Page 1
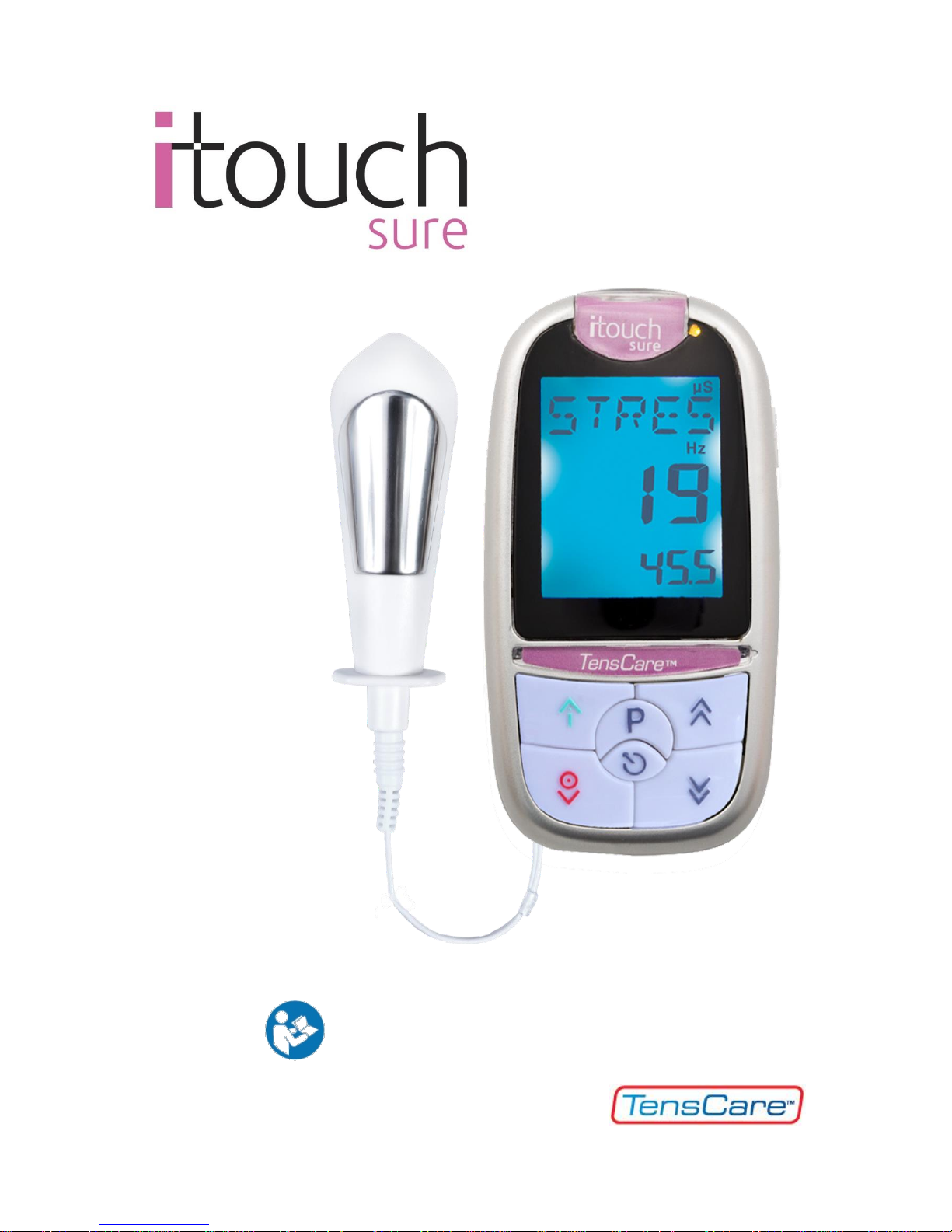
0
INSTRUCTIONS FOR USE
READ CAREFULLY BEFORE USE
Page 2
1
QUICKSTART GUIDE
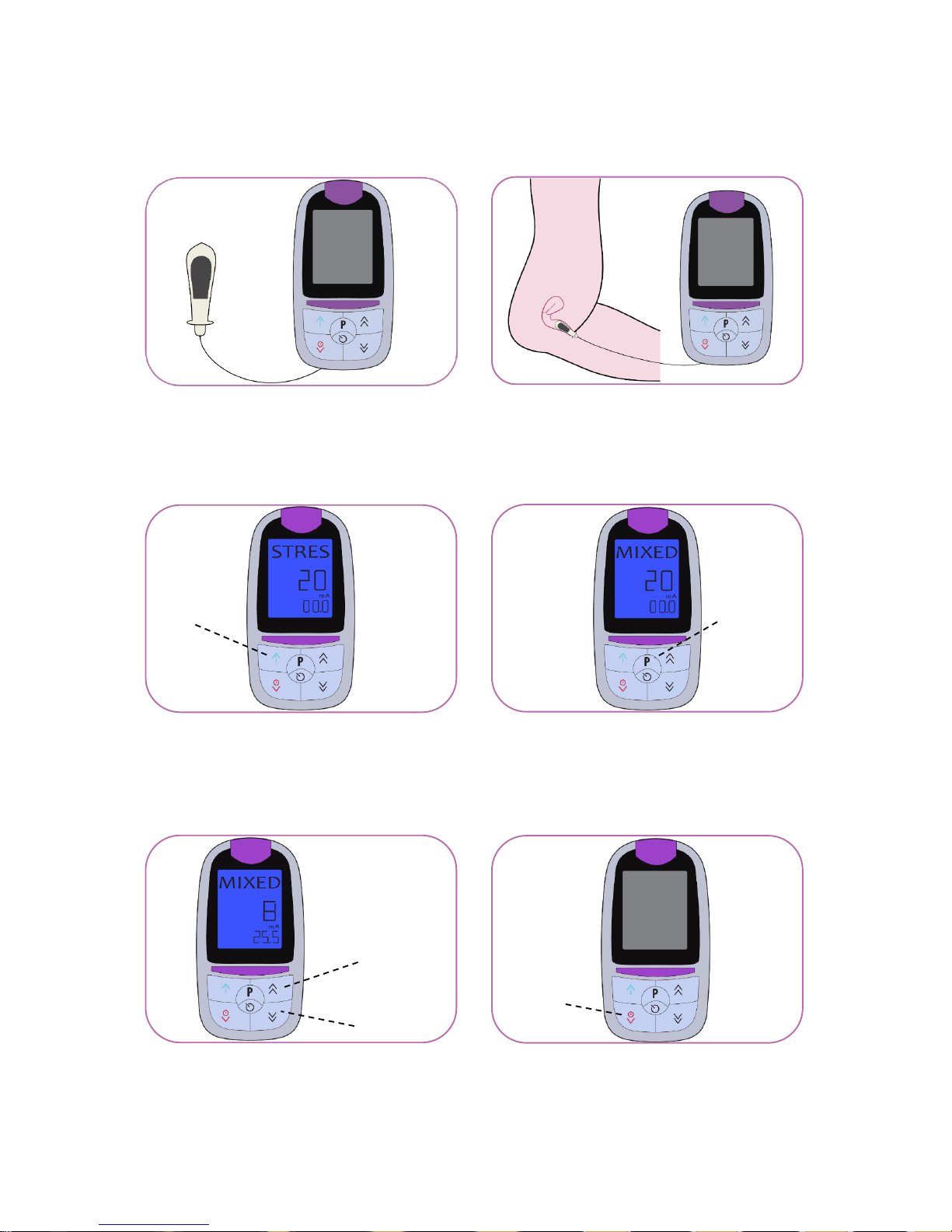
Connect the unit with the
vaginal probe
Insert the vaginal probe
Press and hold the ON button
to switch the device on
Regulate the output intensity with
the buttons ▲ and ▼
Press and hold the OFF button
to switch the device off
ON
Button P
UP
DOWN
OFF
Select the programme by
pressing the button P
1
2
3 4 5
6
Page 3

2
Dear Customer,
Thank you for choosing itouch sure. TensCare stands for high-quality,
thoroughly tested products for the applications in the areas of gentle
electrotherapy, muscle toning, continence management and pain relief
during labour.
Please read these instructions for use carefully and keep them for later
use and observe the information they contain.
Best regards,
Your TensCare Team
Page 4

