Tecnomed Italia MORPHEUS Installation, Use And Maintenance Manual

V.8.00
MORPHEUS SURgical cHaiR
Installation, use and maintenance manual
CODE DE1000X

CONTENTS
ENGLISH
SECTION
1- SYMBOLS
1.1- WARNING LABELS
2- GENERAL WARNINGS
2.1 - GENERAL INSPECTION
2.2 - IN-TRANSIT DAMAGES FOR DELIVERIES IN ITALY
2.3 - IN-TRANSIT DAMAGES FOR DELIVERIES OUTSIDE ITALY
2.4 - TRANSPORT AND STORAGE CONDITIONS
3- SAFETY RULES FOR INSTALLATION
3.1 - WORK ENVIRONMENT
3.2 - MAXIMUM LOADS
4- SAFETY PROVISIONS
4.1 SAFETY REQUIREMENTS
5- PRODUCT DESCRIPTION
5.1 - INTENDED USE AND METHOD OF USE
5.2 - STANDARDS/CERTIFICATIONS
5.3 - TECHNICAL SPECIFICATIONS
5.3.1 - DIMENSIONS
5.4 - IDENTIFICATION PLATES
5.5 - PRODUCT PROFILE
5.6 - CONFIGURATION
6- INSTALLATION
6.1-PRE-ASSEMBLY
6.1.1 - ASSEMBLY SETUP, STANDARD CONFIGURATION
6.1.2 - ASSEMBLY SETUP, FULL CONFIGURATION AT THE CENTRE OF THE ROOM
6.2- INSTALLATION
7- INSTRUCTIONS FOR USE
7.1 - STANDARD HAND CONTROLLER
7.1.1 - HAND CONTROLLER WITH MEMORIES
7.2 - CHAIR MOVEMENT CONTROL WITH JOYSTICK
7.3 - SEAT ROTATION
7.4 - STANDARD HEADREST
7.5 - HOLLOW HEADREST
7.6 - LEG REST
7.7 - STANDARD ARMRESTS
7.8 - RAILS
7.9 - BLOOD SAMPLING ARMRESTS
7.10 - THIGH RESTS
PAGE
4
5
5
6
6
7
7
8
9
9
10
11
12
12
12
13
14
15
16
17
18
18
18
21
23
27
28
28
29
30
30
30
31
31
31
32
33
2

SECTION
7.11 - INFUSION STAND
7.12 - BASE WITH FEET
7.13 - BASE WITH WHEELS
7.14 - UNINTERRUPTIBLE POWR SOURCE
8 - CLEANING AND STERILISING
9 - MAINTENANCE
10- TROUBLESHOOTING
10.1- FIRMWARE
11- RELATED ITEMS
12- DISPOSAL
13- WARRANTY
14.1- DECLARATION OF CONFORMITY
15- CERTIFICATE OF WARRANTY
16- ELECTRICAL TEST
PAGE
ENGLISH
34
34
34
35
36
37
38
39
43
43
44
45
46
47
3
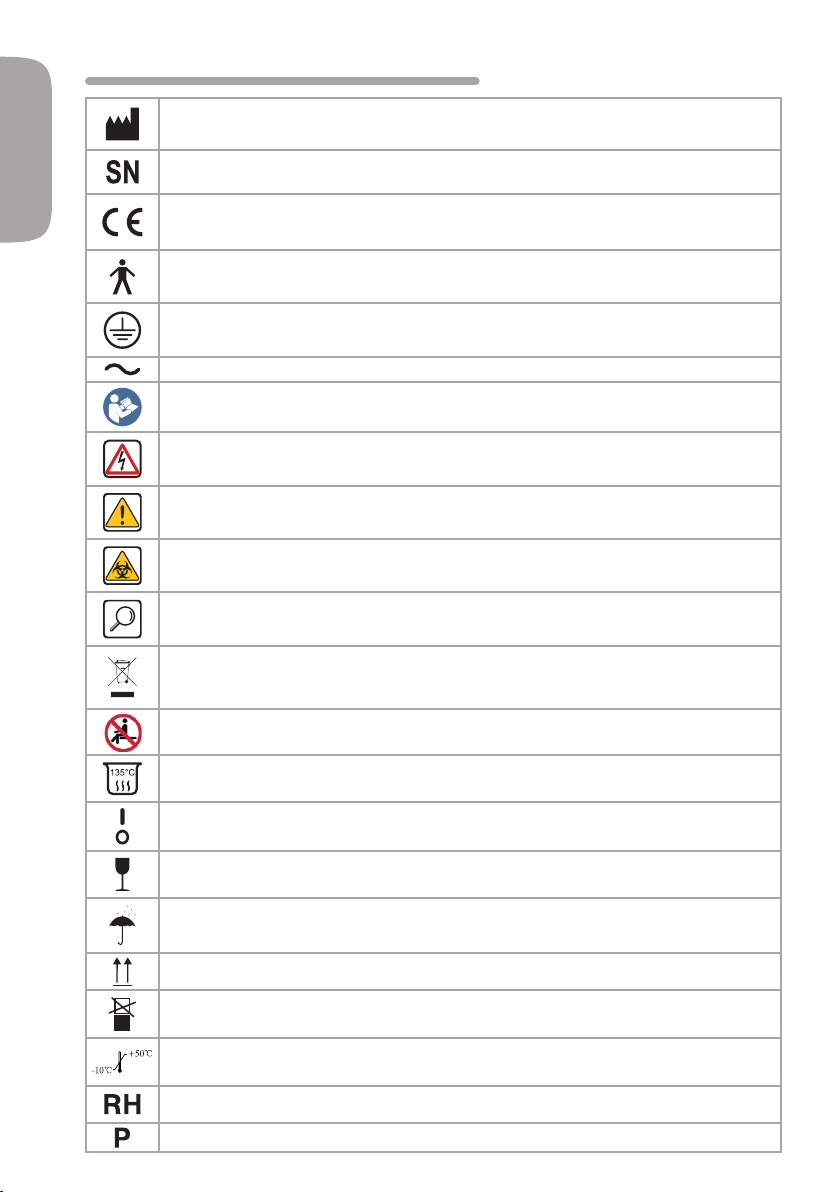
1- SYMBOLS
ENGLISH
MANUFACTURER SYMBOL. This symbol is placed on the product next to the name and
address of the manufacturer.
SERIAL NUMBER SYMBOL. This symbol is placed on the product next to the serial
number of the device.
