Page 1

SynCardia Systems Inc.
temporary Total Artificial
Heart (TAH-t)
INSTRUCTIONS FOR USE
1992 E. Silverlake Road
Tucson, AZ 85713 USA
(520) 545-1234
(866) 771-9437
STERILE EO
CAUTION: Federal (USA) law restricts this device to sale by
or on order of a physician.
0086
16 MAY 2005
950000-001 Rev. 004
Page 2

SynCardia TAH-t Instructions for Use
Table of Contents
Page
1.0 Device Description.…………………………………………...…... 3
1.1 Implantable TAH-t…………………………...………...…… 3
1.2 External Console………………………………….….......... 4
2.0 Indications for Use………………………………………....…..… 4
3.0 Contraindications………………………………………………... 4
4.0 Warnings ...........................………………………………………. 5
5.0 Precautions……………………………………………........…..... 6
6.0 Summary of Clinical Study……………………………………… 7
6.1 Trial Success……………………………………………….. . 7
6.2 Hemodynamics……………………………………………….. 7
6.3 Adverse Events……………………………………………….. 8
6.4 TAH-t Reliability……………………………………………….. 9
7.0 Implant Procedures.......................................................................... 10
7.1 Materials Needed but not Provided………………………… 10
7.2 Preparation.............................................................................. 11
7.3 Removal of Native Ventricles................................................. 11
7.4 Preparing the Atria................................................................... 13
7.5 Outflow Connectors.................................................................. 15
7.6 Connect Artificial Ventricles.................................................... 16
8.0 Operator’s Manual for Console………………………………… 20
8.1 Warnings for Console Operation…………............................. 20
8.2 Readying Console for Clinical Use............................................ 21
8.3 TAH-t Startup Procedure...................................................……… 21
9.0 Explant Procedures…………………………………...……...…… 23
10.0 System Components............................................................................. 24
Appendix A: Patient Selection and Management……………………………….. 25
Patient Selection...……………………………....…...…….……………… 25
Anticoagulation………………………………..…......…………….....…... 25
Exit Site…………………………………………………………………… 26
Appendix B: Outline of Training Program…………………………………….. 27
Appendix C: Materials Matrix…………………………………………………… 28
SynCardia Systems, Inc. Page 2
Page 3

SynCardia TAH-t Instructions for Use
1.0 Device Description
The SynCardia temporary Total Artificial Heart (TAH-t) system is a pulsatile biventricular
device that replaces a patient's native ventricles and valves and pumps blood to both the
pulmonary and systemic circulation. The system consists of the implantable SynCardia
TAH-t and an external console connected by drivelines (Figure 1).
Figure 1: SynCardia TAH-t System
1.1 The Implantable SynCardia TAH-t
The implantable SynCardia TAH-t consists of two artificial ventricles, each made of a
semi-rigid polyurethane housing with four flexible polyurethane diaphragms
separating the blood chamber from the air chamber. The diaphragms allow the
artificial ventricle to fill and then eject blood when compressed by air from the
external console. Mechanical valves, mounted in the inflow (27mm) and outflow
(25mm) ports of each artificial ventricle, control the direction of blood flow. The
maximum dynamic stroke volume of each ventricle is 70 ml, which allows for
generating a flow rate up to 9.5 liters per minute.
The left artificial ventricle is connected via the left atrial inflow connector to the left
atrium, and via the aortic outflow connector to the aorta. The right artificial ventricle
is connected via the right atrial inflow connector to the right atrium and via the
pulmonary artery outflow connector to the pulmonary artery. Each artificial ventricle’s
driveline conduit is tunneled through the chest wall. The right and left artificial
ventricle’s driveline conduits are attached to seven-foot pneumatic drivelines that
connect to the back of the external console.
SynCardia Systems, Inc. Page 3
Page 4

