
Nerivio Migra
®
A neuromodulation device for the acute
treatment of migraine
USER MANUAL

Page | 2
© 2018 Theranica Bio-Electronics LTD. (“Theranica”), all rights reserved. The manual or
any of its parts should not be reproduced without the written permission of Theranica.
Theranica reserves the right to change or improve its products and accompanying
technical literature without specific notice.
Manufacturer:
Theranica Bio-Electronics LTD.
45 Ha-Melakha St.,
Netanya 4250574, Israel
Tel: +972-72-3909-763, Fax: +972-72-3909762
Web: www.theranica.com
2AOH8-NM

Page | 3
Table of content
1. Introduction 4
1.1. About this manual 4
1.2. Product overview 4
1.3. Product functions 5
1.4. Package content 5
2. Glossary 5
3. Labels and symbols 6
4. Safety 8
4.1. Conditions for use 8
4.2. Warnings, precautions and Adverse Events 8
5. What does the treatment feel like? 11
6. Using the device 11
6.1. Starting for the first time 11
6.2. Treating a migraine episode 16
6.3. Storing the device for next use 23
6.4. Reviewing your migraine diary 23
7. Cleaning, maintenance and disposal 23
7.1. Cleaning and maintenance 23
7.2. Disposal 24
8. Troubleshooting 24
8.1. General 24
8.2. Main errors and messages 26
8.3. LED status 28
8.4. Customer support 28
9. Operation specification 28
9.1. Environment operating conditions 28
9.2. Environmental storage and transportation conditions between uses 28
9.3. Environmental trasnportation and storage conditions 29
9.4. Electrical properties 29
10. Technical specifications 29
11. Minimal smartphone requirements 31
12. Potential adverse reactions 32
13. Classification 32
14. EMC statement 32
14.1. Electromagnetic test result summary 33
14.2. Electromagnetic emissions 33
14.3. Electromagnetic immunity 34
14.4. Recommended separation distances 36
15. FCC radio frequency interference statement 36
16. Applicable standards 37

Page | 4
1. INTRODUCTION
1.1. ABOUT THIS MANUAL
This manual provides the information necessary for the user to effectively use the Nerivio
Migra® device.
• Do not attempt to perform any procedure before carefully reading all instructions.
• Always follow product labeling and the manufacturer recommendations.
• For any inquiry, please contact customer support at support@theranica.com.
1.2. PRODUCT OVERVIEW
Nerivio Migra® is a wearable, battery-powered medical device for the acute treatment of
migraine with or without aura. Nerivio Migra® is controlled by a mobile application. The
Nerivio Migra® is intended for self-administration at a home environment.
The device is worn on the upper arm and transmits transcutaneous electrical nerve
stimulation by applying weak electrical pulses that invoke conditioned pain modulation
(CPM) to inhibit migraine pain. Nerivio Migra® is intended for self-administration at the
onset of a migraine episode.
The Nerivio Migra® system includes several main components:
1. The Nerivio Migra device. The device is placed on the arm and produces
electrical signals. The device is good for 12 treatments of 45 minutes.
2. Flexible arm sleevelet. The sleevelet should be wrapped around the device on
the arm to improve the contact between the device and the skin, to secure its
location on the arm and to conceal the device to enable a discreet treatment.
3. Software application
4. Travel bag
The external side of the Nerivio Migra device includes an ON button and a LED indicator
that signals various modes of operation. The internal side includes the electrodes that
deliver neurostimulation signals. The sleevelet holds the device in its location.
The device is controlled by an application which is installed on a smartphone. The
application controls the device, retrieves operational records from the device and stores
the data for further retrospective processing/reviewing.
The application enables the user to activate the stimulation, control the stimulation
intensity, monitor the treatment duration and pause or stop the stimulation. The application
also provides notifications and indications on the connection status and on the remaining
number of treatments. It also offers a migraine dairy feature which enables to track
information about your migraine attacks. The migraine diary can also be accessed via the
web.