3
Page 5

4
Contents
1. INTRODUCTION ................................................................................................. 6
2. INTENDED USE .................................................................................................. 6
3. ITOUCH SURE FEATURES ................................................................................ 7
4. PELVIC FLOOR EXERCISES ............................................................................. 7
4.1. PELVIC FLOOR MUSCLES .......................................................................... 7
4.2. PERFORMING PELVIC FLOOR EXERCISES ............................................. 9
5. TYPES OF INCONTINENCE ............................................................................. 10
6. HOW ‘EMS’ WORKS ......................................................................................... 10
7. CONTRAINDICATIONS, WARNINGS & CAUTIONS ........................................ 11
8. INFORMATION ABOUT THE PROGRAMME SETTINGS ................................ 14
9. PROGRAMMES ................................................................................................ 15
9.1. PROGRAMME SETTINGS ......................................................................... 15
9.2. PRESET PROGRAMMES .......................................................................... 15
10. CONTENT ......................................................................................................... 16
11. UNIT INFORMATION ........................................................................................ 17
11.1. CONTROLS & DISPLAY ......................................................................... 17
11.2. OPERATING INSTRUCTIONS ................................................................ 18
12. SETTING UP AND USING THE ITOUCH SURE ............................................... 20
12.1. INSTALLATION OF BATTERIES ............................................................. 20
12.2. CONNECTING LEAD WIRE .................................................................... 21
12.3. PREPARING FOR SESSION .................................................................. 21
12.4. TRAINING SESSION ............................................................................... 22
12.5. AFTER YOUR TRAINING SESSION ....................................................... 24
13. ANAL PROBE .................................................................................................... 24
13.1. CONDITIONS THAT MAY BE TREATED ................................................ 24
13.2. HOW TO INSERT THE ANAL PROBE .................................................... 25
14. CLEANING ........................................................................................................ 25
15. EMC ................................................................................................................... 26
16. DISPOSAL OF WASTE ELECTRICAL AND ELECTRONIC PRODUCTS ........ 26
17. ACCESSORIES ................................................................................................. 26
18. WARRANTY ...................................................................................................... 27
19. TROUBLESHOOTING ....................................................................................... 29
20. GENERAL SPECIFICATION ............................................................................. 31
NOTES ..................................................................................................................... 33
Page 6

5
SYMBOLS USED
Attention! Please follow the instructions in the user’s instructions for use.
TYPE BF EQUIPMENT: Equipment providing a degree of protection against
electric shock, with isolated applied part. Indicates that this device has conductive
contact with the end user.
This symbol on the unit means “Refer to instructions for use”.
Temperature Limitation: indicates the temperature limits to which the medical
device can be safely exposed.
Lot Number: indicates the manufacturer’s batch code so that the batch or lot can
be identified.
Humidity Limitation: indicates the humidity limits to which the medical device can
be safely exposed.
Serial Number: indicates the manufacturer’s serial number so that a specific
medical device can be identified.
Do not dispose in household waste.
Catalogue Number: indicates the manufacturer’s catalogue number so that the
device can be identified.
This medical device is not water resistant and should be protected from liquids.
Atmospheric Pressure: indicates the atmospheric limits to which the medical device
can be safely exposed.
Date of Manufacture: indicates the date which the medical device was
manufactured. This is included within the serial number found on the device
(usually in the battery compartment), either as “E/Year/Number” (YY/123456) or
“E/Month/Year/Number” (MM/YY/123456).
This medical device is indicated for home use.
The first number 2: Protected against access to hazardous parts with a finger,
and the jointed test finger of 12 mm ø, 80 mm length, shall have adequate
clearance from hazardous parts, and protected against solid foreign objects of 12.5
mm ø and greater.
The second number 2: Protected against vertically falling water drops when
enclosure is tilted up to 15˚. Vertically falling drops shall have no harmful effects
when the enclosure is tilted at any angle up to 15˚ on either side of the vertical.
LOT
S/N
REF
IP22
Page 7
6
1. INTRODUCTION
Device Description & Principles of
Design
Bladder leakage and incontinence are
common problems for both women and
men, affecting their long-term health.
Exercising the pelvic floor muscles is
recognised as the way of preventing
and treating symptoms of incontinence
and pelvic floor weakness.
The itouch sure is a powered muscle
stimulator used for strengthening the
pelvic floor muscles.
It sends a gentle stimulation (similar to
your natural nerve impulses) direct to
your pelvic floor muscles through a
vaginal probe with stainless steel
electrodes. These signals make your
pelvic floor muscles contract. If you
have forgotten how to contract them,
are having trouble getting muscle
response, or simply want to bring back
the condition of your pelvic floor
muscles, the itouch sure can work
them for you to build up their strength
and help you to develop your own
muscle control. It perfectly
complements pelvic floor exercises.
The itouch sure is very easy to use,
with four clearly labelled preset training
programmes and a simple push button
control.
The itouch sure provides relief from
conditions such as:
• Urinary and faecal incontinence:
including stress, urge and mixed
types as well as post prostatectomy
urinary incontinence in men.
Additionally, it may help improve
sexual intimacy by toning the pelvic
floor muscles.
• Treatment of erectile dysfunction
in men* and improvement of pelvic
strength (* requires an anal probe).
2. INTENDED USE
Itouch sure is a medical
device designed to be used in
the home healthcare
environment to treat symptoms of
urinary and/or faecal incontinence and
is suitable for use by all who can control
the device and understand the
instructions.
Do not use the device for any purpose
other than this intended use.
Warning: Not suitable for use
in children without medical
supervision.
Page 8
7
3. ITOUCH SURE
FEATURES
• Single Channel
Single channel unit provides relief from
symptoms of all types of incontinence
via a tampon-shaped probe.
• Comfortable Stimulation
Gentle stimulation in small steps of
intensity, 0.5 mA per step.
• 4 Preset Programmes
Clinically tested programmes for all
types of incontinence including stress,
urge, mixed and a tone aftercare
programme.
• Treatment Timer
Unit defaults to 20 minutes’ treatment to
ensure the pelvic floor muscles are not
overworked. The user can manually
reset this (20,30,45,60 or 90 mins).
• Open Circuit Detection
Automatically resets the strength to
zero and flashes ‘LEADS’ if the
connection comes loose.
• Keypad Lock
Enables the user to manually lock the
controls to prevent any accidental
changes in settings.
• Large Backlit Screen
Clearly shows the operation of the unit
and the programme and intensity
currently being used.
• Memory
Features 3 functions: programme
retention (automatically starts in the last
programme used), number of uses and
time of usage.
4. PELVIC FLOOR
EXERCISES
4.1. PELVIC FLOOR
MUSCLES
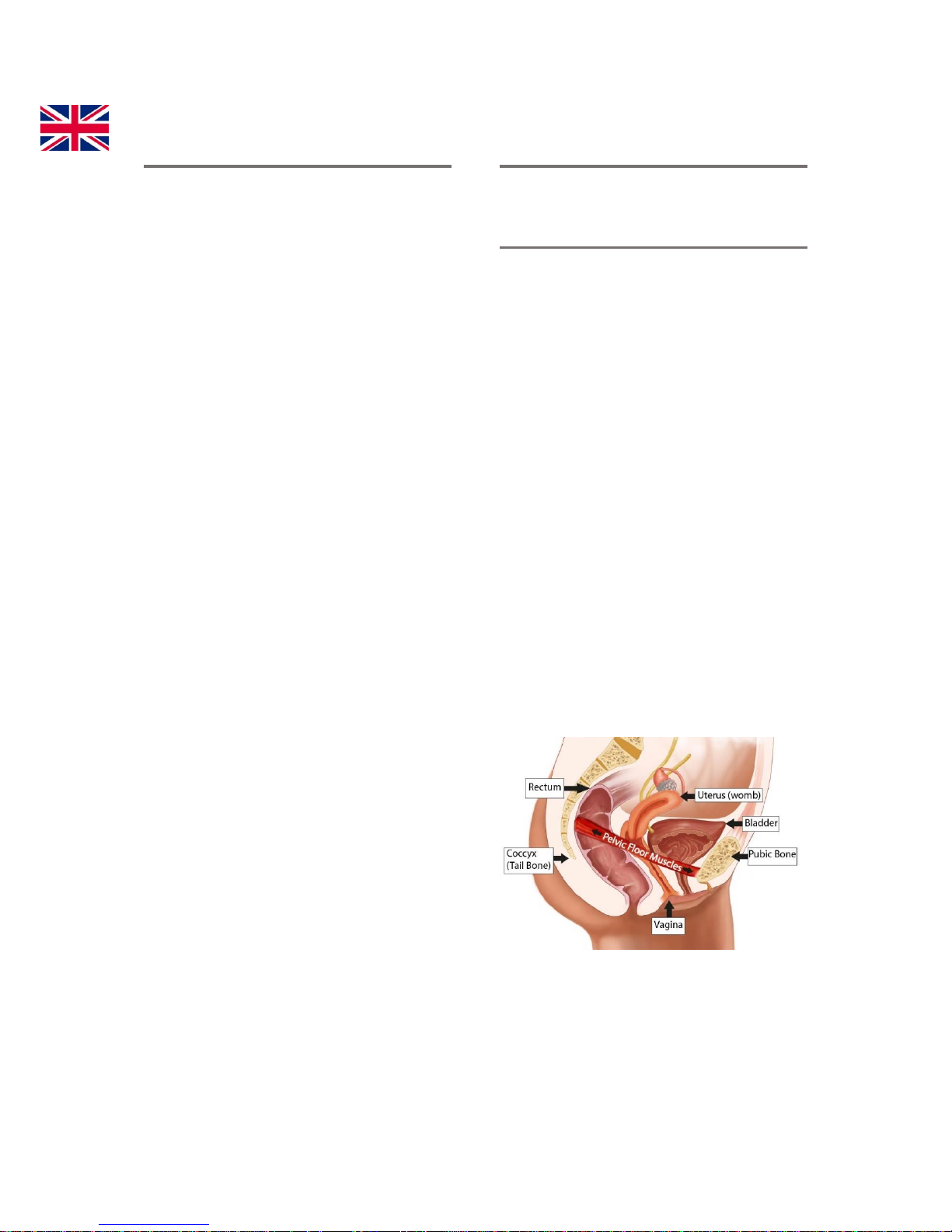
The “FLOOR” of your pelvis is made up
of layers of muscles that support the
bowel, bladder, urethra and uterus.
These muscles are like a hammock, or
the bottom and sides of a bowl, in
shape. They run from the pubic bone in
the front to the end of the spinal column
(or tail bone) in the back.
The pelvic floor muscles:
• Assist in supporting the abdominal
and pelvic organs.
• Work with the abdominal and back
muscles to stabilise and support the
spine.
• In women, also
o provide support for the baby
during pregnancy and
o assist in the birthing process
Pelvic floor muscles are also important
for sexual function in both men and
women:
• In men, it is important for erectile
function and ejaculation.
Page 9