SynCardia TAH-t Instructions for Use
1.2 The External Console
The external console operates and monitors the SynCardia TAH-t. The console
includes a monitoring computer that provides noninvasive diagnostic and monitoring
information to the user. Device rate, dynamic stroke volumes, and calculated cardiac
outputs are displayed on a beat-to-beat basis. Drive pressure and flow waveforms,
along with cardiac output trends are provided. Patient related alarms (e.g., low cardiac
output) are also displayed on the computer screen.
A separate alarm panel on the console provides information on critical drive pressure
and backup air and battery status. In addition, an alarm is generated if the computer is
not monitoring the patient. All alarms generate audio and visual feedback to the user.
A backup air supply (two air tanks) and electrical power (backup power supply and
console battery) are automatically activated if the external compressed air and/or AC
power are interrupted. This can occur during patient transport or in the event of a
failure in the hospital’s air or electrical supply.
The controller is the major component of the external console, and supplies pulses of
pneumatic pressure to the right and left drivelines, which connect into the air
chambers of the respective implanted artificial ventricles. These pulses cause the
diaphragms to distend and thereby eject blood from the right artificial ventricle into
the pulmonary circulation (typically 50-70mmHg) and from the left artificial ventricle
into the systemic circulation (typically 180-200mmHg).
2.0 Indications for Use
The SynCardia temporary Total Artificial Heart (hereinafter called the TAH-t) is
indicated for use as a bridge to transplantation in cardiac transplant-eligible candidates
at risk of imminent death from biventricular failure. The SynCardia TAH-t System is
intended for use inside the hospital.
3.0 Contraindications
The SynCardia TAH-t is contraindicated for use in:
Patients who are not cardiac transplant eligible.
Patients who do not have sufficient space in the chest area vacated by the natural
ventricles. Generally this includes patients who have body surface areas <1.7m², or
who have a distance between the sternum and the 10th anterior vertebral body
measured by computed tomography imaging (CT scan) < 10 cm.
Patients who cannot be adequately anticoagulated on the TAH-t.
SynCardia Systems, Inc. Page 4
Page 5

SynCardia TAH-t Instructions for Use
4.0 Warnings
1) Setup and operation of this device should only be undertaken by personnel trained
in accordance with the SynCardia training program. A thorough understanding of
the technical principles, clinical applications, and risks associated with the device
is necessary. Prior to use, refer to this IFU and to the Circulatory Support System
(CSS) User’s Manual for important operating instructions.
2) Sterile components of the SynCardia TAH-t are intended for single use only. Do
not use if package is opened or damaged. Do not re-sterilize or reuse.
3) Safe use of this system has not been established in pregnant patients.
4) Do not subject patients implanted with the SynCardia TAH-t to magnetic
resonance imaging (MRI) scans.
5) Safety and effectiveness in populations other than those of idiopathic and ischemic
cardiomyopathies has not been established.
6) Do not use this device if the implantable artificial ventricles cannot fit in the chest
area vacated by the natural ventricles. Inferior vena cava and left pulmonary
venous compression are possible consequences.
7) Do not allow any catheter to get near the inflow valves of the SynCardia TAH-t. If
a catheter gets into an inflow valve, the valve could become stuck, limiting flow.
Confirm by x-ray after catheter insertion. A percutaneously inserted central
catheter may migrate into the inflow valve when the patient raises his/her arm.
8) There is a potential for air embolism. De-air the artificial ventricles to minimize
the possibility of air inadvertently entering the device.
9) Do not allow the external drivelines to become kinked. If there is any low cardiac
output alarm, inspect the external drivelines for kinking.
10) A reduction in the maximum stroke volume on the external console’s monitoring
computer to below 50 milliliters may indicate a failure of one of the diaphragms in
an artificial ventricle of the SynCardia TAH-t.
SynCardia Systems, Inc. Page 5
Page 6

SynCardia TAH-t Instructions for Use
5.0 Precautions
1) Measures should be taken to prevent infection or sepsis. Use strict aseptic
techniques during implantation.
2) The outflow grafts must be pre-clotted before use.
3) When closing the chest, a reduction in device output may indicate inflow
obstruction. Reposition the artificial ventricles by anchoring to a rib or moving
into the left plural space.
4) Do not use an antifibinolitic agent like Aprotinine or Amicar with an active
clotting agent like FEIBA.
5) Use only water-soluble antiseptic cleaners around the exit site. Ointments may
delay tissue in-growth into the driveline conduits.
6) Each external console contains a primary and a backup controller. An
additional external console should also be available for use.
7) A sudden reduction in SynCardia TAH-t flow may be due to a kink in the
pneumatic drivelines, or some inflow obstruction to the TAH-t, such as
tamponade. Defibrillation or CPR will not be effective.
8) Flows should be kept at a reasonable output so that proper washing of the
ventricles is established.
SynCardia Systems, Inc. Page 6
Page 7