CE MARKING. This symbol indicates that the product has a CE marking
in conformity with the provisions of Directive CEE 93/42 and subsequent
amendments (Class I Devices).
Protection type against direct and indirect contacts: Class I. Protection against
electric shock: Type B.
EARTHING SYMBOL. This symbol indicates the presence of an earth terminal for
protection against electric shock.
ALTERNATING CURRENT SYMBOL.
INSTRUCTIONS FOR OPERATION. This symbol indicates that you need to consult the
user manual before using the device.
HAZARD SYMBOL. It indicates a dangerous situation that might result in
moderate or severe/fatal injuries unless avoided.
WARNING SYMBOL. It indicates a dangerous situation that might result in
moderate or mild injuries or damage to property unless avoided.
BILOGICAL HAZARD SYMBOL. This symbol indicates the possible presence of risks of
contamination due to contact with infected biological uids or materials.
GENERAL INFORMATION SYMBOL. This symbol indicates a piece of information
that allows you to use the device more efciently.
DISPOSAL SYMBOL. This symbol indicates that the product should not be
disposed of like normal waste, but it should be recycled as per Directive
2002/95/ EC, 2002/96/ EC and 2003/108/ EC.
DO NOT SIT SYMBOL. This symbol indicates that it is forbidden to sit on the armrests.
STERILISABLE. This symbol indicates the possibility of sterilising the product in autoclave.
DEVICE ON/OFF SYMBOL. This symbol shown on the main switch indicates if the
device is on (I) or off (O).
FRAGILE SYMBOL. This symbol indicates that the product inside the packaging is
fragile. Avoid shocks.
PROTECT FROM HUMIDITY SYMBOL. This symbol indicates that the product must
be kept away from water and humidity.
UP SYMBOL. Transport and store the product only in the direction indicated by the
arrows.
DO NOT STACK SYMBOL.
TEMPERATURE LIMIT SYMBOL. From -10° to 50° C.
HUMIDITY LIMIT SYMBOL. From 10 to 90%.
4
ATMOSPHERIC PRESSURE LIMIT SYMBOL. From 500 to 1060 hPa.