8
• In women, voluntary contractions
(squeezing) of the pelvic floor
contribute to sexual sensation and
arousal.
However pelvic floor muscles may
become weak. If your pelvic floor
muscles become stretched or
weakened, your pelvic organs may no
longer be fully supported and you may
lose control of your bladder or bowel
movements.
For some women, the pelvic floor
muscles can also become too tight.
This condition is less common, but it
can lead to pelvic pain and make it
difficult for you to empty your bladder or
bowel completely.
Common signs that can indicate a
pelvic floor problem include:
• Accidentally leaking urine when you
exercise, laugh, cough or sneeze
• Needing to get to the toilet in a hurry
or not making it there in time
• Constantly needing to go to the toilet
• Finding it difficult to empty the
bladder or bowel
• Accidentally losing control of the
bladder or bowel
• Accidentally passing wind
• Pain in your pelvic area
• Painful sex, or
• A prolapse
In women, this may be felt as a bulge in
the vagina or a feeling of heaviness,
discomfort, pulling, dragging or
dropping. This occurs when one or
more of the pelvic organs (bladder,
bowel or uterus) become displaced and
sag down into the vagina. It is very
common in the United Kingdom and
occurs in about 40% of women.
Symptoms tend to become
exacerbated towards the end of each
day and if left untreated, they will
generally worsen over time.
In men, this may be felt as a bulge in
the rectum or a feeling of needing to
use the bowel but not actually needing
to go.
Like other muscles in your body, the
pelvic floor can be strengthened with
regular exercise. Building pelvic floor
strength enables the muscles to better
support your pelvic organs, improves
your bladder and bowel control and can
stop accidental urine, faeces or wind
leakage.
It can also reduce your risk of prolapse,
improve your recovery from childbirth
and gynaecological surgery, and
increase your sexual pleasure. A
continence therapist can help you learn
how to exercise your pelvic floor.
Doing just a few pelvic floor exercises
every day will help to treat bladder
weakness or prolapse symptoms, and
will help to prevent problems later on.
Page 10
9
4.2. PERFORMING PELVIC
FLOOR EXERCISES
It is recommended to make Pelvic Floor
Exercises (sometimes called Kegel
Exercises) part of your daily life.
1) Kegel exercises can be done at any
time and are very discreet so you can
do them almost anywhere; lying in
bed, sitting at the computer or
waiting for a bus. It is a good idea to
try and develop a routine which you
can repeat each day.
2) First, it is important to find your pelvic
floor muscles and feel them working.
So here are a couple of techniques
which might help:
Try inserting one or two clean fingers
into your vagina and then squeezing
the surrounding muscles, lifting up
and towards your belly button – a
squeezing and lifting sensation.
Another way is to try and stop the
flow of urine during urination. If you
are successful, then you know you
are exercising the correct muscles.
Note: These techniques are
just to help you confirm that you
are using the correct muscles. It is
important to have an empty bladder
before starting the exercises.
3) Try to remember the lifting and
squeezing sensation and when you
are ready try to recreate it just using
the muscles you identified earlier;
don't tense the muscles in your legs,
stomach or buttocks and remember
to breathe normally.
4) Aim to hold each squeeze or
'contraction' for three to five
seconds, then release and relax.
You should feel a ‘letting go’ of the
muscles. Rest for five seconds and
then repeat.
5) Try and do about ten squeezes in
this way.
6) Repeat the whole process three or
four times a day.
7) Over a period of time try to increase
the muscle contractions up to about
ten seconds, but remember to rest in
between each squeeze for longer
periods.
Note: It is important to aim for
quality contractions, not
quantity, so a few good hard squeezes
are better than a series of weak ones.
Do not worry if you find holding for 3
seconds difficult at first. Just squeeze
for as long as you feel comfortable to do
so. The more exercise you do, the
stronger the muscles will become and
the longer you will be able to squeeze.
8) Using your itouch sure pelvic floor
stimulator in conjunction with Kegel
exercises will give you a better
understanding of how they work and
how to get the greatest benefit from
them.
Page 11

10
5. TYPES OF
INCONTINENCE
There are three types of incontinence:
Stress, Urge, and Mixed.
Stress Incontinence
If you leak urine when you cough,
sneeze, laugh, strain or make sudden
movements, this is called Stress
Incontinence.
It is particularly common in women who
have had a natural childbirth and occurs
when the bladder neck and the other
mechanisms that act to hold urine in the
bladder are not working properly. The
most common cause is a weak pelvic
floor.
Urge Incontinence
Describes an overactive bladder. A
person may experience a strong and
sudden urge to go to the toilet but are
not always able to hold on, or must go
so frequently that it becomes
inconvenient.
Mixed Incontinence
Is a combination of both Stress and
Urge Incontinence.
6. HOW ‘EMS’ WORKS
E.M.S. stands for Electrical Muscle
Stimulation and has successfully been
used in medical rehabilitation and
training in competitive sports. EMS
produces intensive and effective
muscular contraction.
In rehabilitation, EMS is a wellestablished method for treatment of a
broad field of musculoskeletal
diagnoses as well as pelvic floor
weakness. Electrical stimulation of an
intact peripheral nervous system may
create motor responses in patients with
impaired or lost ability for voluntary
muscle activity.
EMS is a complement to other physical
therapy and should always be
combined with active training such as
Kegel exercises (see section 4.2.).
Advantages of EMS
Use of EMS may lead to faster progress
in the patient’s treatment programme.
The method is simple and appropriate
for treatment in the clinical setting as
well as for self-treatment at home.
How EMS Works
Electrical Muscle Stimulators can play a
vital role in educating women and men
about their pelvic floor and the
sensation they should feel when doing
pelvic floor exercises. Electrical Pelvic
Floor Exercisers (PFE) offer a noninvasive method of producing
contraction of muscles via a gentle
stimulation to the pelvic floor through a
discreet probe or electrode pads when
Page 12