Page | 5
1.3. PRODUCT FUNCTIONS
• The device is battery-powered; the battery is internal, integrated, and nonrechargeable.
• The device includes integrated electrodes, providing the electrical stimulation to the
skin.
• The device is activated by an ON button.
• Flexible arm sleevelet which should be wrapped around the device on the arm to
improve the contact between the device and the skin, to secure its location on the
arm and to conceal the device to enable a discreet treatment.
• An application (app) installed on a smartphone to control and monitor the treatment
(as well as provide other features).
1.4. PACKAGE CONTENT
• Nerivio Migra device
• Flexible arm sleevelet
• Travel bag
• QuickStart guide
2. GLOSSARY
App: Mobile application running on smartphone
LED: Light-Emitting Diode
EMC: Electromagnetic compatibility
MAC: Media access control
Page | 6
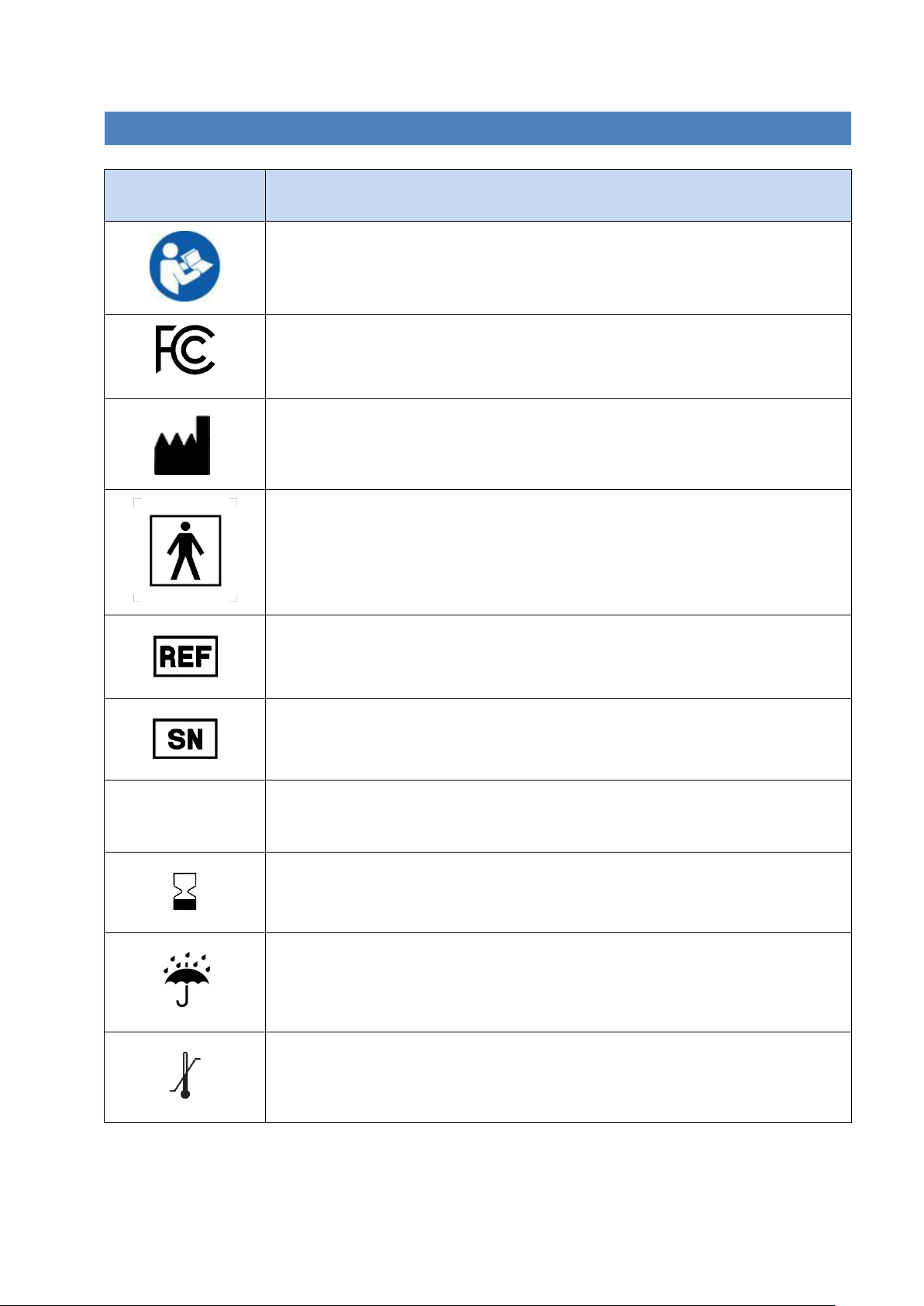
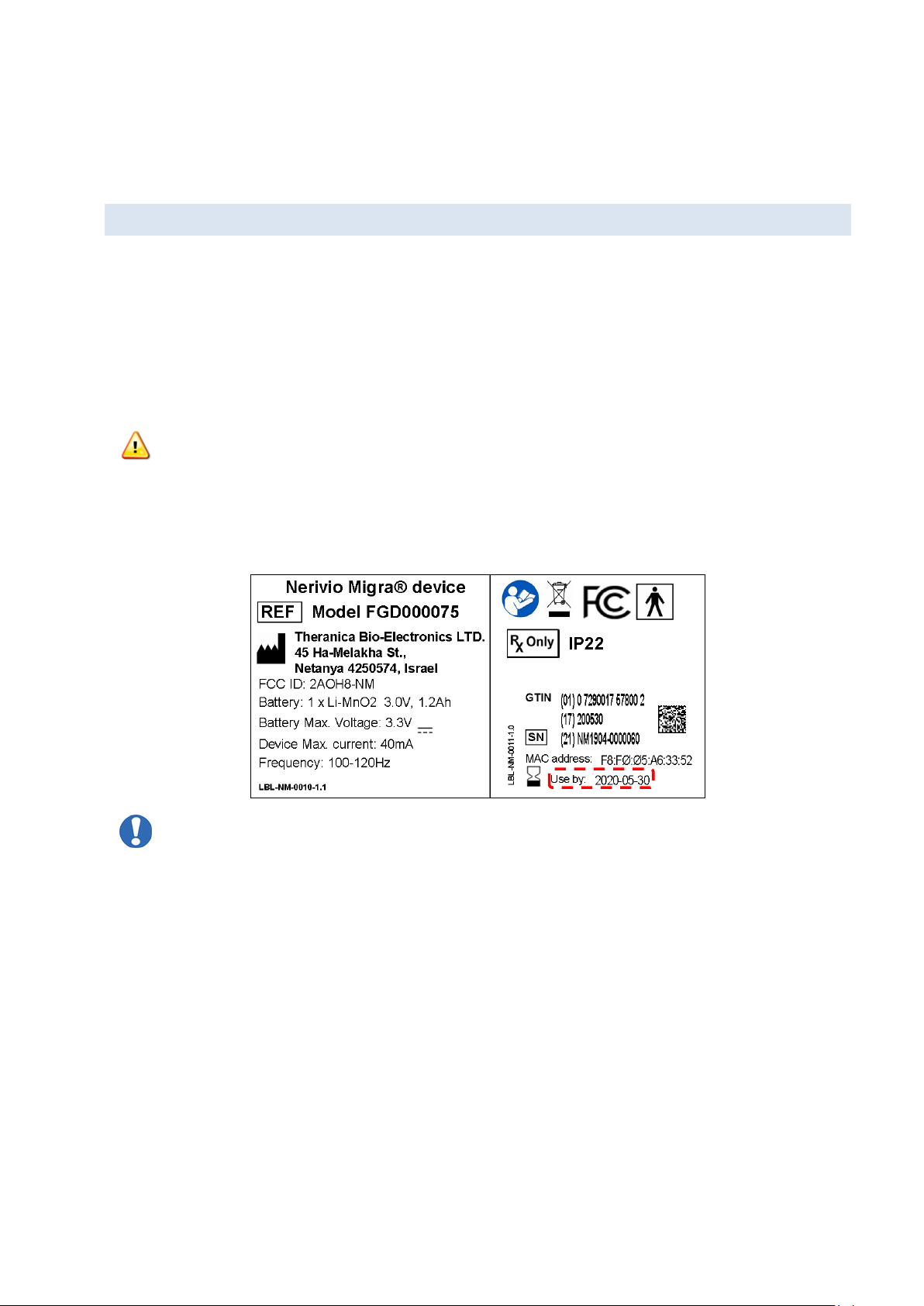
3. LABELS AND SYMBOLS
Symbol
Description
Read and fully understand user manual before using this device
Compliance with FCC Federal Communications Commission Class B – certified for
home use
FCC identifier: 2AOH8-NM
Manufacturer
Type BF applied part (IEC60601-1)
Catalog number
Serial number
IP22
Ingress protection rating
Use by date - indicates the date after which the device is not to be used
Keep dry
Temperature limits

Page | 7
Humidity limitation
Atmospheric pressure limitation
Caution
Keep away from sunlight
Special requirements for waste of electrical and electronic equipment (WEEE
Directive). This product must not be disposed of via municipal waste collection.
Separate collection for electrical and electronic equipment waste per Directive
2012/19/EC in the European Union is required. Contact the manufacturer for
details.
Caution: Federal (US) law restricts this device to sale by or on the order of a
physician.