SynCardia TAH-t Instructions for Use
6.0 Summary of Clinical Study
The multi-center (5) clinical study focused on use of the SynCardia TAH-t as a bridge
to cardiac transplantation in transplant eligible patients at risk of imminent death from
biventricular failure. Ninety-five patients (ages 16-67) were implanted with the
SynCardia TAH-t; 81 (70 males, 11 females) met all inclusion/exclusion criteria and
were designated the core implant group. All patients were in NYHA Class IV at time
of enrollment. Additional characteristics of the core implant group at the time of entry
into the study are: 1) 15 patients were on heart-lung machine/ECMO support, 2) 51
patients had central venous pressure > 18 mmHg, 3) 11 patients had right ventricular
ejection fraction < 20%, and 4) all patients had relative or absolute contraindications
to VAD support as evidenced by refractory arrhythmias or unresuscitatable cardiac
arrest (25), hypokinetic right/left/global ventricles (23), aortic regurgitation, stenosis
or prosthesis (13), massive myocardial infarction or direct myocardial injury that
affects technical insertion of a VAD through the left ventricle (10), failure to wean
from cardiopulmonary bypass with bi-ventricular injury (4), left, right ventricular or
mural thrombus (3) or septal defect (3). All patients were on maximal medical therapy
and at imminent risk of death before a donor heart could be obtained.
6.1 Trial Success
Treatment success was defined as patients who, at 30 days post transplant,
were 1) alive; 2) NYHA Class I or II, 3) ambulatory; 4) not ventilator
dependent; and 5) not on dialysis.
Trial success was achieved in 56 (69%) of the 81 core patients. Sixty-four of
the 81 core patients (79%) reached transplant after a mean time of 79 days
(range 1-414). Fifty eight (72%) survived to 30 days post transplant.
6.2 Hemodynamics
The hemodynamic performance of the SynCardia TAH-t was assessed through
a comparison of pre- and post-implant values of cardiac index, systolic arterial
blood pressure, and central venous pressure. Hemodynamic indices were
effectively restored to near normal values. Average cardiac index increased
from 1.9 to 3.0 L/min/m², average systolic blood pressure increased from
93mmHg to 120mmHg, and average CVP decreased from 20mmHg to
14mmHg.
The average perfusion pressure (mean aortic pressure minus CVP) increased
from 49mmHg to 63mmHg, which was associated with recovery of renal and
hepatic function.
SynCardia Systems, Inc. Page 7
Page 8
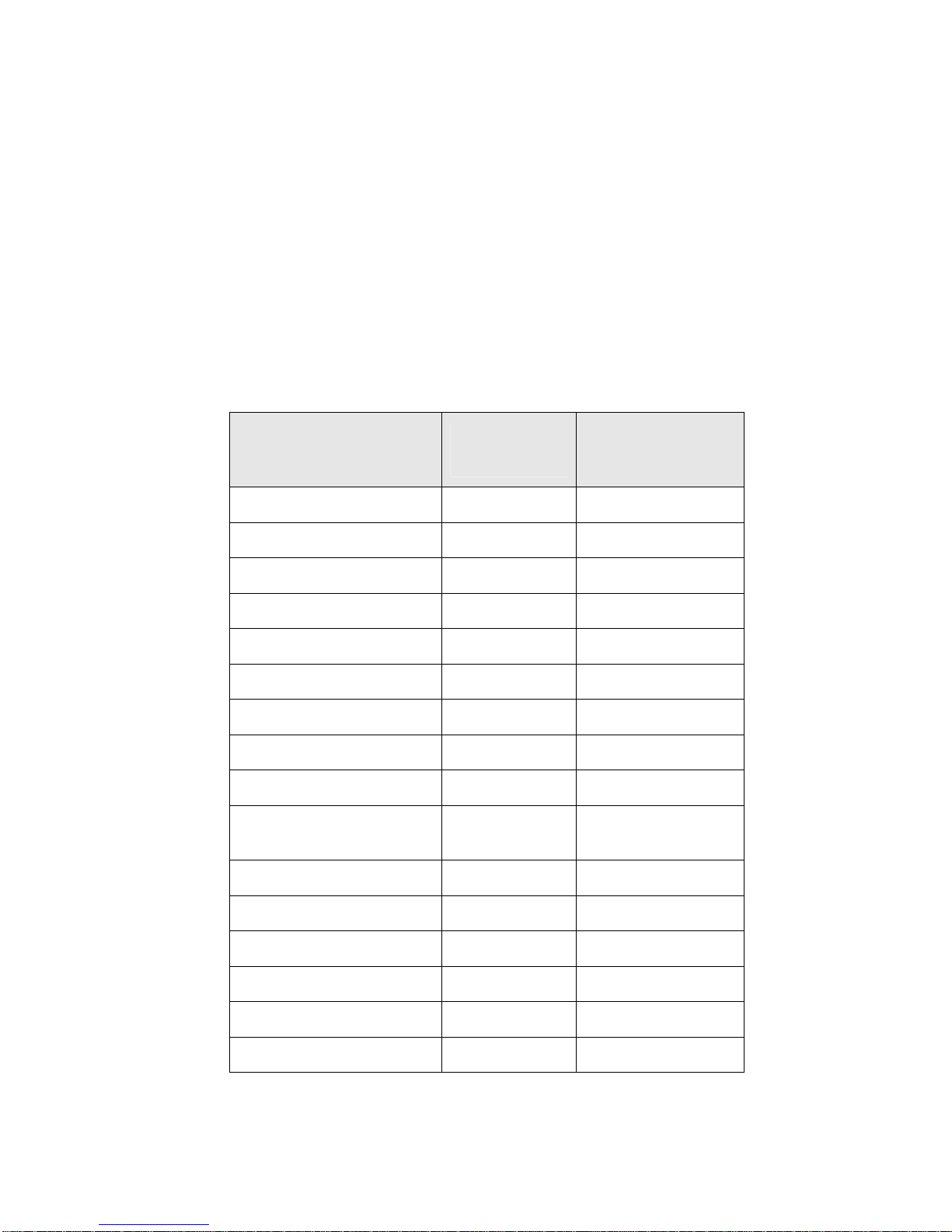
SynCardia TAH-t Instructions for Use
6.3 Adverse Events
Adverse events collected for all 81 core patients while on the SynCardia TAHt device are presented in descending order below. The adverse events
represent 17.6 device years of experience for an overall event rate of 1.9 events
per month while on the device awaiting transplant.
Incidence of Adverse Events in Core Patients During
in Decreasing Order of Frequency
(Represents 17.6 years or 6411 days on the device)
Table 1
Device Implantation,
Adverse Event
Any Adverse Event 400 76 (93.8%)
Infection 125 58 (71.6%)
Bleeding 55 34 (42.0%)
Respiratory Dysfunction 44 24 (29.6%)
Hepatic Dysfunction 30 29 (35.8%)
Neurological Event 26 20 (24.7%)
Renal Dysfunction 23 21 (25.9%)
Reoperation 18 17 (21.0%)
Device Malfunction 18 15 (18.5%)
Peripheral
Thromboembolism
Reduced Blood Pressure 13 12 (14.8%)
Number of
Events
14 9 (11.1%)
Number (%) of
Patients
n=81
Reduced Cardiac Index 11 7 (8.6%)
Technical/Procedural 11 3 (3.7%)
Fit Complication 5 5 (6.2%)
Hemolysis 3 3 (3.7%)
Miscellaneous 3 3 (3.7%)
SynCardia Systems, Inc. Page 8
Page 9
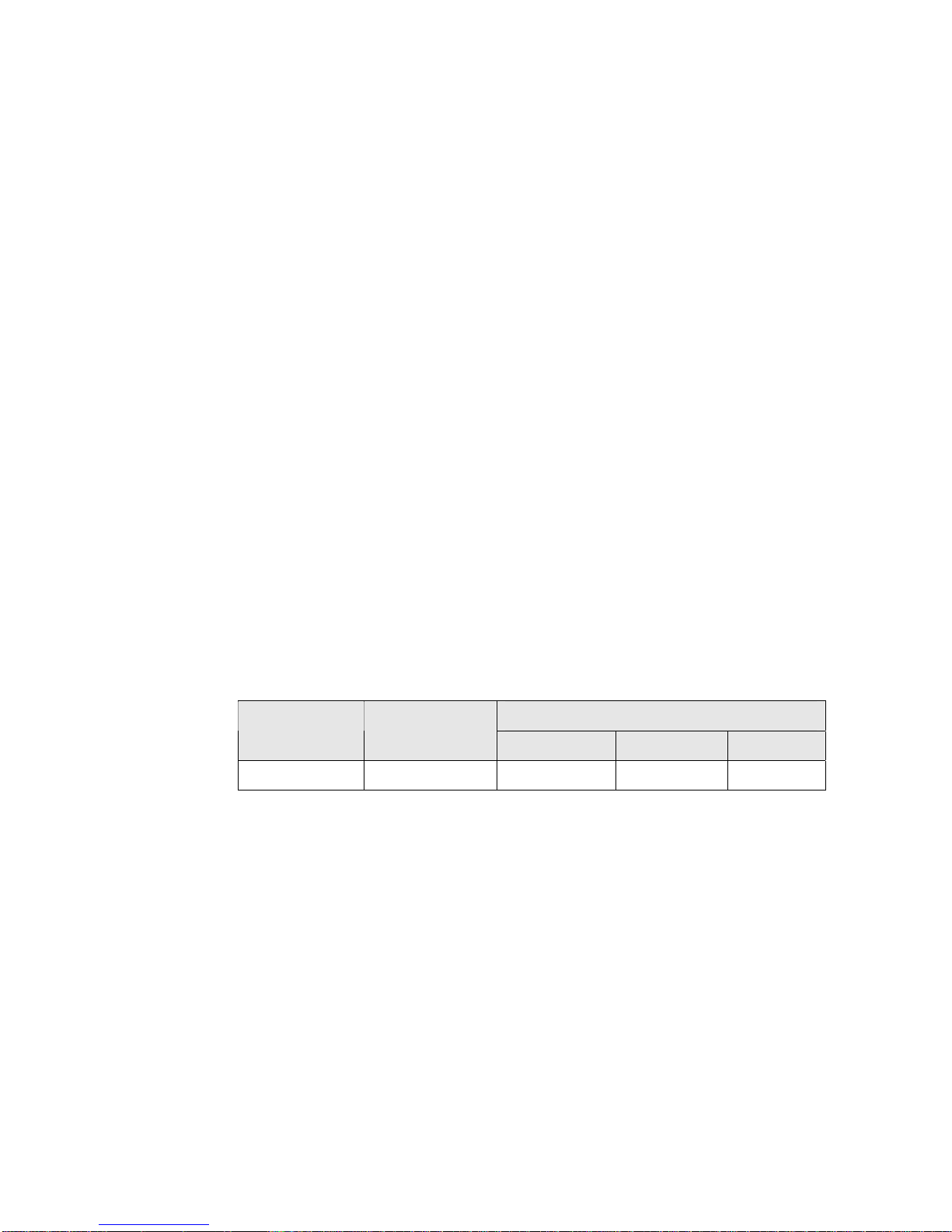
SynCardia TAH-t Instructions for Use
6.4 SynCardia TAH-t Reliability:
Reliability testing was conducted to determine with reasonable assurance how
long a device would perform as intended, without failure.
Three separate sets of in vitro reliability testing were conducted. In one test,
four TAH-t units were run for a period of 180 days. During this time there
were no failures or abnormalities observed.
In a second in vitro reliability trial, four TAH-t units were tested in a “run to
failure” study design and are ongoing. After 35 months of testing, there were
no failures or abnormalities observed.
A third test was initiated using three TAH-t units which had expired their 3
year sterilization expiration date. This provided information about the effects
of long-term storage on the fatigue resistance properties of the TAH-t. After
24 months of testing, there were no failures or abnormalities observed.
In conclusion, a total of eleven units have been run for various lengths of time
over the last six years with no device-related failures. The cumulative number
of days used for calculation was 6715 and there have been no failures or signs
of appreciable wear observed. When the 11 units are used to calculate
reliability with a 90% confidence, the reliability at 30, 60 and 365 days is as
reported in the table below.
Reliability Test Results with 90% Confidence
# days run MTBF*
6715 2916 0.99 0.98 0.88
Table 2
Reliability in number of days run
30 60 365
SynCardia Systems, Inc. Page 9
Page 10