11
they are placed close to the nerve that
controls the pelvic floor muscles. This
current then passes into the nerve
fibres controlling that part of the muscle
stimulating it to contract. So, electrical
stimulation (EMS) artificially activates a
muscle for you enabling you to develop
your own muscle control. These
contractions exercise the muscles and,
as with any kind of exercise if
performed regularly, build strength and
tone.
In urge incontinence, pelvic floor
exercisers work in a slightly different
way. The electrical stimulation is
designed to soothe your bladder
muscles rather than exercise your
pelvic floor. Itouch sure uses a gentler,
low frequency setting which promotes
release of endorphins and reduces
involuntary contractions of the bladder
(detrusor) muscle.
Different frequencies have different
effects; low frequencies (1-10 Hz)
coupled with long impulse times, for
example, have a purifying and relaxing
effect through individual contractions,
whereby the circulation in the treated
muscle is simultaneously improved and
removal of metabolic end products is
supported (lymphatic drainage). The
oxygen supply to the muscle is
improved.
In contrast, by means of a rapid
succession of contractions (fibrillation),
medium frequencies (20-50 Hz) can put
a high level of strain on the muscle, thus
promoting the muscular structure.
Each preset programme has a specific
frequency and pulse width that will offer
the best results for the type of
incontinence treated.
7. CONTRAINDICATIONS,
WARNINGS &
CAUTIONS
In this manual:
A Warning is used when failure
to follow the instructions may
result in serious injury or death.
A Caution is used when failure
to follow the instructions may
result in a minor or moderate injury, or
damage to the device or other property.
Notes are used to provide
clarification or
recommendation.
CONTRAINDICATIONS:
• Do NOT use if you are or may be
pregnant. It is not known whether
TENS may affect foetal
development.
• Do NOT use with optional electrode
pads if you have a pacemaker (or if
you have a heart rhythm problem) or
with any electronic medical devices.
Using this unit with electronic
medical devices may cause
erroneous operation of the device.
Stimulation in the direct vicinity of an
implanted device may affect some
models. Stimulation on the front of
the neck can affect your heart rate.
Very strong stimulation across the
chest may cause an extra heartbeat.
• Do NOT use in the first 6 weeks
following a pelvic surgery or vaginal
childbirth. Stimulation may disrupt
the healing process.
• Do NOT use if you have symptoms
of active urinary tract infection,
vaginal infections, or localized
Page 13

12
lesions. Introducing the probe may
irritate sensitive tissue.
• Do NOT use if you have poor
sensation in the pelvic region. You
may not be able to control the
intensity of stimulation safely.
WARNINGS:
Do NOT use if you are unable
to properly insert the vaginal or
anal probe. If you have a severe
prolapse, or if any discomfort occurs
when inserting the probe, consult your
healthcare professional before use.
Do NOT use when driving,
operating machinery, or similar
actions needing muscular
control. Loose electrode pads,
damaged leads, or sudden changes in
contact may cause brief involuntary
muscle movements.
Do NOT use to mask or relieve
undiagnosed pain. This may
delay diagnosis of a progressive
condition.
Do NOT use if you have, in the
area being treated: active or
suspected cancer or undiagnosed pain
with a history of cancer. Stimulation
directly through a confirmed or
suspected malignancy should be
avoided as it may stimulate growth and
promote spread of cancer cells.
CAUTIONS:
Caution should be used if you
have suspected or diagnosed
epilepsy as electrical stimulation may
affect seizure threshold.
Caution should be observed
when using the device at the
same time as being connected to
monitoring equipment with body
worn electrode pads. It may interfere
with the signals being monitored.
Caution: Simultaneous
connection to high frequency
surgical equipment may result in burns
and damage to the stimulator.
Caution: Strong
electromagnetic fields (electrosurgery/ microwave cookers/ mobile
phones) may affect the correct
operation of this unit. If it appears to
behave unusually, move it away from
these devices.
Caution Do not permit use by
persons unable to understand
the instructions or persons with
cognitive disabilities, i.e.; Alzheimer’s
disease or dementia.
Caution: Insertion of the
vaginal or anal electrode
makes it unsuitable for use in children
without clinical supervision
Caution: Keep away from
children under 5 years of age.
Long cord - risk of strangulation in
infants.
Caution should be observed
when using the itouch sure at
high strength settings. Prolonged use at
high settings may cause muscle injury
or tissue inflammation.
Note: No serious or long term
adverse effects have been
reported. Mild adverse
reactions are very rarely reported, but
these have included muscular pain and
cramps, vaginal tenderness, irritation
and bleeding, mild or short term urge or
faecal incontinence, and tingling
sensation in legs. If you experience any
of these, stop use. When symptoms
have gone, try resuming at a lower
intensity setting.
Page 14

13
PROBE CAUTIONS:
Caution: The itouch sure
vaginal probe is intended for
single patient use only. Do not share
your itouch sure probe with anyone
else. Improper treatment or cross-
infection may occur.
Caution: It is important that the
vaginal probe is cleaned after
each use. Ineffective cleaning may lead
to irritation or infection.
Caution: Never insert or
remove vaginal probe unless
the control unit is powered OFF as
insertion or removal when stimulation is
active may cause discomfort or tissue
irritation.
Caution: If tissue irritation
occurs, discontinue treatment
immediately. Ask your healthcare
professional for advice before
continuing further treatment to prevent
injury.
Caution: Do not use a silicone
based lubricant on the metal
plates of the probe as it may decrease
the effectiveness of itouch sure’s
muscle stimulation.
Caution: The stainless steel in
the probe’s metal plates
contain some Nickel. This could cause
a reaction if you have a Nickel allergy.
Alternative gold probe specially made
without Nickel is available (see X-VPG).
Caution should be used if you
have a copper IUD. If
discomfort occurs, discontinue
treatment immediately and ask your
healthcare professional for advice.
There is a small risk of stimulating the
uterine wall if the IUD is not correctly
positioned.
Caution: Do not use this
device with vaginal probe or
anal probe other than those
recommended by the manufacturer in
section 17. Electrodes with smaller
surface area may cause tissue irritation.
DO NOT PLACE OPTIONAL
ELECTRODE PADS:
• On skin, which does not have normal
sensation. If the skin is numb too
great a strength may be used, which
could result in skin inflammation.
• On broken skin. The electrode pads
could encourage infection.
ELECTRODE PADS CAUTION:
Caution: Do not ignore any
allergic reaction to the
electrode pads: If a skin irritation
develops, stop using TENS, as this type
of electrodes may not be suitable for
you. Alternative electrode pads
specially made for sensitive skin are
available (see E-696-SS).
Caution: Do not use this
device with leads or electrode
pads other than those recommended
by the manufacturer. Performance
may vary from specification. Electrodes
with smaller surface area may cause
tissue irritation.
TO KEEP YOUR DEVICE IN GOOD
WORKING ORDER, OBSERVE THE
FOLLOWING ADDITIONAL
CAUTIONS:
Caution: Do not immerse your
device in water or place it close
to excessive heat such as a fireplace or
radiant heater or sources of high
humidity such as a nebulizer or kettle as
Page 15

