
Safe‐T‐Vue® 10 FAQs
Q: At what temperature should the
blood or blood products be kept prior
to attaching Safe-T-Vue®?
Blood temperature should be as cold
as possible— temperatures between
1.0° C and 4.0°C are recommended. A
low start temperature is critical if the
blood and blood products are to be
delivered within the compliance
temperature. Placing a cold pack
under the bag helps to maintain the
temperature of the blood during the
dispensing process. Keep multiple cold
packs in the blood refrigerator to
assure the cold pack temperature is
the same as the blood bag.
Q: Does the temperature of the room
have any effect on Safe-T-Vue®?
Yes. Ambient temperature can have an
effect on heat transfer. Cooler ambient
temperatures decelerate the warming
rate of blood bags once they are
removed from the refrigerator, while
warmer ambient temperatures
accelerate warming.
Q: Moisture sometimes condenses on
cold blood bags after they are
removed from the blood refrigerator.
Do I need to remove it before
applying a Safe-T- Vue® to the bag?
Remove excess moisture by using a dry
wipe on the surface just before Safe‐T‐
Vue® is applied.
Q: Do busy blood refrigerators affect
blood temperature?
Yes. The air temperature in a blood
refrigerator can increase 3‐4°C when
the door is open for 30 seconds, which
can affect the temperature of the
refrigerated blood.
Q: What are the protocols for packing
blood products that are being sent to
the OR and/or transported to another
location?
When packing with wet ice or reusable
cold packs, follow the packing
instructions provided with your
validated shipping container.
Q: How can I be certain our blood
bags maintain uniform temperature
in our refrigerator?
Uniform temperature of all blood bags
in any given refrigerator depends on
the recovery rate and air circulation of
the refrigerator. You can improve
temperature consistency by storing
blood bags in a vertical position with
air space around them. Bags lying on
top of each other can impede airflow,
and may need longer to reach
refrigerator temperature because they
have less surface area exposed to the
cold air.
Video Instructions
116 The American Road
Morris Plains, NJ 07950
973‐630‐6000
www.williamlabs.com
Instructions & FAQs
Safe-T-Vue® 10
BEGIN HERE
Storage:
• Safe‐T‐Vue® 10 must be refrigerated at the same
temperature as the blood for a minimum of 24
hours prior to use. It is suggested that boxes of
Safe‐T‐Vue® indicators be kept in the blood bank
refrigerator.
• Safe‐T‐Vue® inventory may be stored or shipped at
room temperature.
• Safe‐T‐Vue ® 10 may be kept in the blood bank
refrigerator for up to 2 months. After 2 months,
remove Safe‐T‐Vue®10 from the refrigerator for 24‐
48 hours, and then return to the blood bank
refrigerator. This cycling may be repeated every
2 months.
• Write the date placed in the refrigerator and the
date to remove from the refrigerator in the space
provided on the box.
COMM Instructions for Use
and FAQs STV10 Rev. 7
2017‐10‐01
FDA 510(k) BK910005