1.1- WARNING LABELS
It indicates a dangerous situation that might result in moderate or severe/fatal injuries
unless avoided.
It indicates a dangerous situation that might result in moderate or mild injuries or
damage to property unless avoided.
This symbol indicates the possible presence of risks of contamination due to contact
with infected biological uids or materials.
This icon provides information that allows you to use the device more efciently.
2- GENERAL WARNINGS
• Read this manual carefully before carrying out any operation on the product, follow the
warnings contained in it and keep it for future reference.
• This manual is intended to provide the user with instructions for correct installation and
use of the product.
• The product must be used in accordance with the procedures contained in the manual
and never for purposes other than those provided for therein.
• The user is responsible for the installation, operation and maintenance of the device.
• The manual describes all product versions and optionals, therefore not all instructions are
applicable to your product.
• The product may be equipped with additional components, which are described in this
manual.
• The information, technical specications, drawings contained in this publication are not
binding.
• Tecnomed Italia s.r.l. pursues a policy of continuous product improvement and reserves
the right to make changes to the product or to this manual without prior notice, as long
as said changes do not affect the safe use of the device.
• Keep the manual within reach.
• It is also strictly forbidden any kind of reproduction or appropriation of the text and/or
images in the manual; therefore, some of the instructions, specications and images
contained in this manual may differ slightly from the product purchased by you.
• All the materials contained in this manual are the property of Tecnomed Italia and/
or of the companies represented by it. The images are not binding and are given for
explanatory purposes only.
• This manual must be delivered together with the machine in case of resale.
• The original text of this manual is in Italian.
ENGLISH
DANGER! It is strictly forbidden to make changes to the device.
Tecnomed Italia s.r.l. assumes NO responsibility for personal injury and/or direct or indirect
property damage resulting from non-compliance with this requirement.
DANGER! Tecnomed Italia s.r.l. declines any liability, expressed or implied, and cannot
be held liable for personal injury and/or direct or indirect property damage deriving from
failure to comply with the instructions in this manual and/or from incorrect installation and/
or use of the device and its accessories and/or from improper and/or lack of cleaning and/
or maintenance.
5

2.1 - GENERAL INSPECTION
ENGLISH
ATTENTION! Check the package upon delivery and make sure it is intact and does
not present signs of impact. Otherwise, follow the indications in paragraph 2.2 or 2.3.
2.2 - IN-TRANSIT DAMAGES FOR DELIVERIES IN ITALY
Upon delivery, if the package/parcel shows visible signs of damage, the recipient must
or may sign the delivery note conditionally, reserving the right to claim for any damages.
The Italian law stipulates that “the receipt of goods by paying the amount due without
reserving the right to claim for any damages, renders the rights of the recipient deriving
from the contract void, except in cases of willful misconduct or negligence of the carrier”
(art. 1698 CC). To reserve the right to claim for any damages, ll in the dedicated elds on
the delivery note handed to you by the carrier before signing it. Each carrier has his own
procedure for conditional delivery, therefore you need to ask him how to proceed.
If the package presents clear signs of damage upon delivery, proceed as follows:
1. Check the appearance and the condition of the packages. Make sure they are intact
and complete: if the document mentions several packages, make sure you received
them all. The recipient and the carrier must sign the delivery note subject to verication
after clearly writing down the reason:
- “delivery refused/accepted subject to verication”
- “package with packing tape pulled off, delivery refused/accepted subject to
verication”
- “package with visible signs of damage, cardboard bent, delivery refused/accepted
subject to verication”
- “package extremely damaged and/or partially open, delivery refused/accepted
subject to verication”
2. Leave the product and the packaging as they are. Take a photo and/or a video of the
damaged package/parcel.
3. Do not use the product.
4. Report the damages to the transport company.
5. Report the damages to Tecnomed Italy srl (produzione@dentalastec.it).
6. Do not return the product to Tecnomed Italy srl before receiving an answer and an
authorisation to do so.
7. Send the signed delivery note to Tecnomed Italia srl.
8. Leave the product and the packaging as they are.
9. Do not use the product.
Note: if you suspect that the product might present hidden damages, not visible
from the outside, sign the delivery note conditionally.