SynCardia TAH-t Instructions for Use
7.0 Implant Procedures
This section contains the Implant Procedures. Patients receiving the SynCardia TAH-t
are prepared for the implant per standard hospital procedures for any cardiac surgery.
An arterial line, a central line, and standard artificial ventilation are required prior to
the start of surgery. Transesophogeal echocardiography is recommended.
7.1 Materials Needed but not Provided
• Three 15 by 20 centimeter sheets of membrane are used to create a neo-
pericardium to prevent adhesions.
•
7.2 Preparation
• Pass the SynCardia TAH-t sterile components into the sterile field.
• After a standard median sternotomy is performed and before starting
• Preclot the two arterial outflow connectors three times with the patient's
• Trim the two inflow connectors. Cut edges of the atrial quick connects
• Pass the drivelines conduits through their subcutaneous pathways
• Place a long clamp through the subcutaneous tissue, rectus fascia,
Teflon felt buttresses strips. These are cut to approximately 10-12 mm
in width and are generally 10 cm in length. It most often takes at least
two of these to extend around the entire atrial cuff. (See Section 7.3)
heparin, 1) prepare the arterial outflow connectors, 2) trim atrial inflow
connectors to appropriate size, and 3) tunnel the artificial ventricle
conduits through the skin.
blood before giving the heparin. After exposure to the blood (approx.
30 cc for each connector each time) stretch connector, let dry for about
5 minutes and preclot again. The connectors are coated on the outside
with biologic glue (cryoprecipitate with calcium and topical thrombin).
Stretch again and let dry. This is done before cannulation so there is
plenty of time to obtain sufficient preclotting of the outflow connectors.
If the patient has been heparinized before deciding to implant the
SynCardia TAH-t, the arterial outflow connectors should be preclotted
with a combination of heparinized blood, protamine, and thrombin.
for the atrial anastomoses to a radius extending out
for 5-7 mm. Cut in a completely circular fashion. Then stretch and
invert them.
before heparinization of the patient. Position the left-sided ventricle
conduit in the epigastrium at the level of the midclavicular line and
approximately 2 inches below the costal margin. Make a semicircular
skin flap incision on the left midclavicular line approximately 5 to 10
cm below the costal margin.
rectus muscle, and into the chest as a chest tube would be placed. Use
a similar approach to place the driveline conduit for the prosthetic right
from the connector
SynCardia Systems, Inc. Page 10
Page 11

