
B
C
D
E
F
G
H
SC2M1-54
primers
SC2M1- 6 0
primers
SC2M1-54
primers
SC2M1- 6 0
primers
SC2M1-54
primers
SC2M1- 6 0
primers
SC2M1-54
primers
SC2M1- 6 0
primers
SC2M1-54
primers
SC2M1- 6 0
primers
SC2M1-54
primers
SC2M1- 6 0
primers
SC2M1-54
primers
SC2M1- 6 0
primers
SC2M1-54
primers
SC2M1- 6 0
primers
SC2M1-55
primers
SC2M1- 61
primers
SC2M1-55
primers
SC2M1- 61
primers
SC2M1-55
primers
SC2M1- 61
primers
SC2M1-55
primers
SC2M1- 61
primers
SC2M1-55
primers
SC2M1- 61
primers
SC2M1-55
primers
SC2M1- 61
primers
SC2M1-55
primers
SC2M1- 61
primers
SC2M1-55
primers
SC2M1- 61
primers
SC2M1-56
primers
SC2M1-62b
primers
SC2M1-56
primers
SC2M1-62b
primers
SC2M1-56
primers
SC2M1-62b
primers
SC2M1-56
primers
SC2M1-62b
primers
SC2M1-56
primers
SC2M1-62b
primers
SC2M1-56
primers
SC2M1-62b
primers
SC2M1-56
primers
SC2M1-62b
primers
SC2M1-56
primers
SC2M1-62b
primers
SC2M1-57
primers
SC2M1- 6 3
primers
SC2M1-57
primers
SC2M1- 6 3
primers
SC2M1-57
primers
SC2M1- 6 3
primers
SC2M1-57
primers
SC2M1- 6 3
primers
SC2M1-57
primers
SC2M1- 6 3
primers
SC2M1-57
primers
SC2M1- 6 3
primers
SC2M1-57
primers
SC2M1- 6 3
primers
SC2M1-57
primers
SC2M1- 6 3
primers
SC2M1-58
primers
SC2M1-64
primers
SC2M1-58
primers
SC2M1-64
primers
SC2M1-58
primers
SC2M1-64
primers
SC2M1-58
primers
SC2M1-64
primers
Note: Reactions using the same cDNA sample have identical color coding.
Note: The layout above is for querying the entire S gene. If only a subset of amplicons is to be
analyzed, the layout can be adjusted accordingly.
SC2M1-58
primers
SC2M1-64
primers
SC2M1-58
primers
SC2M1-64
primers
SC2M1-58
primers
SC2M1-64
primers
SC2M1-58
primers
SC2M1-64
primers
SC2M1-59
primers
SC2M1- 6 5
primers
SC2M1-59
primers
SC2M1- 6 5
primers
SC2M1-59
primers
SC2M1- 6 5
primers
SC2M1-59
primers
SC2M1- 6 5
primers
SC2M1-59
primers
SC2M1- 6 5
primers
SC2M1-59
primers
SC2M1- 6 5
primers
SC2M1-59
primers
SC2M1- 6 5
primers
SC2M1-59
primers
SC2M1- 6 5
primers
Note: Positive and negative control samples can be run on the same or a dierent plate; the negative
control is a no-template control (NTC).
3.2. In each well of a 96-well PCR plate, combine:
• 1.5 µL of 10X sequencing amplification primer mix in duplicate
(as suggested in the table above)
• 5 µL of 2X BigDye Direct PCR Master Mix (supplied in kit)
• 1 µL of cDNA sample from completed step 2.3
– Leftover cDNA sample can be frozen at –20°C.
• Water to 10 µL total volume
3.3. Seal the plate; vortex for 2–3 seconds, then centrifuge briefly (5–10 seconds) at 1,000 x g.
3.4. Place the plate into a thermal cycler and run the following program:
Stage/step
Polymerase
activation
Temperature 95°C 96°C 62°C 68°C 4°C
Time 10 min 3 sec 15 s ec 30 sec Indefinitely
Cycling (40 cycles)
HoldParameter Denaturation Annealing Extension
Note: Samples can be held at 4°C or on ice for up to 8 hours; for longer storage, freeze at –20°C.
Note: Do not exceed 10 µL total reaction volume. The cycle sequencing and BigDye Xterminator
steps have been optimized for 10 µL input volumes.
Page 6