If the product is damaged but the packaging presents no visible signs of damage,
proceed as follows:
1. Inform the transport company no later than 7 days after delivery.
2. Report the damages to Tecnomed Italy srl (produzione@dentalastec.it).
3. Leave the product and the packaging as they are.
4. Do not use the faulty product.
ATTENTION! If the recipient fails to comply with any of the aforementioned provisions,
the damage shall be considered as if arising after delivery.
6
2.3 - IN-TRANSIT DAMAGES FOR DELIVERIES OUTSIDE ITALY
ATTENTION! Tecnomed Italy srl shall not be liable for damages occurred during transit.
Check the goods as soon as you receive them!
If the package presents clear signs of damage upon delivery, proceed as follows:
1. The recipient must note down the missing parts or the damage on the delivery note.
The recipient and the carrier must sign the delivery note. The recipient may claim the
replacement of the product due to in-transit damages only based on such evidence.
2. Leave the product and the packaging as they are.
3. Do not use the product.
If the product is damaged but the packaging presents no visible signs of damage, proceed
as follows:
1. Inform the transport company no later than 30 days after delivery.
2. Leave the product and the packaging as they are.
3. Do not use the faulty product.
ATTENTION! If the recipient fails to comply with any of the aforementioned provisions,
the damage shall be considered as if arising after delivery.
2.4 - TRANSPORT AND STORAGE CONDITIONS
The product inside the packaging is fragile. Avoid shocks.
Keep the package away from water and humidity.
Transport and store the product only in the direction indicated by the arrows.
ENGLISH
Do not stack.
Temperature limit: from -10° to 50° C.
Humidity limit: from 10 to 90%.
Atmospheric pressure limit: from 500 to 1060 hPa.
7

3- SAFETY RULES FOR INSTALLATION
ENGLISH
DANGER! Tecnomed Italia s.r.l. declines any liability, expressed or implied, and cannot be
held liable for personal injury and/or direct or indirect property damage deriving from failure to
comply with the instructions in this manual and/or from incorrect installation and/or use of the
device and its accessories and/or from improper and/or lack of cleaning and/or maintenance.
ATTENTION! For safety reasons, Tecnomed Italia s.r.l., manufacturer of the Morpheus
surgical chair, recommends that the installation, maintenance and repair operations are
carried out only by authorised technical staff of Tecnomed Italia s.r.l.
DANGER! In case of faults, the components are to be replaced using only original spare parts.
DANGER! Any technician not authorised by Tecnomed Italia s.r.l., who makes changes to
the product replacing parts or components with spare parts different from those recommended
by the manufacturer, assumes a responsibility similar to that of the manufacturer.
DANGER! It is strictly forbidden to make changes to the device. Tecnomed Italia s.r.l.
assumes NO responsibility for personal injury and/or direct or indirect property damage
resulting from non-compliance with this requirement.
ATTENTION! The Morpheus surgical chair can be either xed or mobile.
DANGER! Comply with the conditions of use provided in the “Technical Specications”
chapter and do not exceed the recommended values.
ATTENTION! Before connecting the power cord, make sure the electrical contacts are
perfectly dry. If necessary, use compressed air to dry them.
DANGER! The electrical system available at the installation site must comply with
standards CEI 64-8/7 and subsequent amendments. The device shall be installed only by
technical staff authorised by Tecnomed Italia s.r.l.
DANGER! To avoid the risk of electric shock, this device must be connected to an
earthed electrical system.
ATTENTION! Make sure the mains voltage matches the power supply voltage specied
on the label .
DANGER! Do not install the device in environments subject to anaesthetic or ammable gases.
ATTENTION! Do not expose the device to direct sunlight or to sources of UV light.
DANGER! Install the device in a place protected against collisions or against accidental
spills of water or liquids.
DANGER! Do not install the device next to heat sources. Install it so as to ensure proper
ventilation inside it.
DANGER! Do not use the product with accessories not approved by Tecnomed Italia
s.r.l. and without CE marking or tted with standardised interfaces. The patient and the dentist
might get injured or the device might get damaged. Install only accessories authorised by
Tecnomed Italia s.r.l. The company assumes NO responsibility for personal injury and/or direct
8

or indirect property damage resulting from non-compliance with this requirement.persone
e/o danni a cose diretti o indiretti derivati dell’inosservanza di tale prescrizione.
ATTENTION!
wear-related damages, the product requires routine and extraordinary maintenance interventions
(specied in this manual) that must be carried out at specied intervals by your Tecnomed Italia
s.r.l. dealer. For more information, see the paragraph “Maintenance” on page 37.