SynCardia TAH-t Instructions for Use
ventricle, approximately 4 to 5cm medial to the left ventricle conduit so
that no necrosis between the two exit sites will result.
• Enlarge pathway by opening the clamp and inserting a 1-inch Penrose
drain through the pathway. Place the end of the conduit in the Penrose
drain and advance approximately 8-10 cm. Pull Penrose drains through
the pathway that delivers the driveline conduit. Position the artificial
ventricles lateral to the wound and cover with a towel while the rest of
the procedure takes place. This provides ample opportunity for small
bleeders in the driveline pathway to clot.
7.3 Removal of the Native Ventricles
• Cannulation of the aorta and both superior and inferior vena cava is
done in a standard fashion. Umbilical tape chokers are used on the
cavae. Dissection around the aorta and pulmonary artery is limited to
the proximal portion of the aorta in anticipation of transplantation, thus
leaving some untouched areas that will not be very fibrotic.
Cardiopulmonary bypass is instituted and the heart is fibrillated. Total
bypass is instituted by pulling on the choker tapes.
• The heart is fibrillated and excision of the heart begun. The excision is
different from that used for transplantation. It seeks to preserve the
annulus of both the tricuspid and mitral valves. Thus, an incision is
made on the ventricular side of the AV groove of the right ventricle
(Figure 2).
Figure 2: First Incision of Ventricle Excision
SynCardia Systems, Inc. Page 11
Page 12