14
this may cause it to cease to operate
correctly.
Caution: Keep the device
away from sunlight, as longterm exposure to sunlight may affect
the rubber causing it to become less
elastic and crack.
Caution: Keep the device
away from lint and dust, as
long-term exposure to lint or dust may
affect the sockets or cause the battery
connector to develop a bad contact.
Caution: Temperature &
Relative Humidity of storage: 10°C to +60°C, 8 to 93% R.H.
Temperature & Relative Humidity of
operation: 0°C to +40°C, 10 to 93%
R.H.
Caution: Do not attempt to
open or modify the TENS unit. This may
affect the safe operation of the unit and
will invalidate the warranty.
8. INFORMATION ABOUT
THE PROGRAMME
SETTINGS
Each programme has its own
combination of Frequency and Pulse
Width settings which allow for different
sensations through the probe or
optional electrode pads and help
treating the different types of
incontinence.
• Frequency (measured in Hz -
pulses per second)
Low frequencies (1-10 Hz) have a
purifying and relaxing effect through
individual contractions.
Medium frequencies (20-50 Hz) can put
a high level of strain on the muscle, thus
promoting the muscular structure
• Pulse Width (measured in μs -
millionths of a second)
The itouch sure unit has pulse widths
of 200 to 300 μs. Generally speaking,
the higher the pulse width, the more
"aggressive" the stimulation feels, if the
pulse width is set high enough, it will
usually elicit a muscle contraction,
which is required for an effective toning
of the pelvic floor muscles.
Page 16

15
9. PROGRAMMES
9.1. PROGRAMME SETTINGS
9.2. PRESET PROGRAMMES
The itouch sure has 4 preset
programmes. One for each type of
incontinence (stress, urge and mixed),
and one aftercare programme for
toning the pelvic floor muscles (TONE).
STRESS INCONTINENCE:
Shown on the screen as: STRES
The STRES incontinence programme
strengthens the muscles of the pelvic
floor using gentle stimulation. Once
these muscles are stronger they are
better able to resist urinary leakage
caused by external pressure being
applied to the bladder such as with a
cough, sneeze or physical exertion.
The stimulation causes the muscles to
contract and work. This builds their
strength. Like other fitness training,
successful treatment requires
stimulation once a day for one to three
months. Improvement starts becoming
apparent after about four weeks.
The sensation is like a strong drawing
in of the muscles of the vagina, pulling
up the pelvic floor. Your natural reaction
will be to pull your muscles in and up,
and this exercises and strengthens
them.
URGE INCONTINENCE:
Shown on the screen as: URGE
The URGE programme works in a
different way to the STRES
programme. The gentle continuous
stimulation soothes the bladder
(detrusor) muscle, reducing its
involuntary contractions. This prevents
the unwanted and unexpected
emptying of the bladder.
Successful treatment requires
stimulation once a day and
improvements can sometimes be seen
in as little as two weeks.
The sensation is a softer, vibrating,
stimulation. Nevertheless, when the
programme finishes, and your pelvic
floor relaxes, it will become apparent
how much your pelvic floor has been
exercised.
MIXED INCONTINENCE:
Shown on the screen as: MIXED
This programme is perfect if you are
suffering from both Stress and Urge
incontinence. It is a combination of the
STRES and URGE programmes.
Prog
STRES
URGE
MIXED
TONE
Freq.
(Hz)
50
10
20
35
Pulse
width
(μs)
300
200
250
250
Ramp Up
& Down
(s)
1 1 2
2
Plateau
(s)
5 5 3
3
Rest (s)
10
10
20
6
Default
duration
(min)
20
20
20
20
Page 17

16
TONE:
Shown on the screen as: TONE
Once the pelvic floor muscles have
been strengthened with itouch sure,
continue to exercise them.
Regular use of this programme, about
twice a week, will ensure that your
muscles remain fit and toned.
The TONE programme may also be
used as an alternative treatment for
stress incontinence.
The sensation when using the TONE
programme is a mixture of a strong
drawing in of the muscles and then
releasing.
A strong and fit pelvic floor may
increase sexual health and enjoyment.
10. CONTENT
The pack contains:
• 1 x itouch sure continence
stimulator unit
• 1 x Lead wire (L-IT1-NEW)
• 1 x Liberty vaginal probe (X-VP)
• 2 x AA 1.5V alkaline batteries
• 1 x Storage pouch
• 1 x Instructions for use booklet
Page 18

17
11. UNIT INFORMATION
11.1. CONTROLS & DISPLAY
Programme
selected
Programme
selector
Time selector
OFF button
ON button
Lock button
Time remaining
(min)
Strength
selected (mA)
Strength up
Strength down
Low Battery
Output active
indicator
Page 19

18
11.2. OPERATING
INSTRUCTIONS
ON/OFF
To turn the unit on, press the ON
button and hold for 3 to 5 seconds
until the display shows.
At first use, or after changing batteries,
the display shows that the unit is
automatically set in programme STRES
at zero strength.
When switched on the unit will
automatically start in the programme
which was being used when it was last
switched off.
The strength returns to 50% of the level
that was being used when the unit was
switched off.
During start up to stop the increase in
strength at any time, press any key
once.
To turn the unit off, press the OFF
button and hold for 3 to 5 seconds
until the display stops.
Alternatively, press and release the
OFF button until the LCD screen
displays 0, any further press will switch
the unit off.
The unit will turn off automatically:
• When the timer reaches zero,
• If it is left at zero strength for more
than 5 minutes.
Note: Always check unit is
OFF before applying or
removing probe or pads.
The screen is illuminated whenever a
button is pressed.
STRENGTH CONTROLS
The buttons marked ▲ and ▼
are the strength controls.
To increase strength, press
and hold down the button ▲
until required strength is
achieved.
To decrease the strength, press and
release the button ▼.
To increase strength in steps of 0.5 mA,
press and release the button ▲.
The unit will remain in the WORK part
of the cycle for 5 seconds while
intensity is being adjusted.
The strength levels are shown on the
LCD.
The strength control buttons will not
operate until the unit is properly
connected to you (probe inserted
correctly). Itouch sure detects a
disconnection and automatically
returns the strength to zero.
The unit has 198 levels of strength (0 to
99.5 mA). If you hold down the button
▲ for 3 to 5 seconds, the strength will
start scrolling.
You may feel nothing over the first few
presses. Continue pressing until the
sensation is strong but comfortable.
Further increases during use may be
necessary if your body becomes used
to the sensation.
PROGRAMME CONTROL
The button marked P is the
programme control. The
itouch sure has four preset
programmes. At first use, the unit
automatically goes to programme
▲
▼
P
Page 20

19
STRES. Next time it is switched on, it
will default to the programme used last.
Each time you press and release the P
button, the programme changes and is
shown on the LCD.
Each time you change the programme,
the strength level reverts back to zero.
This is a safety feature to alleviate any
sudden feeling of a surge, as each
programme gives a different sensation.
TREATMENT TIMER
The TIMER button can be
used to set the session
duration. When you switch the unit on,
it is automatically set at 20 minutes for
all the programmes.
By repeatedly pressing and releasing
the TIMER button you can manually
override and select 10, 30, 45, 60, 90
minutes or continuous time.
The length of time that you have
selected will be shown on the screen.
The minutes will start to count down
once you start the programme.
If you hold down this key for 3 to 5
seconds, you can PAUSE the timer,
allowing you to interrupt the treatment
session, and resume it later. Return to
main screen by pressing any other key.
The LCD shows the session duration
next to the clock symbol. The unit
automatically counts down the minutes
set and switches off when it reaches 0.
LOW BATTERY
A low battery indicator BATT will show
when you need to change the batteries.
The unit will shut down about 2 minutes
after this.
LOCK BUTTON
The LOCK button on the top right
hand side of the unit can be used
to lock all controls except for the
▼ and OFF buttons.
To activate, press and hold down the
button for 3 to 5 seconds until LOCK is
displayed on the screen. Repeat to
deactivate.
LEADS ALARM
The itouch sure monitors the
connection and the contact between
you and the probe, or the pads. This is
to prevent sudden changes if a broken
connection is re-made. If either of these
goes outside of a standard range while
the strength is above 20.0 mA, the unit
will flash LEADS, bleep three times,
and return the strength to 00.0 mA.
Check the lead and if necessary,
lubricate the probe with a water based
lubricant such as TensCare Go Gel
(see K-GO). Please see section 19 for
more troubleshooting tips.
MEMORY
The itouch sure has a Memory with
three functions:
1) Programme Retention. When you
switch the unit on, it will automatically
start in the programme which was being
used when it was switched off.
2) Usage. Press the TIMER button and
▲ together and hold down for 3 to 5
seconds. The display will show the
duration of use in hours and the
average strength used.
Press the same buttons again to return
to normal controls.
Page 21