Page | 8
4. SAFETY
4.1. CONDITIONS FOR USE
4.1.1. INDICATION FOR USE
The Nerivio Migra® is indicated for acute treatment of migraine with or without aura, for
patients 18 years of age or older who do not have chronic migraine. It is a prescription
use, self-administered device for use in the home environment at the onset of migraine
headache or aura.
4.1.2. CONTRAINDICATIONS
I. The device should not be used by people with congenital heart failure (CHF),
severe cardiac or cerebrovascular disease.
II. The device should not be used by people with uncontrolled epilepsy
III. The device should not be used by people with active implantable medical device,
such as a pacemaker, hearing aid implant, or any implanted electronic device.
Such use could cause electric shock, electrical interference or serious injuries or
medical conditions.
4.2. WARNINGS, PRECAUTIONS AND ADVERSE EVENTS
The following icons are used throughout this user manual:
Warning: Indicates a potentially hazardous situation which, if not avoided, could
result in serious injury or death.
Precaution: Indicates a potentially hazardous situation which, if not avoided,
may result in minor or moderate injury to the user or patient or damage to the
equipment or other property.
Note: indicates important information regarding the use of the system
Warnings
Do not attempt to perform any procedure before carefully reading all the
instructions
Do not use the device on the heart, chest, neck, head or any other body location
other than the upper arm, because this could cause severe muscle spasms
resulting in closure of your airway, difficulty in breathing, or adverse effects on
heart rhythm or blood pressure

Page | 9
Do not use the device over skin conditions, such as open wounds or rashes, or
over swollen, red, infected or inflamed areas or skin eruptions or fragile skin on
your upper arm at the treatment location
Do not share the device with other people. The device is intended to be used by
a single person to avoid skin disease or any transmissible disease
Do not disassemble, crush, incinerate or short-circuit the battery. This could
cause a fire, injury, burns, or other hazards
Precautions
Federal Law restricts this device to sale by or on the order of a physician
The device should not be applied over areas of skin that lack normal sensation.
If one upper arm is insensitive to physical sensation, use the other upper arm
Do not use the device over or in proximity to cancerous lesions
Do not use the device on an arm with a metallic implant. In such cases, consider
using it on the other upper arm
Do not use the device simultaneously with another electrical stimulation device
Do not use the device while driving, cycling, or operating any vehicle or machinery
Do not use this device on wet skin or when bathing, showering, during exercise,
while sweating or in high humidity
Do not use the device in the presence of electronic monitoring equipment (e.g.,
cardiac monitors, ECG alarms)
Do not use the device in a magnetic resonance imaging (MRI) environment
The long-term effects of chronic use of the device are unknown
The device has not been evaluated for use in pregnant women and people less
than 18 years of age.
The safety and effectiveness of the device has not been demonstrated for
subjects with chronic migraine
The safety and effectiveness of the device has not been demonstrated for the
preventive treatment of migraine headache

Page | 10
Do not use the device past expiration date
Check the device for damages. If the device is damaged return it to the
manufacturer or contact customer support
If the device was damaged, do not touch exposed electronics
Do not use the device if the electrodes become significantly dirty or damaged
Keep the device in a dry environment. Moisture may damage the device
Do not start a treatment before placing the device on your arm
In case of device malfunction, remove the device from your arm and contact
customer support
It is recommended that your smartphone will be protected by a password (or other
security mechanism) to refrain from unwanted people to activate the device
Interference to the Bluetooth connectivity may occur in the vicinity of equipment
emitting RF (e.g. microwave, routers, WIFI devices)
To minimize moisture loss, when unused, the electrodes should be covered with
the provided protective film and the device should be stored in its original package
Do not expose the device to moisture and/or high humidity. If exposed, dry the
device as soon as possible
Before or after a treatment, rub the electrodes with your finger using a drop of
water to improve their adhesiveness
Do not clean the device with soap, alcohol, submerge in water, or scrub with
abrasive material
Do not disassemble or modify the device by yourself
Do not attempt to recharge or detach the battery
Keep the device out of the reach of infants, toddlers, children and pets
The device uses Bluetooth technology; it may therefore be interfered with by other
equipment utilizing RF technology, even that other equipment complies with
CISPR emission requirements

Page | 11
The device should not be used adjacent to or stacked with other equipment and
if adjacent or stacked use is necessary, the Nerivio Migra® should be observed
to verify normal operation in the configuration in which it will be used
Do not use devices which generate strong electrical or electromagnetic fields,
near the Nerivio Migra® device. This may result in incorrect operation of the device
and create a potentially unsafe situation. In order to reduce the risk of EM
interference, it is recommended to keep a minimum distance of 30 cm (12 inches)
between the device and other electromagnetically radiating devices. Verify
correct operation of the device in case the distance is shorter. During the
immunity tests the device operated normally
Adverse reactions
During the treatment you might experience a temporary sensation of warmth, local
tingling or numbness in the arm or redness of the skin, which should disappear shortly
after the end of the treatment.
Refer to Theranica’s website at http://theranica.com/clinical-data/ for a complete
listing of clinical data and adverse events information
5. WHAT DOES THE TREATMENT FEEL LIKE?
The device transmits electrical pulses. You may feel a strong sensation at first, but it will
typically fade to a comfortable level after a couple of seconds. You will then need to set
the treatment intensity level by increasing it to the highest level that feels strong yet
comfortable and painless (see instructions below). If the sensation is uncomfortable or
painful, you should decrease the intensity. If you experience hand numbness and/or
muscle twitching, try changing the location of the electrodes on the arm.
6. USING THE DEVICE
6.1. STARTING FOR THE FIRST TIME
Before using the device for the first time, the application must be installed, and the device
should be connected to the app. Make sure the Bluetooth connection on your
smartphone is enabled.
Page | 12
Do not attempt to perform any procedure before carefully reading all the
instructions
Do not use the device if the package was damaged during shipment
6.1.1. DOWNLOADING AND INSTALLING THE APPLICATION
Step 1: Verify that your phone operating system is compatible with the Nerivio Migra app
(Android versions 6 to 9 and iOS versions 9 to 12.1.4).
Step 2: Download and install the “Nerivio Migra” application via Google play or
App store (depending on your operating system).
Step 3: Open the application. When the app is opened for the first time, you will need to
confirm the privacy policy and end-user license agreement (EULA). Scroll down the
privacy policy/EULA page and touch "Confirm". You will then be asked to create an
account. Follow the app instructions. This login is only required when the app is opened
for the first time. For safety reasons, you are advised to lock your smartphone screen with
a password.
Page | 13
Step 4: A quick introduction will be presented. Read it by swiping the screens to the left
and touch “Done” when finished. You can skip it by touching “Skip intro”.
Step 5: As you begin using the app, it may ask for permissions. Please allow these
permissions so that the app works properly.
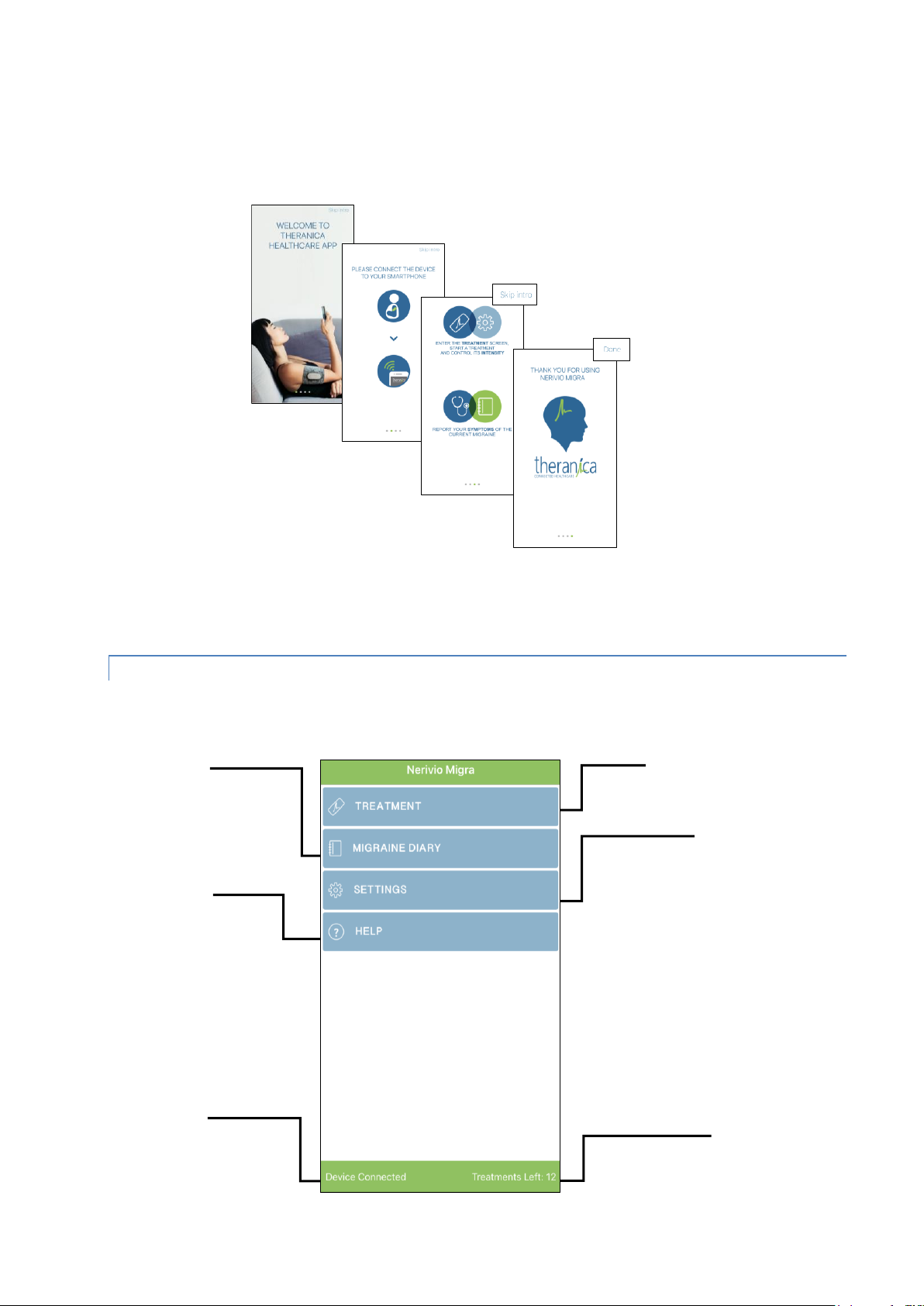
6.1.2. THE APP HOME SCREEN
The home screen provides access to information about your migraine treatments and the
Nerivio Migra system.
Remaining treatments
Status of
connection
between the
smartphone and
the device
Migraine diary
Touch to review
the history of
migraine
treatments
Settings
Touch to change
device settings and
to pair the device
to your smartphone
Treatment
Touch to
start a
treatment
Help menu
Touch to
read the
user manual