4. Cycle sequencing
4.1. Once the PCR in step 3.4 is complete, the plate can be used directly for cycle sequencing.
4.2. Remove the seal from the plate.
4.3. To each well of the plate, add:
• 2 µL of BigDye Direct Sequencing Master Mix (supplied in kit)
• 1 µL of BigDye Direct M13 Forward or M13 Reverse primer (supplied in kit)
Note: It is important to add the M13 Forward primer to one of the duplicate PCR reactions, and the
M13 Reverse primer to the other reaction. An example based on the plate setup is shown below.
1 2 3 4 5 6 7 8 9 10 11 12
A
SC2M1-54
M13 F or
SC2M1-54
M13 R ev
SC2M1-55
M13 F or
SC2M1-55
M13 R ev
SC2M1-56
M13 F or
SC2M1-56
M13 R ev
SC2M1-57
M13 F or
SC2M1-57
M13 R ev
SC2M1-58
M13 F or
SC2M1-58
M13 R ev
SC2M1-59
M13 F or
SC2M1-59
M13 R ev
B
C
D
E
F
G
H
SC2M1- 6 0
M13 F or
SC2M1-54
M13 F or
SC2M1- 6 0
M13 F or
SC2M1-54
M13 F or
SC2M1- 6 0
M13 F or
SC2M1-54
M13 F or
SC2M1- 6 0
M13 F or
SC2M1- 6 0
M13 R ev
SC2M1-54
M13 R ev
SC2M1- 6 0
M13 R ev
SC2M1-54
M13 R ev
SC2M1- 6 0
M13 R ev
SC2M1-54
M13 R ev
SC2M1- 6 0
M13 R ev
SC2M1- 61
M13 F or
SC2M1-55
M13 F or
SC2M1- 61
M13 F or
SC2M1-55
M13 F or
SC2M1- 61
M13 F or
SC2M1-55
M13 F or
SC2M1- 61
M13 F or
SC2M1- 61
M13 R ev
SC2M1-55
M13 R ev
SC2M1- 61
M13 R ev
SC2M1-55
M13 R ev
SC2M1- 61
M13 R ev
SC2M1-55
M13 R ev
SC2M1- 61
M13 R ev
SC2M1-62b
M13 F or
SC2M1-56
M13 F or
SC2M1-62b
M13 F or
SC2M1-56
M13 F or
SC2M1-62b
M13 F or
SC2M1-56
M13 F or
SC2M1-62b
M13 F or
SC2M1-62b
M13 R ev
SC2M1-56
M13 R ev
SC2M1-62b
M13 R ev
SC2M1-56
M13 R ev
SC2M1-62b
M13 R ev
SC2M1-56
M13 R ev
SC2M1-62b
M13 R ev
SC2M1- 6 3
M13 F or
SC2M1-57
M13 F or
SC2M1- 6 3
M13 F or
SC2M1-57
M13 F or
SC2M1- 6 3
M13 F or
SC2M1-57
M13 F or
SC2M1- 6 3
M13 F or
SC2M1- 6 3
M13 R ev
SC2M1-57
M13 R ev
SC2M1- 6 3
M13 R ev
SC2M1-57
M13 R ev
SC2M1- 6 3
M13 R ev
SC2M1-57
M13 R ev
SC2M1- 6 3
M13 R ev
SC2M1-64
M13 F or
SC2M1-58
M13 F or
SC2M1-64
M13 F or
SC2M1-58
M13 F or
SC2M1-64
M13 F or
SC2M1-58
M13 F or
SC2M1-64
M13 F or
4.4. Seal the plate. Vortex for 2–3 seconds, then centrifuge briefly (5–10 seconds) at 1,000 x g.
4.5. Place the plate into a thermal cycler and run the following program:
SC2M1-64
M13 R ev
SC2M1-58
M13 R ev
SC2M1-64
M13 R ev
SC2M1-58
M13 R ev
SC2M1-64
M13 R ev
SC2M1-58
M13 R ev
SC2M1-64
M13 R ev
SC2M1- 6 5
M13 F or
SC2M1-59
M13 F or
SC2M1- 6 5
M13 F or
SC2M1-59
M13 F or
SC2M1- 6 5
M13 F or
SC2M1-59
M13 F or
SC2M1- 6 5
M13 F or
SC2M1- 6 5
M13 R ev
SC2M1-59
M13 R ev
SC2M1- 6 5
M13 R ev
SC2M1-59
M13 R ev
SC2M1- 6 5
M13 R ev
SC2M1-59
M13 R ev
SC2M1- 6 5
M13 R ev
Stage/step
Post PCR
cleanup
Post PCR
inactivation
Polymerase
activation
Cycling (25 cycles)
HoldParameter Denaturation Annealing Extension
Temperature 37°C 80°C 96°C 96°C 50°C 60°C 4°C
Time 15 m in 2 min 1 min 10 sec 5 sec 75 sec Indefinitely
Page 7