20
3) Memory Reset. To reset memory to
zero, hold down the TIMER and ▼
buttons together for 3 to 5 seconds.
12. SETTING UP AND
USING THE ITOUCH
SURE
12.1. INSTALLATION OF
BATTERIES
1) Remove the battery cover: Press
down in the centre and slide
backwards in direction of raised
arrows.
2) Insert batteries: Ensure that the
batteries are inserted the right way
as shown in battery compartment
and that the ribbon is behind them.
3) Replace the battery cover.
When the batteries are running low, a
low battery indicator BATT will show on
the screen and it is important to change
the batteries as soon as possible.
Rechargeable batteries
The unit will work with rechargeable
batteries, but the display may appear
dim.
Storage
Remove batteries from your itouch
sure if the unit is unlikely to be used for
a long period. Some types of batteries
may leak corrosive fluid.
Battery Life
Batteries should last at least 15 hours
at 50 mA, 300 μs, 50 Hz.
Unused batteries have a nominal shelf
life of 3 years, but will usually last longer
than this.
Battery Warnings
Do NOT pierce, open,
disassemble, or use in a humid
and/or corrosive environment.
Do NOT expose to
temperatures over 60°C(140F).
Do NOT put, store or
leave near sources of heat, in
direct strong sunlight, in a high
temperature location, in a pressurized
container or in a microwave oven.
Do NOT immerse in water or
sea water, or get wet.
Do NOT short-circuit.
Do NOT connect the device
unless the battery cover is in
place.
If battery leakage occurs and comes in
contact with the skin or eyes, wash
thoroughly with lots of water.
Average
strength
used
Time used
in hours
Page 22

21
Keep batteries out of the reach of small
children.
Caution NEVER attempt to
recharge an alkaline battery.
Risk of explosion.
Caution Do not mix old, new or
different types of batteries as
this may lead to battery leakage or low
battery indication.
Disposal: Always dispose of batteries
responsibly according to local
government guidelines. Do not throw
batteries onto a fire. Risk of explosion.
12.2. CONNECTING LEAD
WIRE
Insert the lead wire plug into the base of
the unit.
Connect the lead from the base of
the unit to the lead in the probe.
Push the pin ends firmly into the pigtail
ends of the probe lead.
The lead wires may be damaged by
rough handling, and should be treated
with care.
Lead wire colour coding.
The ends of the lead wire are coloured
black or red. This coding is provided for
some professional uses. For most
purposes, the orientation makes no
difference, and you can ignore this
colour coding.
12.3. PREPARING FOR
SESSION
1) Before using itouch sure you will
need to visit the toilet.
2) Lubricate the metal electrode
surfaces and probe tip with a waterbased lubricant, such as TensCare
Go Gel or water (see K-GO).
Caution: Do not use a silicone
based lubricant on the
stimulation contacts as it may
decrease the effectiveness of the
itouch sure’s muscle stimulation.
3) Choose a comfortable position, such
as lying down on your bed on your
side with your knees raised.
Warning: Ensure that the
itouch sure is switched OFF
before inserting the probe.
4) After the wire is securely connected,
insert the probe into your vagina, in
Page 23

22
the same way as you would a
tampon, until only the plastic rim at
the base of the probe is visible.
5) The metal parts conduct the
electrical pulse and should be in
contact with the main part of the
muscle at all times. The tissues close
to the entrance are more sensitive,
so you should avoid stimulating
them.
Ensure that the probe is aligned so
that the contact plates are to the
sides and the longer sides of the
positioning end rim are vertical.
The probe will naturally position itself
with the widest part of the flange
vertically.
Note: The flange should not be
inserted into the vagina and
should remain outside of the vagina at
all times.
12.4. TRAINING SESSION
1) Press and hold the ON button on the
control unit for 3 to 5 seconds to
switch the control unit on.
2) You can select from the four preset
programmes. Details in section 9 will
help you identify the best programme
to suit you.
To change between the
programmes, press the programme
selector, which is labelled P, in the
centre of the control unit keypad.
3) With the required programme
selected, you can adjust the intensity
of the muscle stimulation until you
reach a comfortable level. Once you
have reached a comfortable level, 5
seconds after you stop pressing the
button, the intermittent work/rest
phase will start. The machine will
take itself to 0 mA for a rest period
and then take itself back up to the
level of intensity you chose, to work
the muscle. This cycle will continue
for the 20 minutes of the programme.
Note: The strength required
varies widely between users some will use the itouch sure at full
power – 99.5 mA. The itouch sure
strength will go up at 0.5 mA
increments.
Initially the sensation through the probe
may be limited but will improve during
treatment. Take care not to use too
much strength and thereby over
stimulate the muscles until normal
sensation is restored. The sensation
may not be even as it may vary
depending on the sensitivity of the
nerves.
The LCD display shows the strength of
intensity used. The aim is to increase
this over a few days. But remember
there is no hurry, so only increase the
strength of the stimulation as and when
you are comfortable and ready to
progress.
Note: If the sensation becomes
uncomfortable, reduce the
intensity using the button ▼.
Metal contact Vertical
plates flange
Page 24

23
When switched on, in STRES and
TONE programmes the unit will go
through an exercise programme for 4-5
seconds, followed by a rest period of 810 seconds. The itouch sure causes a
sensation which feels like a strong
drawing in of the vagina and pulling up
of the pelvic floor. The natural reaction
will be to pull in and up with the
muscles.
At lower strength settings, you may not
feel any sensation at all, this depends
very much on the individual and any
pre-existing physical conditions, so
slowly increase the intensity by
repeatedly pressing the ▲ button until
you begin to feel the muscles around
your vagina contract.
For best results in these programmes
try to contract the pelvic floor muscles
along with the itouch sure, and to
sustain the contraction into the rest
interval. If possible, link the contraction
to your breathing to get into a gentle
rhythm.
Increase the strength as high as it is
comfortable, and then take it back down
one step.
The strength display on the control unit
will reduce to 00.0 mA and flash during
the rest period.
The URGE programme works
differently. There is no need to have a
contraction. The strength should be
comfortable, but always remain
noticeable. You may need to increase it
over the course of the treatment.
The length of each session is
automatically set to 20 minutes. The
length of each session for muscle
strengthening will also depend on your
ability to contract and your resistance to
fatigue. Be careful not to overuse early
on, as the resulting aches may not be
felt until the next day.
Note: If you experience
cramping, switch the unit off
until the symptoms go away
then continue the session with the
intensity set at a lower level.
Optional skin surface electrode
placement for URGE
An alternative method to a vaginal
probe is to stimulate areas of the skin
that are close to the nerves that go to
the bladder and urethra. These come
from the parts of the spinal cord
segment called S2-S3.
The electrodes are placed on the skin
between the anus and the genitals, or
at the very bottom of the spine near
your coccyx or “tail”. See electrode
placement pictures below.
The stimulation should be strong
enough to make your anus contract
slightly.
Page 25