DANGER! Do not disassemble the device. In case of malfunction, please contact an
authorised Tecnomed Italia s.r.l. centre.
In order to ensure the functionality and safety of the product and to avoid any
TECNOMED ITALIA S.R.L. IS RESPONSIBLE FOR THE SAFE USE OF THE PRODUCT AND
GUARANTEES THE PRODUCT ONLY IF THE CONDITIONS PROVIDED IN THIS MANUAL
ARE RESPECTED.
device and the serial number. For information on technical support: produzione@dentalastec.it
For any request, always indicate the date of purchase, the model of the
3.1 - WORK ENVIRONMENT
FLOOR COMPOSITION
The quality of the oor must meet the capacity standards standards specied in DIN1055
sheet 3 and present the pressure resistance laid down in DIN 18560 T1.
ENVIRONMENTAL REQUIREMENTS:
• Environmental temperature: from +10°C to + 40°C.
• Optimum environmental temperature: from 15°C to 30°C.
• Relative air humidity: from 30 to 75%.
• Ambient pressure from 600 to 1060 hpa
3.2 - MAXIMUM LOADS
• The maximum allowed load on the surgical chair is 200 Kg. Do not exceed this value.
• The maximum allowed load on the sampling armrest is 10 Kg. Do not exceed this value.
• The maximum allowed load on the blood sampling armrest is 10 Kg. Do not exceed this value.
• The maximum allowed load on the thigh rests is 30 Kg. Do not exceed this value.
• The maximum allowed load on the headrest is 8 Kg. Do not exceed this value.
ENGLISH
DANGER! Tecnomed Italia s.r.l. denies any liability, expressed or implied, and cannot
be held liable for injuries to persons and/or direct or indirect damages to property, deriving
from failure to comply with the indications above.
9

4- SAFETY PROVISIONS
ENGLISH
DANGER! Tecnomed Italia s.r.l. declines any liability, expressed or implied, and cannot
be held liable for personal injury and/or direct or indirect property damage deriving from
failure to comply with the instructions in this manual and/or from incorrect installation and/
or use of the device and its accessories and/or from improper and/or lack of cleaning and/
or maintenance.
DANGER! Before using the product on patients, it is recommended to ask them if they
have pacemakers or other implants!
DANGER! The device must be used exclusively by specialised and suitably trained
personnel. Use the device only for the intended use that is foreseen for it. Failure to observe
this prescription may cause serious injuries to the patient, the operator, and damages to
the device.
DANGER!
s.r.l. and without CE marking or tted with standardised interfaces. The patient and the dentist
might get injured or the device might get damaged. Use only accessories authorised by
Tecnomed Italia s.r.l. The company assumes NO responsibility for personal injury and/or direct
or indirect property damage resulting from non-compliance with this requirement.
DANGER! The device and its accessories are supplied non-sterile. At the rst use and
after each treatment, the device and its accessories must be cleaned and/or sterilised
following the instructions in paragraph “Cleaning and sterilising”, on page 46.
DANGER! Cut off the power supply to the equipment by pressing the green ON/OFF
light button placed on the base of the surgical chair before carrying out any maintenance or cleaning operation.
ATTENTION! After sterilising the components in the autoclave, wait for them to cool
down before using them.
Do not use the product with accessories not approved by Tecnomed Italia
s.r.l. Inappropriate products might damage the surface of the device, affect its operation,
lead to contamination or cause injuries to the patient and/or the operator. Tecnomed
Italia s.r.l. assumes NO responsibility for personal injury and/or direct or indirect property
damage resulting from non-compliance with this requirement. For more information, see
paragraph “Cleaning and sterilising”, on page 46.
standards CEI 64-8/7 and subsequent amendments. The device shall be installed only by
technical staff authorised by Tecnomed Italia s.r.l.
earthed electrical systems.
the end of the day (if present). Tecnomed Italia s.r.l. shall not cover damages caused by
failure to comply with the indications above.
10
CAUTION! Use only care and cleaning products authorised by Tecnomed Italia
DANGER! The electrical system available at the installation site must comply with
ATTENTION! To avoid any electric shock risks, the device must be connected only to
DANGER! Do not use the device in environments subject to anaesthetic or ammable gases.
ATTENTION! Do not expose the device to direct sunlight or to sources of UV light.