5. Sequencing cleanup
5.1. Spin the reaction plate at 1,000 x g for 1 minute, then remove the seal.
5.2. Prepare a mix with SAM Solution and BigDye XTerminator™ Solution in an appropriately
sized tube.
5.2.1. Calculate the amount of SAM Solution and XTerminator Solution needed for all samples.
You will need 45 µL of SAM Solution and 10 µL of XTerminator Solution per well.
5.2.2. Add the calculated volume of SAM Solution to a new tube using a conventional pipette tip.
Note: Make sure there are no particulates in the SAM Solution before pipetting. If there are
particulates, heat the SAM Solution to 37°C and mix to dissolve. Cool to room temperature
before using.
5.2.3. Vortex the bulk container of XTerminator Solution at maximum speed for at least
10 seconds, until the solution is homogeneous.
5.2.4. Using a wide-bore pipette tip, add the calculated volume of XTerminator Solution to
the tube.
IMPORTANT: Avoid pipetting from the top of the liquid.
5.2.5. Mix the tube of combined reagents until homogeneous.
5.3. Add 55 µL of the SAM Solution/XTerminator Solution mix to each well.
IMPORTANT: Avoid pipetting from the top of the liquid. When aliquoting into the plate, re-vortex the
SAM Solution/XTerminator Solution mix every 8–10 wells to homogenize the bead mixture.
5.4. Seal the plate with Applied Biosystems™ MicroAmp™ Optical Adhesive Film. Make sure the plate
is sealed well.
5.5. Vortex the reaction plate for 40 minutes.
5.6. In a swinging-bucket centrifuge, spin the plate at 1,000 x g for 2 minutes.
Page 8

6. Collect data
6.1. Make sure the instrument is calibrated with the correct sequencing standard (Z-dye set matrix
and sequencing standard)
• For details, see the Applied Biosystems™ 3500/3500xL Genetic Analyzer User Guide or SeqStudio™
Genetic Analyzer Getting Started Guide.
6.2. Remove the MicroAmp film and replace it with a 96-well plate septum.
6.3. Load plates into the genetic analyzer.
6.4. Select or create an appropriate run module according to your capillary length, number of
capillaries, and polymer type on your instrument. The recommended default run modules are
listed below:
• For 3500xL instruments with 50 cm capillaries:
– Instrument protocol: BDxFastSeq50_POP7xl_Z
Note: Replace 50 with 36 in the instrument protocol name if you have a 36 cm capillary installed.
– Analysis Module: BDTv3.1_PA_Protocol-POP7
• For SeqStudio instruments:
– MedSeqBDX
Page 9

7. Analyze results using a sequencing program
Sequence Scanner v2.0 is free software for viewing electropherograms. It provides an easy way to
perform a high-level sequencing data quality check or general data review that includes summary
tables and electropherograms as well as a general raw or analyzed data view for .ab1 files.
7.1. To obtain the software, go to thermofisher.com/pages/WE28396/
7.2. Using Sequence Scanner Software v2.0, generate a QC report. For each sequencing trace,
determine the trace score, CRL, and the QV20+ score.
7.3. Suggested acceptance criteria:
• A sequencing trace is acceptable as positive if two of the three thresholds are met:
– Trace score greater than 31
– CRL greater than 50
– QV20+ greater than 50
• A sequencing trace is acceptable as negative if two of the three thresholds are met:
– Trace score less than 14
– CRL less than 24
– QV20+ less than 24
• Sequencing traces that do not fit the above criteria are indeterminate and should be repeated
7.4 Using BLAST™ alignment or another sequence alignment tool, align positive traces to the
SARS-CoV-2 genome.
• Alignments greater than 85% over read length are considered homologous to the
SARS-CoV-2 genome
• Discard any sequences that are not homologous to SARS-CoV-2
7.5 For variant analysis in any of the amplicons, these criteria should be met:
• Positive (passable) traces in both directions (7.3)
• Homology to the SARS-CoV-2 genome (7.4) in regions outside the putative variant
• Negative traces in NTC reactions (7.3)
7.6. Test runs that fail for reasons not attributable to system performance, such as equipment
malfunction, operator error, or other demonstrable cause, will be designated as invalid runs.
Invalid runs will be retested and documented in the study report(s).
Page 10

References
1. van Dorp L et al.(2020) No evidence for increased transmissibility from recurrent mutations in SARS-CoV-2.
Nat Commun 11, 5986. https://doi.org/10.1038/s41467-020-19818 -2
2. Wise J (2020) Covid-19: New coronavirus variant is identified in UK. BMJ Dec 16;371:m4857.
doi: 10.1136/bmj.m4857
3. Tegally H et al. (2020) Emergence and rapid spread of a new severe acute respiratory syndromerelated coronavirus 2 (SARS-CoV-2) lineage with multiple spike mutations in South Africa. medRxiv
https://doi.org/10.1101/2020.12.21.20248640
4. Conceicao C et al. (2020) The SARS-CoV-2 Spike protein has a broad tropism for mammalian ACE2 proteins.
PLoS Bio 18(12):e3001016. https://doi.org/10.1371/journal.pbio.3001016
5. Paden CR et al. (2020) Rapid, sensitive, full-genome sequencing of severe acute respiratory syndrome coronavirus 2.
Emerg Infect Dis. 26(10):2401-2405. doi.org/10.3201/eid2610.201800
Find out more at thermofisher.com/sangercoronavirus
For Research Use Only. Not for use in diagnostic procedures. © 2021 Thermo Fisher Scientific Inc. All rights reserved.
All trademarks are the proper ty of Thermo Fisher Scientific and its subsidiaries unless other wise specified. B LA ST is a
trademark of the National Liberty of Medicine. COL34063 0321
 Loading...
Loading...