24
12.5. AFTER YOUR TRAINING
SESSION
When the timer reaches zero, your
session is complete and the unit turns
off.
1) Check that the control unit is off. If it
is not, hold down the OFF button to
switch off then remove the probe
from your vagina by holding the
positioning end rim and gently pulling
outwards.
2) Wash and thoroughly dry the probe
and return it to the storage pouch.
3) The itouch sure will not only
improve your pelvic floor muscles but
also help you to recognise the
correct sensation you need to feel
when doing your Kegel exercises
(explained in section 4.2.).
Note: When removing the
probe, DO NOT PULL ON THE
LEAD WIRE.
13. ANAL PROBE
13.1. CONDITIONS THAT MAY
BE TREATED
An anal probe such as TensCare XPR13 can be purchased. This probe
can be used for urinary and faecal
incontinence in both males and
females.
This anal probe may be used to treat
Urinary and Faecal Incontinence in a
similar way to the vaginal probe.
Because the stimulation cannot be
restricted to one muscle group, and the
mucosal tissue has different electrical
characteristics, anal stimulation is less
comfortable than vaginal.
You should consult your healthcare
professional before starting treatment.
Faecal Incontinence
Faecal incontinence can be the result of
weakened or poorly functioning anal
sphincter muscles or damage to the
nerves controlling them. The purpose is
to re-educate the anal sphincter and
other muscles of the pelvic floor to
contract. The treatments aim to
progress towards graduated active
exercises, in order to improve pelvic
floor muscles’ strength and endurance
and to regain function.
You may benefit from the itouch sure
if you either have no active anal
sphincter contraction, or a weak or
poorly sustained contraction. Use the
STRES or TONE programmes.
Intensity should be as strong as
possible without being painful. When
possible, try to contract the muscles at
the same time as the itouch sure.
Post Prostatectomy Urinary
Incontinence
Electrical stimulation has been found to
help urinary incontinence in men after
radical prostatectomy in some trials.
Use the same programmes as for
vaginal stimulation. Increase intensity
in STRES, MIXED, or TONE
programmes to the highest tolerable.
Page 26

25
13.2. HOW TO INSERT THE
ANAL PROBE
1) Before using itouch sure you will
need to visit the toilet.
2) Lubricate the metal electrode
surfaces and probe tip with a waterbased lubricant, such as TensCare
Go Gel or water (see K-GO).
Caution: Do not use a silicone
based lubricant on the
stimulation contacts as it may
decrease the effectiveness of the
itouch sure’s muscle stimulation.
3) Choose a comfortable position, such
as lying down on your bed on your
side with your knees raised.
Warning: Ensure the itouch
sure is switched OFF before
inserting the probe.
4) After wires are securely connected,
insert the probe into the anus whilst
‘bearing down’ (as in the action of
passing stool) to a comfortable limit
until the base of the flange on the
probe touches the anus. The metal
parts conduct the electrical pulse
and should be in contact with the
main part of the muscle at all times.
The tissues close to the entrance are
more sensitive, so you should avoid
stimulating them. It is recommended
that the probe is inserted past the
sphincter muscles of the anus,
unless directed otherwise by a
healthcare professional.
5) Anal probes with long electrodes (the
metal part) that run up and down the
length of the attachment should
always be inserted with the metal
parts facing hip-to-hip. Anal probes
with circular electrodes (the metal
part) should be inserted simply to the
desired depth.
Note: Sometimes the wearing
of tight fitting undergarments or
a tight pair of jeans will help to
keep the probe in place and maintain
correct contact during the programme.
For Faecal incontinence, the aim is to
stimulate the external sphincter and/or
pubo-rectal muscle, so circular
electrodes should be placed so that the
external ring is just inside the sphincter.
For Urinary Stress incontinence, the
aim is to stimulate the levator muscles
and the probe should be inserted
deeper.
14. CLEANING
It is important that the probe is cleaned
after each use. Clean with either an
alcohol-free antibacterial wipe such as
TensCare Wipes (see X-WIPES) or by
wiping with warm soapy water. Rinse
and dry thoroughly and return the unit
to the storage pouch. Do not immerse
the probe in a liquid.
Clean the case of the unit and lead wire
at least once a week using the same
method.
• Do not immerse your itouch sure
unit in water.
• Do not use any other cleaning
solution.
Page 27

26
15. EMC
Wireless communications equipment
such as wireless home network
devices, mobile phones, cordless
telephones and their base stations,
walkie-talkies can affect this equipment
and should be kept at least a distance d
= 3,3 m away from the equipment.
(Note. As indicated in Table 6 of IEC
60601-1-2:2007 for ME EQUIPMENT,
a typical cell phone with a maximum
output power of 2 W yields d = 3,3 m at
an IMMUNITY LEVEL of 3 V/m).
Note: For hospital use, full EMC
advice tables are available on
request.
16. DISPOSAL OF WASTE
ELECTRICAL AND
ELECTRONIC
PRODUCTS (WEEE)
One of the provisions of the European
Directive 2002/96/CE is that anything
electrical or electronic should not be
treated as domestic waste and simply
thrown away. To remind you of this
Directive all affected products are now
being marked with a crossed-out
wheelie bin symbol, as depicted below.
To comply with the Directive, you can
return your old electro-therapy unit to us
for disposal. Simply print a postagepaid PACKETPOST RETURNS label
from our website www.tenscare.co.uk,
attach this to an envelope or padded
bag with the unit enclosed, and post it
back to us. Upon receipt, we will
process your old device for components
recovery and recycling to help conserve
the world’s resources and minimise
adverse effects on the environment.
17. ACCESSORIES
Expected Service Life
• The machine will often last for more
than 5 years, but is warrantied for 2
years. Accessories (lead wire, probe,
and batteries) are not covered by the
warranty.
• Lead life depends greatly on use.
Always handle the leads with care.
We recommend to replace the lead
wires regularly (about every 6
months).
• Replace the probe every 6 months to
ensure hygiene.
• Optional electrode pads should last
12-20 applications, depending on
skin condition and humidity.
• AA alkaline batteries should last at
least 15 hours at 50 mA, 300 μs, 50
Hz.
Replacement electrode pads, new
batteries and lead wires are available
from your supplier or distributor (see
back cover for contact details), by mail
order from TensCare, by telephone
using a credit or debit card, or through
the TensCare website.
The following replacement parts may
be ordered from TensCare at
www.tenscare.co.uk or +44(0) 1372
723434.
Page 28

27
X-VP Liberty Vaginal Probe (28
mm dia.)
X-VPM Liberty Plus Vaginal
Probe (32 mm dia.)
X-VPG Gold Vaginal Probe (26
mm dia.)
L-IT1-NEW Replacement lead wire,
1.25 m length
X-PR13 Anal probe (19.6 mm dia.)
E-CM5050 Pack of 4 electrode pads
(50x50 mm)
K-GO Go Gel Personal Water-
based Lubricant
B-AAA-8 8 AA batteries 1.5V
X-WIPES Pack of 30 wipes
Note: You should only use the
probe supplied with the unit or
the replacements above as
performance may vary with other
electrodes.
Warning: Do NOT use silicone
based or hybrid (mixed water
and silicone) lubricants.
CHANGE OF LEAD
Please note that from serial number
E14/009931 (since 2014) the itouch
sure has an all-plastic connecting plug
on the lead to comply with current
safety regulations. Please ensure that
you purchase the replacement lead LIT1-NEW, see picture below.
The connection to plug
into the unit is made of
plastic (not metal).
18. WARRANTY
This warranty refers to the unit only. It
does not cover, electrode pads, battery,
or the lead wires.
PRODUCT WARRANTY
INFORMATION
This product is warranted to be free
from manufacturing defects for 2 years
from date of purchase.
This warranty is void if the product is
modified or altered, is subject to misuse
or abuse; damaged in transit; lack of
responsible care; is dropped; if
incorrect battery has been fitted; if the
unit has been immersed in water; if
damage occurs by reason of failure to
follow the written instruction booklet
enclosed; or if product repairs are
carried out without authority from
TensCare Ltd.
We will repair, or at our option replace
free of charge, any parts necessary to
correct material or workmanship, or
replace the entire unit and return to you
during the period of the warranty.
Otherwise, we will quote for any repair
which will be carried out on acceptance
of our quotation. The benefits conferred
by this warranty are in addition to all
other rights and remedies in respect of
the product, which the consumer has
under the Consumer Protection Act
1987.
Our goods come with guarantees that
cannot be excluded under the UK
consumer Law. You are entitled to have
Page 29