Page | 14
The home screen includes:
Treatment – This section enables to initiate, control, monitor and stop a treatment
session.
Migraine diary – This section enables you to review and edit your migraine symptoms.
Setting – This section provides access to some of the technical aspects of the app:
About- select this to view the software version
Profile – select this to view your account details
Connect - select this option to connect the device to the Nerivio Migra app
Log out- select this to log out of your account
Help – This section provides access to the Nerivio Migra user manual and to
instructional videos that explain how to connect the device to the app and how to treat a
migraine episode with the device.
6.1.3. CONNECTING THE DEVICE TO THE APP FOR THE FIRST TIME
Step 1: Turn on the device using the ON button located at the external part of the device.
A slow flashing (mostly on) green light indicates the device is on.
Check the device for damages. If the device is damaged return it to the
manufacturer or contact customer support
ON button
LED indicator

Page | 15
Step 2: In the home screen touch “Settings” and then touch “Connect”. The device and
the smartphone should be in proximity of less than 1 inch. When the device is
successfully connected to the app, there will be a fast flashing green indicator light on
the device. The connection status can be viewed in the app on the bottom left corner of
the screen.
If a connection has not been established, make sure that Bluetooth is enabled in your
smartphone. The app may prompt you to connect manually using the device MAC address.
Locate the MAC address on the device label located on the protective film and on the
package and follow the app instructions.
Step 3: After the connection has been established, the app will prompt you to enter a
personal PIN code required for the initial pairing (for example, 1111 or 1234). Remember
the PIN code for future use.
Step 4: Place the device in its original package or in the travel bag to store it for next use
or start a treatment following the instructions below. If the device was on for over 5 minutes
Page | 16
when no treatment was initiated, it automatically shuts down. Turn it back on to start a
treatment.
6.2. TREATING A MIGRAINE EPISODE
The treatment should be performed at the onset of a migraine episode and takes 45
minutes. For effective results, you should start the treatment as soon as you feel the first
symptoms of the migraine and within the first hour (60 minutes) of the migraine symptoms
onset (headache and/or aura).
Before you begin, make sure the smartphone Bluetooth connection is enabled.
Do not share the device. The device is intended for a single user.
Step 1: Check the device expiration date on the label located on the protective film and
on the package.
Do not use the device past expiration date
Step 2: Make sure that your arm skin is clean, dry and free from lotion.
Step 3: Turn on the device. A slow flashing (mostly on) green light indicates the device is
on. If the LED is still off or is solid green, please contact customer support.

Page | 17
Step 4: Open the app and confirm the device is connected successfully. The connection
status can be viewed in the app on the bottom left corner of the screen. Also verify that
there is at least one remaining treatment on the bottom right corner of the app screen.
Step 5: Carefully remove the protective film from the electrodes and save it for storing the
device and maintaining the electrode adhesiveness between uses.