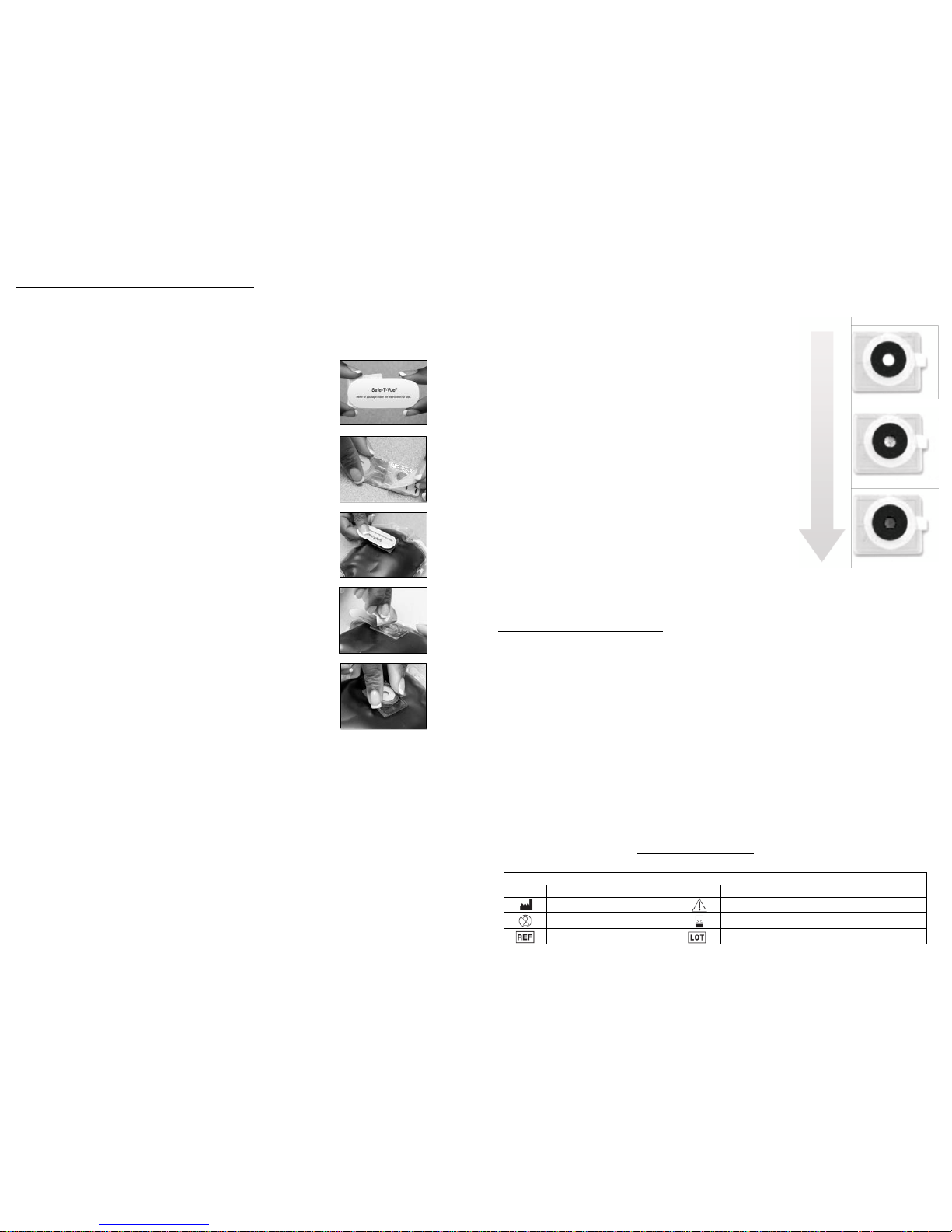
Safe‐T‐Vue® 10 Ins
tructions
Preparation
•Refrigerate Safe‐T‐Vue® for a minimum of 24 hours before activating.
•Use a refrigerated cold pack beneath blood products to keep them
cold during the dispensing process (recommended).
Handling
•Hold the Safe‐T‐Vue® indicator by the outer
edges.
•Do not hold or touch the temperature sensitive
indicator areas, as your hands can pre‐warm
the indicator and affect its accuracy.
Application
1. Apply the Safe‐T‐Vue® indicator to the blood bag
•When the blood is requisitioned and dispensed
from the blood bank, OR
•When the blood is received into your blood
bank inventory
Color Change Temperature Indication
•The white dot will begin to gradually fill in
with red dots at approximately 9°C.
•The appearance of small red dots (typically
starting around 9°C) is an indication that the
blood should be cooled or re‐refrigerated
immediately.
•The temperature‐sensitive dot on Safe‐T‐Vue®
10 will turn from white to completely red
when the blood product temperature reaches
10°C.
•Extensive testing has shown that the color
change from white to completely red should
be clear and unambiguous if Safe‐T‐Vue has
been handled properly.
Validation
< 9° C
≈ 9.5° C
≥ 10° C
2. Remove the adhesive backing and apply
Safe‐T‐Vue® to the lower area of the blood bag,
where there is the largest blood volume.
3. Be careful not to touch the center indicator areas.
Activation
1. Pull back the foil to expose the indicator’s active
areas. Again, be careful not to touch the center
indicator areas.
2. Activate the indicator by folding the rounds
together and snap shut.
3. The blood is ready for transport to destination,
or can be placed back into the refrigerator until
needed.
A detailed Validation Protocol, is available for download in MSWord format at
www.williamlabs.com/support
. Many factors such as ambient temperature,
bag size, indicator handling, and air flow can influence the results of your
validation testing. To account for these variables, the validation protocol can
be edited and modified to meet the requirements of your facility. Once
validated, you may continue to use the indicators according to your validated
procedure. Contact customer service at 973‐630‐6050 for support.
Recommended equipment and processes:
•An electronic thermometer and thermister probes (±0.1°C stated accuracy)
placed inside simulated blood bags are recommended for valida
tion
of Safe‐T‐
Vue® 10. Avoid surface measurement such as infrared thermometers (±1.5°C
typical stated accuracy) or glass thermometers with contact only on the
surface of the bag.
•Simulated blood products may be prepared using the appropriate volume of
water with glycerol‐ see www.williamlabs.co
m for simulated blood product
recipes.
Symbols Glossary: Refer to ISO 15223-1 Standard
Symbol
Meaning
Symbol
Meaning
Manufacturer
Caution: Consult the Instructions
Do not re‐use
Use By Date
Catalogue or Part Number
Batch Code
 Loading...
Loading...