SynCardia TAH-t Instructions for Use
• Incision can be done with a knife and extended with a knife or scissors.
It is extended anteriorly across the right ventricular outflow tract and
just proximal to the pulmonary valve. Posteriorly, it is extended to the
interventricular septum and across the septum, staying on the left side
of the arterioventricular (AV) groove and preserving the entirety of the
mitral annulus. The anterior and posterior lines of incision are dissected
apart from each other out to the level of the pulmonary bifurcation.
• Trim the excess muscle on the right and left sides down to near the AV
valves. All chordae are trimmed away, and a 2 mm edge of valve tissue
along with the annulus is left intact. The atrial cuff generally extends 1
cm beyond the AV valves and consists of residual ventricular muscle
and fat in the AV groove. The portion of the cuff in the left ventricular
outflow tract consists of the residual anterior leaflet of the mitral valve
and some aortic tissue. Most of the aortic tissue is trimmed away;
however, some is left intact because it is felt to present strong tissue for
the sewing of the inflow connector. The great vessels are then
separated from the remaining ventricular myocardium above the
valvular level. The great vessels are separated from each other (Figure
3).
Over-sew the coronary sinus entrance into the right atrium. This
prevents backflow of blood through the coronary sinus and out to the
cut vessels on the AV groove.
SynCardia Systems, Inc. Page 12
Figure 3: Ventricles Removed
Page 13

SynCardia TAH-t Instructions for Use
• Three 15 by 20 centimeter sheets of m embrane are used to create a neo-
pericardium to prevent adhesions. On the right side a sheet is anchored
with non-absorbable suture to the pericardial reflection at the level of
the superior vena cava, pulmonary veins and inferior vena cava. On the
left side, a second sheet is sutured to the pericardial reflection just
anterior to the left pulmonary veins. On the diaphragmatic side, a third
sheet is sutured so as to cover the entire diaphragmatic pericardial
surface. The 3 sheets are then folded upon themselves to keep them out
of the operative field while the SynCardia TAH-t is implanted.
7.4 Preparing the Atria
The outer walls of the entire right and left atrial cuff complex are encircled
with Teflon felt buttresses. These are placed in such a way that they can be
used for strengthening the anastomosis to the inflow connector and also to
tamponade and control all possible bleeding from the AV groove portion of the
connector. These are cut to approximately 10-12 mm in width and are
generally 10 cm in length. It most often takes at least two of these to extend
around the entire atrial cuff. They are placed on the outer edge of the cuff and
sewn in place with a running 3-0 polypropylene (Figure 4). A long needle is
used to accomplish this (MH needle) and, after completing this, the left and
right atrial cuffs are surrounded by Teflon felt buttresses.
SynCardia Systems, Inc. Page 13
Figure 4: Atrial Sutures
Page 14