Page | 18
Step 6: Place the device on your upper arm so that the electrodes are in contact with your
skin and the LED indicator is facing outwards. The device should be located midway
between the elbow and the shoulder. Place the device directly on the skin and not on your
shirt.
Step 7: Wrap the sleevelet around the device on your arm to improve the contact between
the device and your skin.
Do not use the device on the heart, chest, neck, head or any other body location
other than the upper arm
Do not use the device over skin conditions, such as open wounds or rashes, or
over swollen, red, infected or inflamed areas or skin eruptions or fragile skin on
your upper arm at the treatment location
It is important to use the device only when positioned correctly on the arm. The
device should be located midway between the elbow and the shoulder, on the
outer side of the arm.
Step 8: To start the treatment, touch “Treatment" in the main menu of the home screen.
Do not start a treatment before placing the device on your arm

Page | 19
Do not use the device on wet skin or when bathing, showering, during exercise,
while sweating or in high humidity
Do not use the device while driving, cycling, or operating any vehicle or
machinery
Do not use the device if the electrodes become significantly dirty or damaged
If the device was damaged, do not touch exposed electronics
Do not use the device in the presence of electronic monitoring equipment (e.g.,
cardiac monitors, ECG alarms)
Keep the device in a dry environment. Moisture may damage the device
Do not use the device in a magnetic resonance imaging (MRI) environment
The long-term effects of chronic use are unknown
In case of device malfunction, remove the device from your arm and contact
customer support
In case the device fails to properly adhere to the skin, contact customer support
Step 9: Touch “Start” to begin the treatment and then set the treatment intensity level, so
it feels strong yet comfortable and painless.
Setting intensity level:
1. Start the treatment
2. Increase the intensity
3. When the
stimulation is painful
and/or
uncomfortable,
reduce the intensity
to the previous level

Page | 20
a) Start increasing the stimulation intensity using the "+" button. Each press will
increase the intensity by 1 unit.
b) When the stimulation is painful and/or uncomfortable, reduce the intensity to the
previous level using the "-" button. Each press will decrease the intensity by 1
unit.
Once you find the strongest and convenient stimulation intensity level, relax and continue
with the treatment. If during the treatment the sensation is not strong, if it feels
uncomfortable or painful, adjust the intensity level using the "+" and "-" buttons.
• The default starting intensity level is 12%.
• Note that long/continuous presses should be avoided.
• If you have significantly increased the intensity and still do not feel the stimulation,
please refer to troubleshooting or contact customer support.
Step 10: 60 seconds after the treatment has begun, questions on your migraine symptoms
will be automatically displayed. You can record your migraine symptoms or skip this by
touching “Skip”.
Note that the treatment is still in progress.
For effective and convenient treatment, the intensity level is individually set so
it feels strong yet comfortable and painless
You should monitor the activity of the device throughout its operation

Page | 21
6.2.1. TREATMENT IN PROGRESS
The progress of the treatment can be monitored by the treatment progress bar and by the
specified amount of time that has passed out of the total treatment duration time (45
minutes).
You can pause the treatment session for 5 minutes by touching ‘Pause’. Press ‘Resume’
to restart. Each session can only be interrupted 3 times. If a treatment is not resumed
within 5 minutes, it will be stopped automatically.
The treatment can be stopped early at any time by touching “Stop”. Do not remove the
device before the treatment has ended or has been stopped.

Page | 22
During the treatment, you may experience slight muscle spasm, numbness of the hand
and irritation of the skin. These sensations should resolve soon after the end of treatment.
If you experience an uncomfortable or painful sensation that does not resolve by
decreasing the intensity, stop the treatment using the “Stop” button or remove the device
from your arm.
Note that if the device was on for over 3 minutes when no treatment was in progress, it
automatically shuts down. Turn the device back on to start a treatment.
It is recommended that your smartphone will be protected by a password (or
other security mechanism) to refrain from unwanted people to activate the
device
Interference to the Bluetooth connectivity may occur in the vicinity of
equipment emitting RF (e.g. microwave, routers, WIFI devices)
For effective results, it is recommended to avoid using other electrical devices
during treatment
If the “Stop” button does not respond, you can remove the device from your
arm
6.2.2. TREATMENT COMPLETED
Step 1: When the treatment is completed, remove the sleevelet and the device from your
arm. The device will turn off automatically one minute after the treatment session has
ended (the green light will turn off).
Step 2: Apply the protective film on the electrodes (the protective film is reusable).

Page | 23
Step 3: Place the device in its original package or in the travel bag store it for next use.
Step 4: Close the app.
Note that if your migraine headache is not aborted 30 minutes following treatment, you
may administer additional treatments.
6.3. STORING THE DEVICE FOR NEXT USE
Once the treatment has been completed, the device needs to be stored until the next
treatment.
Step 1: Verify that the electrodes are covered with the protective film.
Step 2: Store the device in its original package or in the travel bag in an indoor
environment, away from direct sunlight and according to storage enviroment conditions
specified in the user manual.
To minimize moisture loss, when unused, the electrodes should be covered with
the provided protective film and the device should be stored in its original
package
Do not expose the device to moisture and/or high humidity. If exposed, dry the
device as soon as possible
The device should be stored and cleaned according to the recommended
conditions describe in the user manual
6.4. REVIEWING YOUR MIGRAINE DIARY
Keeping detailed records of migraine episodes can help in migraine management. The
symptoms recorded during a treatment will be saved in a migraine diary that can be
reviewed at any time via the app. In the home screen touch “Migraine diary” to review your
migraine symptoms. The migraine diary can also be accessed via the web. Type
http://theranica.com/userlogs/#/ in your web browser and enter your login details. To view
your login details in the app, touch “settings” and then touch “profile”.
7. CLEANING, MAINTENANCE AND DISPOSAL
7.1. CLEANING AND MAINTENANCE
• The device can be cleaned with a dry cloth (except for the electrodes).
• If the electrodes begin losing adhesion, gently rubbing one or two drops of water
onto the gel surface may extend usage.
Page | 24
• The sleevelet can be machine washed at 80°F or 30°C. No bleach products should
be used. Do not tumble dry.
• To minimize moisture loss, when unused, the electrodes should be covered by the
provided protective film and the device should be stored in its original package.
• Contact customer support if the package and/or device labeling are damaged.
• The life of the electrodes varies depending on skin conditions, skin preparation,
storage and climate.
Before or after a treatment, rub the electrodes using a drop of water to improve
their adhesiveness
Do not clean the device with soap, alcohol, submerge in water, or scrub with
abrasive material
Do not disassemble or modify the device by yourself
7.2. DISPOSAL
• This product should be disposed of in accordance with all applicable
federal, state and local regulations related to the disposal of electronic
equipment and batteries.
• If the battery has been fully discharged before use and before product
expiration date, please contact customer support.
• Contact customer support for further information on the appropriate
disposal of device components.
8. TROUBLESHOOTING
This section lists problems or observations that you may have, the possible cause(s) and
recommended actions. Before addressing the troubleshooting table, please check and
confirm the following:
1. Make sure that Bluetooth connection is enabled in your phone
2. Make sure that there are treatments left in your device
8.1. GENERAL
Problem
What it may mean
What to do
The device does not power
on
The device is not
working
Contact customer support at
support@theranica.com

Page | 25
The ON button was not
held long enough
Press the ON button continuously for
2-3 seconds
The LED is flashing very
rapidly (5 times per second)
There is an error
message on the screen
View the error message in the app and
follow the instructions. If the error does
not appear on the screen, wait for the
device to automatically turn off and
then turn it back on
There are no treatments
left in the device
Check in the app how many treatments
you have left. The device is good for
12 treatments of 45 minutes. If there
are no treatments left, dispose the
device.
The LED is solid green
Device malfunction
Contact customer support at
support@theranica.com
No communication between
the app and the device
The device is turned off
If the LED is off, turn on the device
Bluetooth connection is
disabled on the phone
Enable the Bluetooth feature on your
phone and try to reconnect
The phone and the
device are not close
enough
Bring the phone closer to the device, to
a range of 1 inch
The device was
automatically shut down
since the treatment
ended or has not been
initiated for a prolonged
duration of time.
If the LED is off, turn on the device
The stimulation is not felt
The treatment has not
started yet or has been
stopped or paused
Touch “Start” or “Resume” in the
“Treatment” screen
The stimulation
intentisty is too low
Increase the stimulation using the “+”
button in the “Treatment” screen, until
you feel the stimulation
The protective film was
not removed
Remove the protective film from the
electrodes
The electrodes begin
losing adhesion
Gently rub with your finger one or two
drops of water onto the gel surface of
the electrodes
The adhesive surface of
the device is damaged
Replace the device