ATTENTION! Disconnect the pneumatic, hydraulic and electrical power supply at
DANGER! Before every treatment, always check that the device works perfectly
and that the accessories are efcient. Always check that there is no water underneath
the device. Do not perform the treatment if you notice any malfunctions. In case of
anomalies, contact a service centre authorised by Tecnomed Italia s.r.l.
DANGER! Pay attention when using the product, as the automatic movements might
give rise to dangerous situations, even if minor.
DANGER! The patient’s arms and the feet should rest on the padding of the surgical
chair.
DANGER! It is strictly forbidden to make changes to the device. Tecnomed Italia s.r.l.
assumes NO responsibility for personal injury and/or direct or indirect property damage
resulting from non-compliance with this requirement.
ATTENTION! In order to ensure the functionality and safety of the product and to avoid
any wear-related damages, the product requires routine and extraordinary maintenance
interventions at specied intervals (contact a service centre authorised by Tecnomed
Italia s.r.l.). For more information, see the paragraph “Maintenance” on page 37.
DANGER! For any malfunctions not described herein, put the device out of service
and contact an authorised Tecnomed Italia s.r.l. technician.
DANGER! Do not disassemble the device. In case of malfunction, please contact an
authorised Tecnomed Italia s.r.l. centre.
4.1 SAFETY REQUIREMENTS
Tecnomed Italia s.r.l. declines any liability, expressed or implied, and cannot be held
liable for personal injury and/or direct or indirect property damage deriving from failure
to comply with the instructions in this manual and/or from incorrect installation and/or use
of the device and its accessories and/or from improper and/or lack of cleaning and/or
maintenance.
Tecnomed Italia srl cannot be held responsible, expressly or implicitly, for any type of
injuries to persons and/or damages to property inicted by the user of the product and its
accessories, and which have taken place in the following cases:
• If the device is used for other purposes than those for which it is intended;
• If the device is not used in compliance with the instructions and requirements provided
in this manual;
• Lack of stock materials to be used in the event of device stop due to fault or
inconveniences;
• Use of accessories not authorised by Tecnomed Italia srl;
• If the electrical system available at the installation site does not comply with the
application standard and the appropriate requirements;
• Assembly and/or repairs carried out by staff not authorised by Tecnomed Italia srl;
• If the storage conditions do not comply with the requirements specied in chapter
“Transport and storage conditions”.
ENGLISH
11

5- PRODUCT DESCRIPTION
5.1 - INTENDED USE AND METHOD OF USE
ENGLISH
Morpheus is a multifunctional surgical chair designed for the following sectors: Dentistry,
Maxillofacial surgery, Reconstructive microsurgery, Dermatology, Otolaryngology, Gynaecology, Sampling, Diagnostics. This device is not intended for use in potentially explosive
atmospheres.
It is the responsibility of the user to:
1- Use only EC certied devices in perfect state.
2- Protect himself, the patients and any third parties from any hazards.
3- Avoid any contamination of the product.
When using the device, follow the applicable regulations in force in the country of use, especially:
1- The provisions in force regarding safety at work.
2- The accident prevention measures in force.
DANGER! he device must be used exclusively by specialised and suitably trained personnel.
Use the device only for the intended use that is foreseen for it. Failure to observe this prescription
may cause serious injuries to the patient, the operator, and damages to the device.
DANGER! Tecnomed Italia s.r.l. declines any liability, expressed or implied, and cannot
be held liable for personal injury and/or direct or indirect property damage deriving from
failure to comply with the instructions in this manual and/or from incorrect installation and/
or use of the device and its accessories and/or from improper and/or lack of cleaning and/
or maintenance.
5.2 - STANDARDS/CERTIFICATIONS
Medical device
The MORPHEUS surgical chair is tted with CE marking.
The Morpheus device
Directive 93/42/CEE.
Protection class: Class I (EN 60601-1)
Part applied: type B
The surgical chair is also compliant with the following standards:
Number Title
EN ISO 14971:2012 Medical devices – Application of risk management
EN 60601-1:2006 Third Ed.