SynCardia TAH-t Instructions for Use
• The atrial inflow connector is sewn first. It is inverted and placed inside
the left atrial cuff on the lateral wall. 3-0 polypropylene is used with an
MH needle with a running stitch, taking care to tailor the atrial cuff and
the inflow connector into a single hemostatic suture line. The suture
line includes both free walls of the atrium, buttressed with Teflon felt in
the atrial septum, which has no buttressing material. A similar
procedure is done with the right inflow connector. The connector is
inverted, placed in the atrium, the suture line is run, and after
completing both suture lines, the inflow connectors are returned to their
normal position (Figure 5).
Figure 5: Inflow connector inverted for suturing (left), finished normal position (right)
• Check for hemostasis with the plastic leak tester made to fit within the
inflow connector. A syringe (60-100 cc) is used to inject into a threeway stopcock connected with the tester to test the left atrial suture line.
The surgeon places his hand posterior to the left atrium and compresses
the right and left pulmonary veins, while the assistant injects saline
mixed with a small amount of blood into the left atrium. Observe for
leaks. A dental tool is used to break the seal between the tester and
connector. If there are any leaks, sutures are placed at this time. On
the right side, fluid is simply injected into the right atrium under
pressure, since the inferior and superior vena cava are already
obstructed by the caval tapes. Again, closure of leaks with a 3-0 MH
polypropylene suture is done at this time.
SynCardia Systems, Inc. Page 14
Page 15

SynCardia TAH-t Instructions for Use
7.5 Outflow Connectors
• Great vessel connections are made. The pulmonary artery anastomosis
is made first. The lengths of the outflow connectors are determined by
placing the artificial ventricles in position within the pericardial cavity.
Place the outflow connector between the aortic or pulmonic valve and
its respective great vessel and measure the
connectors to the appropriate lengths, usually 3 to 5 cm.
• The pulmonary artery anastomosis is made with a running 4-0
polypropylene suture in an end-to-end fashion, beginning with lateral
wall and running the back wall of the anastomosis from the inside
(Figure 6).
distance. Cut outflow
• A similar anastomosis is made with the aortic suture line. Then, the
outflow connector leak tester is used, which is inserted into the aortic
outflow connector. Saline is injected under pressure, observed for
leaks, and then any leaks are closed with a 4-0 polypropylene suture.
The pulmonary artery needs to be cross-clamped in order to test the
integrity of the pulmonary artery to connector anastomosis. The
pulmonary artery and aortic tester is the same, but smaller, than the one
utilized for the atrial inflow connector.
SynCardia Systems, Inc. Page 15
Figure 6: Outflow Connector Suturing
Page 16

SynCardia TAH-t Instructions for Use
7.6 Connect Artificial Ventricles
• Once adequate hemostasis of all suture lines is established, placement
of the artificial ventricles begins. First, the left artificial ventricle is
connected (Figure 7).
• Grasp the left inflow connector with two large Mayo clamps placed
side by side, with a good hold of the connector. The opposite side of
the plastic fitting for the connector of the artificial left ventricle is
placed within the connector, and the operator pulls with the Mayo
clamps and pushes the artificial left ventricle into the inflow connector.
The position in which the heart sits for the duration of the support of
the patient is determined by the orientation of the artificial left ventricle
as it is placed into the left atrial inflow connector. Therefore, a careful
assessment of exactly where the aortic outflow connector will connect
to the artificial left ventricle and the anticipated position of the artificial
left ventricle should be made before the connection of the atrial inflow
connector is completed. It is then an easy matter to snap on the aortic
SynCardia Systems, Inc. Page 16
Figure 7: Connect Ventricles
Page 17