28
the goods repaired or replaced if the
goods fail to be of acceptable quality.
Before you send your unit for service
Before sending in your unit for service,
please take a few minutes to do the
following:
Read your manual and make sure you
follow all the instructions.
Returning your unit for service
Should repair be needed within the
warranty period, enclose the tear off
section of the warranty card (see page
32) and your proof of purchase receipt.
Please ensure all relevant details are
completed before sending your unit in
for service. Please ensure your contact
details are still current and include a
brief description of the problem you are
experiencing together with your
purchase receipt.
For hygiene reasons, please do not
include used probe or electrode pads.
Send only the unit and the lead wire.
Please return the unit and warranty
card (see page 32):
TensCare Ltd
PainAway House,
9 Blenheim Road,
Longmead Business Park,
Epsom, Surrey
KT19 9BE, UK
Should you require any further
information please do not hesitate to
contact us by calling our number:
+44 (0) 1372 723 434.
Page 30

29
19. TROUBLESHOOTING
If your itouch sure is not working properly, please check the following:
Problem
Possible causes
Solution
No display
Flat batteries.
Replace batteries.
Batteries inserted
incorrectly.
Remove plastic wrap
Check + / - .
Damaged springs in
battery compartment.
Contact supplier.
Low battery
display
Low batteries.
Replace batteries.
Controls won’t
work
Keypad is locked.
If LOCK is shown on display, press and hold the
LOCK button for 3 to 5 seconds to unlock.
If no LOCK is showing, remove and replace the
batteries.
No sensation
and LEADS
alarm showing
The itouch sure has a safety feature which will not allow the intensity to pass
20.0 mA if the machine detects a connection error. If a connection error is
detected the intensity will return to 00.0 mA and the screen will flash LEADS.
This safety feature will prevent the machine from giving any uncomfortable
stimulation should the contact break between the machine and your skin. This
will also prevent anyone from increasing the intensity to a high level without
firm contact between the machine and the skin.
A connection error can occur if:
1. A break has developed
within one of the two lead
wires.
If this happens, you can try to test the unit by
holding the probe in your hand:
i) Dampen your hand with water and a little table
salt. Squeeze the probe firmly and make sure
your skin is covering the metal parts of the probe
and carefully increase strength until you can feel
something. Most people will start to feel the
stimulation in their hand at around 25.0 mA.
ii) If the LEADS alarm shows and the unit will not
allow you to pass 20.0 mA. The lead wires need
to be replaced.
If you have tried the test
above and DO have
sensation when the probe
is in your hand, then it may
be that:
2. The skin is dry,
meaning poor conductivity
between the metal plates
If this happens, you can try the below solutions:
i) Using a water–based lubricant such as
TensCare Go Gel (see K-GO), which will
improve conduction.
ii) Crossing your legs and squeezing to increase
pressure on the probe, which should improve
the connection. If this enables you to use the
unit, you should find that in a few weeks of
Page 31

30
on the probe and your
skin.
stimulation the contact improves. If it does not,
this unit may not be suitable for you. You may
need to contact your healthcare professional to
discuss other suitable options.
iii) The probe supplied with the unit has a 28 mm
diameter. An optional 32 mm probe, part no. X-
VPM, is available.
No sensation
and no LEADS
alarm showing
Intensity level is not high
enough and/or reduced
sensitivity in the area
being treated.
i) Please make sure you are increasing the
intensity high enough. Most people will start to
feel the stimulation in their hand at around 25.0
mA and with the probe inserted you will need to
increase the intensity higher to around 40.0 mA
to 60.0 mA. Max power is 99.5 mA. Everyone is
different so just keep increasing the intensity
until you can feel it. The intensity increases in
very small steps of 0.5 mA.
ii) You may have reduced sensitivity due to
previously damaged or desensitised pudendal
nerves (this can happen in childbirth or some
surgical procedures). Please consult your
healthcare professional.
No sensation
on one side of
the probe (or
electrode)
Position is not optimal –
needs adjusting.
The current flows from one side of the probe to the
other, so it is not possible to have one side “not
working”. However, the strength of the sensation
depends on how close to the nerve the current
flows, and also in which direction it flows relative
to the nerve. You can try slightly adjusting the
position on the probe, or exchanging the
connection of the wires in the probe.
Sudden
change in
sensation
If you disconnect and reconnect a few minutes
later, the signal will feel
quite a lot stronger.
Always return strength to zero after disconnecting
the lead or the probe.
The patient is an intended operator. There are no user-serviceable parts inside the
unit, and no calibration is required.
If the above review has failed to resolve your problem, or to report unexpected
operation or events, call TensCare or your local dealer (address on back cover) for
advice.
Contact TensCare customer service on +44 (0) 1372 723 434. Our staff are trained to
assist you with most issues you may have experienced, without the need to send your
product in for service.
Page 32

31
20. GENERAL SPECIFICATION
Waveform
Asymmetrical rectangular
Amplitude (over 500 Ohm load)
99.5 mA +/- 10%
Max intensity
50V zero to peak
Constant voltage over 470-1500 Ohm
Constant current over 160-470 Ohm
Output plug
Fully shielded
Channels
Single channel
Max Pulse energy
Total output limited to 25 μC per pulse
Batteries
2 x AA alkaline (two AA batteries) or 2 x AA NiMH
Weight
90 gms without batteries
Dimensions
102 x 53 x 30 mm
Safety Classification
Internal power source.
Environmental Specifications:
Operating:
Storage:
Temperature range: 0 to +40⁰C
Humidity: 10 to 93% RH non-condensing
Atmospheric pressure: 700hPa to 1060hPa
Temperature range: -10 to +60⁰C
Humidity: 8 to 93% RH non-condensing
Atmospheric pressure: 700hPa to 1060hPa
TYPE BF
EQUIPMENT
Equipment providing a degree of protection against
electric shock, with isolated applied part.
Designed for continuous use.
This symbol on the unit means “Refer to Instructions
for Use”
IP22
The unit is not water resistant, and should be
protected from liquids.
Complies with EU WEEE regulations
Applied Part
Vaginal and anal electrodes. Optional skin surface
electrode pads. See section 17.
Contact duration: At least 10 minutes.
Note: The electrical specifications are nominal and subject to variation from
the listed values due to normal production tolerances of at least 5%.
Page 33

32
PLEASE RETAIN THIS WARRANTY CARD.
RETURN THIS PORTION ONLY WHEN YOU RETURN YOUR PRODUCT FOR
REPAIR UNDER WARRANTY.
NAME:
ADDRESS:
POSTCODE:
DAYTIME TELEPHONE:
E-MAIL:
MODEL:
DATE OF PURCHASE:
ATTACH PROOF OF PURCHASE
DO NOT SEND IN PROBE OR ELECTRODE PADS
RETAILERS NAME:
RETAILERS ADDRESS:
RETAILERS POSTCODE:
BRIEF DESCRIPTION OF PROBLEM YOU ARE EXPERIENCING:
WARRANTY IS VOID UNLESS THE ABOVE INFORMATION IS COMPLETED AND
CORRECT.
Page 34

33
NOTES
Page 35

34
TensCare aim to give you the best possible product and service. We listen to your
suggestions and are constantly trying to improve our products. We also want to learn
about the way our products are used, and the benefits they give. If you have anything
you would like to share with us, please contact:
www.tenscare.co.uk
Follow us:
https://www.facebook.com/pages/TensCare-Ltd
https://twitter.com/TensCareLtd
https://www.linkedin.com/company/tenscare-limited
https://plus.google.com/+TenscareLtdEpsom
https://uk.pinterest.com/TensCareLtd/
https://tenscareltd.wordpress.com/
https://www.youtube.com/channel/UCzpik9dmLlJ3j0aHOpQ-0sg
EC Declaration of Conformity
TensCare Ltd hereby declare that an examination of the production quality assurance
system has been carried out following the requirements of the UK national legislation
according to Annex V of the Directive 93/42/EEC on medical devices. We certify that
the production quality system conforms with the relevant provisions of the
aforementioned legislation, and the result entitles the organization to use the CE 0088
marking on this product.
Page 36

35
Distributed by:
Manufactured by:
TensCare Ltd, 9 Blenheim Road,
Epsom, Surrey KT19 9BE, UK
Tel: +44(0) 1372 723434
www.tenscare.co.uk
Pub No.: I-ITS-UK Version 4.1 05/17
 Loading...
Loading...