EN 60601-1-6:2007 Medical electrical equipment – General requirements usability
Dir. 93/42/CEE European directive concerning medical devices
Law Decree 46/97 Implementing directive 93/42
Dir 2007/47/CE Updating directive 93/42
Law Decree 37/10 Implementing directive 2007/47
EN 1041: 2010
ISO 15223-1:2012
EN ISO 13485:2012 Medical devices. Quality management systems
ISO 6875:2011 Dentistry - Dental patient chair
EN 62366:2007 Application of Usability engineering to Medical Devices
12
is classied as class IIa medical device based on rule 1 of annex IX of
Medical electrical equipment – General requirements for
Information supplied by the manufacturer with the Medical
Medical devices -Symbols to be used with medical device labels, labelling and information to be supplied - Part 1: General
requirements
safety.
Devices
5.3 - TECHNICAL SPECIFICATIONS
Model name:
Power: 90 VA
Voltage: 230 Volt ~ 50-60HZ
Maximum air pressure: 8 bar
Weight: 180 Kg
Protection with 3,15A
Operating mode: continuous operation with intermittent load, suitable for use in dental
practices.
Electrical power supply: 3 x1.5 mm
Floor-mounted free standing end with side covering furniture: 300 mm
ATTENTION! It is essential to install a wall-mounted thermal magnetic circuit breaker of
250 Volt 30mA upstream of the unit. The 6 mm2 earthing conductor must be connected to
the equipotential node of the site.
Fuses
Identication Value Dimensions Protection Position
FUSE F1
FUSE F2
The scope of supply includes two spare fuses.
Packaging dimensions:
•PARCEL 1 TECNOMED ITALIA- Box with cart
Dimensions 1200 x 800 x 1300 mm (width x depth x height). Weight 180 kg (approx).
Morpheus
T 3.15 A
250V
T 6.3 A
250V
5x20
5x20
230 VAC
on control unit
30 VDC
on control unit
On the side of the power button
On the side of the power button
ENGLISH
13
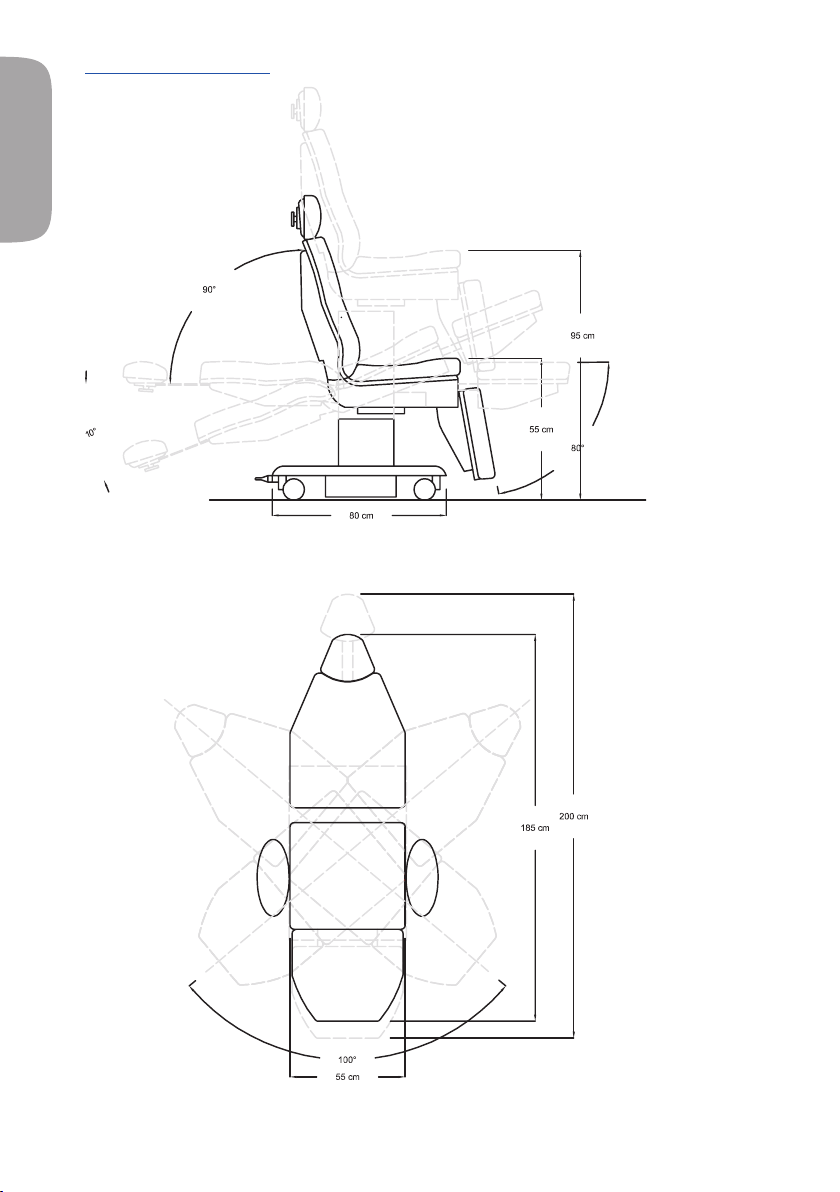
5.3.1 - DIMENSIONS
ENGLISH
14
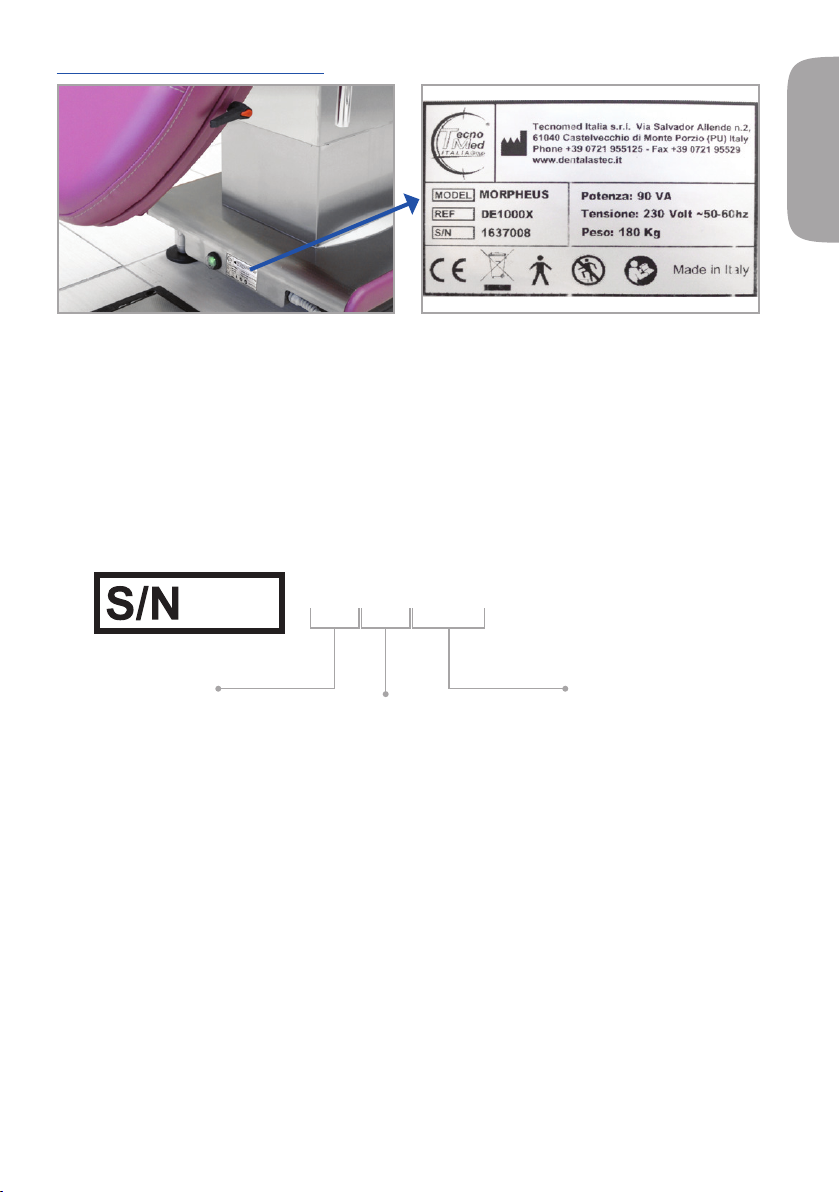
5.4 - IDENTIFICATION PLATES
Data on the plate
• Name of the manufacturer.
• Name of the device.
• Product code.
• Serial number.
• Power.
• Voltage.
• Weight.
Guide to reading the serial number
1308001
ENGLISH
Year of manufacture
Progressive number
Week of production
15
 Loading...
Loading...