Page | 26
8.2. MAIN ERRORS AND MESSAGES
Errors and messages
displayed on the screen
What it may mean
What to do
Bluetooth is off
The Bluetooth connection of
your smartphone is disabled
preventing from your
smartphone to connect to the
device
Enable Bluetooth connection
in your smartphone
The device is not properly
placed on your arm. Place the
device on the arm as
described in the QuickStart
guide or user manual.
The device is not properly
placed on the body
Make sure the protective film
was removed from the
electrodes
The device should be placed
directly on the arm and not on
your shirt
Device malfunction. The
device is shutting down.
Please wait, turn it back on
and try again
Device malfunction
Turn the device on. For further
support, contact customer
support.
There are no remaining
treatments on this device.
Please replace the device or
order additional units if
required
No remaining treatments
The device cannot be used.
Replace the device or order a
new device.
Unable to start a treatment
(error code is: 0X20B). Please
contact customer support at
support@theranica.com
Device malfunction or no
remaining treatments
Contact customer support at
support@theranica.com
The treatment screen of the
app is not responding
No communication
between the app and
the device
See “what to do” in the problem of “No
communication between the app and
the device”
The device is turned off
If the LED is off, turn on the device
Device malfunction
Contact customer support at
support@theranica.com
The app is not working
Restart the app
Page | 27
Unable to connect using
proximity. Do you want to
enter device MAC address?
The device could not be
paired with the app by mere
proximity
Enter device mac address for
manual paring. Locate the
mac address of the device on
the label in the external part of
the device. The mac address
includes 12 letters and digit
separated by colons in the
format of: xx:xx:xx:xx:xx:xx.
Enter it to the MAC address
field without the colons.
The device is shutting down
since no treatment is
performed. Turn it back on to
start a treatment
The device was on for over 3
minutes and no treatment was
performed
Turn on the device
Device not found
The mac address entered is
incorrect
Re-enter the correct device
mac address.
The device is turned off
Turn on the device
The device is too far from the
smartphone
Bring the phone closer to the
device, to a range of 1-2
meters
Invalid device MAC address
The device MAC address field
has been left empty or the
MAC address has been
entered in the wrong format
Locate the mac address of the
device on the label in the
external part of the device.
The mac address includes 12
letters and digit separated by
colons in the format of:
xx:xx:xx:xx:xx:xx. Enter it to
the MAC address field without
the colons.
The OS version on your
device is newer than
supported by the current
version of the Nerivio Migra
app. To ensure flawless
functionality, please upgrade
the app as soon as possible
via App store
Incompatible mobile phone
OS and app versions
Update the app
Page | 28
8.3. LED STATUS
LED indication
Status
Flashing very rapidly (5 times per second)
An error occurred or there are no treatments
left in the device
Solid green
Device malfunction
Flashing slowly (mostly on)
The device is ready to be connected to the
app
Flashing rapidly
The device is connected to a smartphone
Flashing slowly (mostly off)
The device is in a treatment process
8.4. CUSTOMER SUPPORT
Customer support is available to answer any questions you may have about your Nerivio
Migra® device.
The service lifetime of the Nerivio Migra® is until product expiration date.
The battery operation time is 540 minutes if stored at ambient temperature of
23±2℃ (i.e., 12 treatments of 45 minutes)
Theranica Bio-Electronics LTD.
Address: 45 Ha-Melakha St.
Netanya 4250574, Israel
Email: support@theranica.com
Web: www.theranica.com
Fax: +972-72-390-9762
9. OPERATION SPECIFICATION
9.1. ENVIRONMENT OPERATING CONDITIONS
Operating temperature range: +5º to +40º C (41'F-104'F)
Relative humidity range: 35%-65%
Atmospheric pressure: 70-106 kPa
9.2. ENVIRONMENTAL STORAGE AND TRANSPORTATION CONDITIONS
BETWEEN USES

Page | 29
Temperature range: +10º to +27º C (50'F-80.6'F)
Relative humidity range: 40%-50%, with no condensing
Atmospheric pressure: 70-106 kPa
9.3. ENVIRONMENTAL TRASNPORTATION AND STORAGE CONDITIONS
Temperature range: +10º to +27º C (50'F-80.6'F)
Relative humidity range: 40%-50%, with no condensing
Atmospheric pressure: 70-106 kPa
9.4. ELECTRICAL PROPERTIES
Battery type: Primary cell Li-MnO2, 3.0 V, 1.2 Ah
Maximum Voltage: 3.3V
Maximum Current 40mA
Frequency 100-120Hz
Charger Input: N/A – the battery is not rechargeable in the device
Charger output: N/A – the battery is not rechargeable in the device
Battery lifetime 540 minutes if stored at ambient temperature of
23±2℃.
Do not disassemble, crush, incinerate or short-circuit the battery. This could
cause a fire, injury, burns, or other hazards.
Do not attempt to recharge or detach the battery
Recycle or dispose the device in accordance with disposal instructions in the
user manual
10. TECHNICAL SPECIFICATIONS
Number of channels
1
Waveform
Biphasic rectangular, modulated
Net charge (µC per pulse)
0 (charge is balanced by using a symmetrical, biphasic
pulse)
Max output voltage

Page | 30
500Ω
20V
2KΩ
60V
10KΩ
60V
Max output current
500Ω
40mA
2KΩ
30mA
10KΩ
6mA
Maximum phase charge
500Ω
8 µC
Maximum average current
500Ω
1.76mA
Maximum current density
(peak) 500Ω
1.6 mA/cm²
Maximum current density
(r.m.s) 500Ω
0.34 mA/cm²
Maximum average current
density (abs value) 500Ω
0.07 mA/cm²
Maximum average power
density 500Ω
1.41mW/cm²
Frequency
100-120Hz, average 110Hz
Primary phase duration
[µSec]
200
Pulse Duration [µSec]
400
Burst mode
No
Program duration [min]
45
Electrode area
25cm2
Electrode compliance
with 21 CFR 898
Yes
Electrode cable
No
Indication display
Device LED
Via the mobile application, if connected
-On/off status
Yes
Yes

Page | 31
-Wireless connection
Yes
Yes
-Low battery
No
Yes (remaining number of treatments)
-Current level
No
Yes (stimulation intensity)
-Output mode
Yes
Yes (stimulation time indicator)
-Time to cut-off
No
Yes (stimulation time indicator)
Power source
Integrated, non-rechargeable, primary cell Li-MnO2 battery
Operation time: 540 minutes (12 treatments of 45 minutes).
Processor control
Yes
Wireless control
Yes
Wireless communication
Frequency range: 2.400-2.4835 GHz
Modulation: Gaussian frequency shift
Output power: ≤0 dBm
Automatic overload trip
Yes, limiter for max current and voltage
Automatic no load trip
Yes, out-of-range load detection
Automatic shutdown
Yes, timer
Simulation intensity control
Yes, current amplitude is adjustable by the user
Wireless communication interference
This device operates in the 2.400-2.4835 GHz ISM band. In case this device is used
around other wireless devices such as microwave and wireless LAN, which operate at the
same frequency band as this device, interference between this device and such other
devices may occur. If an interference occurs before the treatment has begun, the treatment
may not start. Once the treatment has started, the device maintains the treatment
parameters (shape and frequency of pulses during stimulation, intensity and duration)
autonomically and does not require any further control. However, the app may not enable
you to stop the treatment or adjust the intensity, which may result in an uncomfortable
feeling. If such sensation occurs, please remove the device from your arm without touching
the electrodes, stop the operation of the other devices or move away from the interfering
source.
11. MINIMAL SMARTPHONE REQUIREMENTS
• At least 4.0 inches multi-touchscreen with at least 640 x 1136 pixels (~326 ppi
pixel density) resolution

Page | 32
• 800MHz or more CPU, internal memory of 512kB or more, and internal storage of
at least 4GB
• BT4.0 and above connectivity capabilities
• Supported operating system - iOS versions 9-12.1.4 and Android versions 6-9.
12. POTENTIAL ADVERSE REACTIONS
• People with sensitive skin may experience a rash or redness of the skin under the
electrodes.
13. CLASSIFICATION
• Internally powered ME Equipment
• Type BF applied part
• Enclosure IP22
• Continuous operation
14. EMC STATEMENT
With the increased number of electronic devices such as PC’s and mobile (cellular)
telephones, the Nerivio Migra® device may be susceptible to electromagnetic interference
from other devices, even if they comply with CISPR emission requirements.
Electromagnetic interference may result in incorrect operation of the Nerivio Migra device
and create a potentially unsafe situation.
The Nerivio Migra® device should not interfere with other devices.
In order to regulate the requirements for EMC (Electro Magnetic Compatibility) with the
aim to prevent unsafe product situations, the IEC60601-1-2 standard has been
implemented. This standard defines the levels of immunity to electromagnetic
interferences as well as maximum levels of electromagnetic emissions for medical
devices.
The Nerivio Migra® medical device conforms with the IEC60601-1-2:2014 standard for
both immunity and emissions.
The Nerivio Migra® device requires special precautions regarding EMC and needs to be
installed and used according to the EMC information provided in this manual:
• Do not use any unspecified accessories with the Nerivio Migra® device. This may
result in increased emissions or decreased immunity of the device.
• The Nerivio Migra® device should not be used adjacent to or stacked with other
equipment. In case adjacent or stacked use is necessary, the Nerivio Migra® device
should be monitored to verify normal operation in the configuration in which it is
used.
• Do not use devices which generate strong electrical or electromagnetic fields in
proximity to the Nerivio Migra® device. This may result in incorrect operation of the
device and create a potentially unsafe situation. In order to reduce the risk of EM

Page | 33
interference, it is recommended to keep a minimum distance of 30 cm (12 inches)
between the device and other electromagnetically radiating devices. Verify correct
operation of the device if the distance is shorter.
The Nerivio Migra® device complies with immunity tests described below.
14.1. ELECTROMAGNETIC TEST RESULT SUMMARY
Test
Standard
Class /
Severity level
Test
result
Emission
Radiated emission
Frequency range:
30-1000 MHz
IEC 60601-1-2 section 7 /
CISPR 11
Group 1 Class B
Complies
ETSI EN 301 489-1 section 8.2;
ETSI EN 301 489-17 section
7.1/EN 55032
Class B
Complies
Radiated emission
Frequency range:
1.0 GHz-6.0 GHz
ETSI EN 301 489-1 section 8.2;
ETSI EN 301 489-17 section 7.1
EN 55032
Class B
Complies
Immunity
Immunity from
Electrostatic
discharge (ESD)
IEC 60601-1-2 section 8, Table 4 /
IEC 61000-4-2
8 kV contact &
15 kV air discharges
Complies
ETSI EN 301 489-1 section 9.3;
ETSI EN 301 489-17 section 7.2/
EN 61000-4-2
4 kV contact &
8 kV air discharges
Complies
Immunity from
radiated
electromagnetic fields
IEC 60601-1-2 section 8, Table 4 /
IEC 61000-4-3
10 V/m,
80 MHz ÷ 2.7 GHz,
80% AM, 1 kHz
Complies
Immunity from
Proximity field
from wireless
communications
equipment
IEC 60601-1-2 section 8, Table 9 /
IEC 61000-4-3
List of frequencies,
from 9 V/m up to 28 V/m,
PM (18 Hz or 217 Hz),
FM 1 kHz
Complies
Immunity from
radiated
electromagnetic fields
ETSI EN 301 489-1 section 9.2;
ETSI EN 301 489-17 section 7.2/
EN 61000-4-3
3 V/m,
80 MHz - 6 GHz,
AM 80% @ 1 kHz
Complies
Immunity from power
frequency magnetic
field
IEC 60601-1-2 section 8, Table 4 /
IEC 61000-4-8
30 A/m @ 50 Hz & 60 Hz
Complies
Note: this table is formatted based on IEC60601-1-2:2014.
14.2. ELECTROMAGNETIC EMISSIONS
The Nerivio Migra® is intended for use in the electromagnetic environment specified below.
Please assure that the device is used according to these specifications.
Note: the following tables is formatted based on IEC60601-1-2:2007.

Page | 34
Electromagnetic emissions IEC 60601-1-2
Emissions Test
Compliance
Electromagnetic Environment - Guidance
RF emissions
CISPR 11
Group 1
The Nerivio Migra® uses RF energy only for
its internal function. Therefore, its RF
emissions are very low and are not likely to
cause any interference in nearby electronic
equipment.
RF emissions
CISPR 11
Class B
The Nerivio Migra
®
is suitable for use in all
establishments, including domestic
establishments and those directly
connected to the public low-voltage power
supply network that supplies buildings used
for domestic purposes
Harmonic emissions
IEC 61000-3-2
Not applicable
Voltage fluctuations /
Flicker emissions
IEC 61000-3-3
Not applicable
14.3. ELECTROMAGNETIC IMMUNITY
The Nerivio Migra® is intended for use in the electromagnetic environment specified below.
Please assure that the device is used according to these specifications.
Electromagnetic immunity IEC 60601-1-2
Immunity Test
IEC 60601-1-2 test
level
Compliance Level
Electromagnetic EnvironmentGuidance
Electrostatic
discharge, ESD
(IEC 61000-4-2)
Contact discharge:
±8 kV
Contact discharge:
±8 kV
The relative humidity should be at
least 5%
Air discharge:
±15 kV
Air discharge:
±15 kV
Electrical fast
transient / burst
(IEC 61000-4-4)
Power supply lines:
±2 kV
Not Applicable
Longer input /
output lines: ±1 kV
Not Applicable
Surge on AC
mains lines
(IEC 61000-4-5)
Common mode:
±2 kV
Not Applicable
Differential mode:
±1 kV
Not Applicable
Voltage dips,
short interruptions
and voltage
variations on
power supply
lines
(IEC 61000-4-11)
0% UT
for 0.5 cycle
Not Applicable
0% UT
for 1 cycle
Not Applicable
70% UT
for 25 cycles
Not Applicable
0% UT
for 5 s
Not Applicable
Power frequency
(50/60 Hz)
magnetic field
(IEC 61000-4-8)
30 A/m
30 A/m

Page | 35
Electromagnetic immunity IEC 60601-1-2
Immunity Test
IEC 60601-1-2 test
level
Compliance Level
Electromagnetic EnvironmentGuidance
Note: UT is the A.C. mains voltage prior to application of the test level.
Electromagnetic immunity IEC 60601-1-2
Portable and mobile RF
communications equipment should
be used no closer to any part of the
Nerivio Migra®, including cables,
than the recommended separation
distance calculated from the
equation applicable to the frequency
of the transmitter.
Recommended separation
distance
Conducted RF
(IEC 61000-4-6)
3 V rms
150 kHz to 80 MHz
Not Applicable
d = 1.16 √P 150 kHz to 80 MHz
6 V rms
The ISM bands and
the amateur radio
bands between 150
kHz to 80 MHz
Not Applicable
d = 0.58 √P 150 kHz to 80 MHz
(The ISM bands and the amateur
radio bands)
Radiated RF
(IEC 61000-4-3)
10 V/m
80 MHz to 2.7 GHz
IEC 60601-1-2:2014
Table 9
(Up to 28 V/m at
certain frequencies)
10 V/m
80 MHz to 2.7 GHz
IEC 60601-12:2014 Table 9
(Up to 28 V/m at
certain frequencies)
d = 0.35 √P 80 MHz to 800 MHz
d = 0.7 √P 800 MHz to 2.7 GHz
Where P is the maximum output
power rating of the transmitter in
watts (W) according to the transmitter
manufacturer and d is the
recommended separation Distance in
meters (m).
Field strengths from fixed RF
transmitters, as determined by an
electromagnetic site survey,a should
be less than the compliance level in
each frequency range.
Interference may occur in the vicinity
of equipment marked with the
following symbol:
Note 1: At 80 MHz and 800 MHz, the higher frequency range applies.
Note 2: These guidelines may not apply in all situations. Electromagnetic propagation is affected by
absorption and reflection from structures objects and people.

Page | 36
Electromagnetic immunity IEC 60601-1-2
a
Field strengths from fixed transmitters, such as base stations for radio (cellular/cordless) telephones and land mobile radios,
amateur radio, AM and FM radio broadcast and TV broadcast cannot be predicted theoretically with accuracy. To assess the
electromagnetic environment due to fixed RF transmitters, an electromagnetic site survey should be considered. If the measured
field strength in the location in which the Nerivio Migra is used exceeds the applicable RF compliance level above, the Nerivio
Migra® should be observed to verify normal operation. If abnormal performance is observed, additional measures may be
necessary, such as re-orienting or relocating the Nerivio Migra.
14.4. RECOMMENDED SEPARATION DISTANCES
Recommended separation distance between portable and mobile RF communications equipment and the NM
Nerivio Migra
®
is intended for use in an electromagnetic environment in which radiated RF
disturbances are controlled. The customers or the users of Nerivio Migra
®
can help prevent
electromagnetic interference by maintaining a minimum distance between portable and mobile
RF communications equipment (transmitters) and Nerivio Migra
®
as recommended below,
according to the maximum output power of the communications equipment.
Output Power of
Transmitter in Watt
Separation distance according to frequency of transmitter in meter
150 kHz to 80 MHz
80 MHz to 800
MHz
800 MHz to 2.7
GHz
d = 1.16 √𝑷
d = 0.58 √𝑷
d = 0.35 √𝑷
d = 0.7 √𝑷
0.01
0.12
0.06
0.04
0.07
0.1
0.37
0.18
0.11
0.22
1
1.16
0.58
0.35
0.7
10
3.67
1.8
1.1
2.2
100
11.6
5.8
3.5
7.0
For transmitters rated at a maximum output power not listed above, the recommended
separation distance d in meters (m) can be estimated using the equation applicable to the
frequency of the transmitter, where P is the maximum output power rating of the transmitter in
watts (W) according to the transmitter manufacturer.
Note: At 80 MHz and 800 MHz, the separation distance for the higher frequency range applies
Note: These guidelines may not apply in all situations. Electromagnetic propagation is affected
by absorption and reflection from structures, objects, and people.
15. FCC RADIO FREQUENCY INTERFERENCE STATEMENT
FCC Registration Number (FRN): 0027054477.
This equipment has been tested and found to comply with the limits of Class B digital
device, pursuant to part 15 of the FCC Rules. These limits are designed to provide
reasonable protection against harmful interference in a residential installation. This
equipment generates, uses and can radiate radio frequency energy and, if not installed
and used in accordance with the instructions, may cause harmful interference to radio
communications. However, there is no guarantee that interference will not occur in a
installation. If this equipment causes interference with other devices, which may be
determined by turning the equipment off and on, the user is encouraged to try to correct
the interference by one or more of the following measures:

Page | 37
• Reorient or relocate the device receiving the interference
• Increase the separation between the equipment
• Connect the equipment into an outlet on a circuit different from that to which the device
is connected.
• Consult the manufacturer or field service technician for help
Theranica Bio-Electronics Ltd. is not responsible for any radio or communication
interference caused by using other than specified or recommended cables and battery or
by unauthorized changes or modifications to this equipment. Changes or modifications not
expressly approved by the manufacturer could void the user’s authority to operate the
equipment.
This device complies with part 15 of the FCC Rules. Operation is subject to the following
two conditions:
1. This device may not cause harmful interference, and
2. This device must accept any interference received, including interference that may
cause undesired operation.
16. APPLICABLE STANDARDS
• IEC/EN 60601-1 edition 3.1 Medical electrical equipment, part 1: General
requirements for basic safety and essential performance.
• IEC/EN 60601-1-2 edition 4.0 Medical electrical equipment- Part 1-2: General
requirements for safety – collateral standard: Electromagnetic compatibility –
Requirements and tests.
• IEC/EN 60601-2-10 edition 2.1 Requirements for the safety of nerve and muscle
stimulators

Page | 38
Nerivio Migra®
Theranica Bio-Electronics LTD.
45 Ha-Melakha St.,
Netanya 4250574, Israel
Tel: +972-72-3909-763, Fax: +972-72-3909762 © 2018 Theranica Bio-Electronics LTD. All Rights Reserved.
www.theranica.com ART No. LBL-NM-0014-1.1, Rev. 1.1, May 2019
 Loading...
Loading...