SynCardia TAH-t Instructions for Use
outflow connector, taking care not to twist the connector or aorta.
While this is being done, the artificial left ventricle should be filled
with saline through the aortic valve as well as the outflow connector.
Once the connection is made, the patient is placed in a steep
Trendelenburg position and large vent sites are placed in the highest
point of the aortic outflow connector and the aorta for removal of air.
• The artificial right ventricle is then connected. The atrial connection is
made first, again taking care with the orientation of the artificial right
ventricle so that the direction of flow from the outlet valve is
appropriate for the anatomy of the patient. After the atrial connection
is made, the pulmonary outflow connection is made, again, taking care
not to twist. Before connecting the pulmonary outflow connector graft,
the chokers on the superior and inferior vena cava should be removed.
This allows a flow of blood into the right atrium and the right artificial
ventricle, and flushes air out as the connection to the pulmonary artery
is made (Figure 8).
• With the patient in extremely steep Trendelenburg position and lungs
being slowly ventilated, begin pumping at a very slow rate (40 BPM,
40%SYS, 180mmHg-LDP, 60mmHg-RDP, 0mmHg-VAC). Agitation
of the artificial ventricles, as well as atria, is done at this time. If
available, monitor for air bubbles in the atria and aorta with
transesophageal echo to help decide when the device has been
completely de-aired. As air is slowly removed from the device,
increase pumping rate and pressure. Generally, this process takes about
10 minutes and should be done with patience and attention to remove
SynCardia Systems, Inc. Page 17
Figure 8: SynCardia TAH-t Final Position
Page 18

SynCardia TAH-t Instructions for Use
air before the SynCardia TAH-t takes over from the heart-lung
machine. Decrease flow on the heart-lung machine temporarily to help
move air through the lungs and into the device. Once satisfied that all
air is out of the device, close vent sites and begin full pumping as the
heart-lung machine is weaned off. The patient should be kept in steep
Trendelenburg for an additional 15-20 minutes.
• As the table is flattened out, try to position the artificial ventricles
within the mediastinum. The pleura on both sides should not be opened
and the pericardium should be left intact for closure. In smaller
patients, there may be a need to force the right ventricle under the left
edge of the sternum. Care should be taken to examine the left
pulmonary veins and the inferior vena cava for evidence of
compression. This is facilitated with trans-esophageal echo.
•
Check for hemostasis. After protamine has been administered and
hemostasis obtained, a trial closure of the sternum is done using towel
clips. If the fit of the device is judged adequate by hemodynamic
stability and by transesophageal echo examination of the caval and
pulmonary venous flows, reopen the chest and bring together the edges
of the Gortex sheets to form a tent or neo-pericardium. Take care to
make a loose fit, without impingement upon the cavae and tension on
the device. Prior to closure of the cephalic part of the neo-pericardium,
pass a rectangular piece of Gortex membrane around the proximal
ascending aorta and anchor with non-absorbable suture. This is to
provide a surgical plane at explant between the aorta and pulmonary
artery to facilitate encircling and cross clamping the aorta.
• One chest tube is placed in the neo-pericardium and a second in the
native pericardial space. Irrigate with antibiotic solution before
closure. Close the sternum and remaining incision in a routine fashion.
Check device output, central venous pressure, and device filling when
the chest is closed, because chest closure may alter the anatomy,
causing pressure on the left-sided pulmonary veins, inferior vena cava,
and occasionally the right-sided pulmonary veins. If decreased flow is
noted, the chest must be reopened and changes made in the position of
the device. One change has been to mobilize the diaphragmatic
attachment of the
in the chest. This requires opening the left pleura, allowing the
SynCardia TAH-t to slightly migrate into the left pleural space. If
decreased flow is still observed, the right artificial ventricle may need
to be anchored to a rib using umbilical tape (Figure 9).
pericardium, allowing the device to sit more leftward
SynCardia Systems, Inc. Page 18
Page 19

SynCardia TAH-t Instructions for Use
Figure 9: Solution to a Fit Problem
SynCardia Systems, Inc. Page 19
Page 20

SynCardia TAH-t Instructions for Use
8.0 Circulatory Support System (CSS) User’s Manual
The Circulatory Support System (CSS) User’s Manual, Part #950001, contains
detailed information on the setup, operation and troubleshooting of the SynCardia
TAH-t system. A brief description of the contents is given as a reference.
Introduction to the External Console: Describes the system overview,
indications for use, and warnings.
Features and Operations: Describes the operation of the controller, power
supply, air supply, vacuum pump, UPS, alarms and computer.
Unpacking and Initial Setup: Covers unpacking instructions and initial setup.
Performance Verification: Describes the console test procedure and the
preparation for standing by for an implant.
Clinical Use: Describes console operation, readying system for clinical use,
SynCardia TAH-t startup, patient transport, transfer to backup controller, and
console replacement.
Specifications: Describes the SynCardia TAH-t physical and performance
specifications.
Routine Maintenance and System Checkout: Describes console checkout,
batteries, cleaning, and checkout procedure.
Field Service Guide: Describes air tank replacement, scheduled servicing, air
tank connector O-ring replacement, controller pilot pressure calibration,
controller replacement, fuse replacement, inactive storage, and crating
instructions.
8.1 Warnings for Console Operation
• DO NOT operate or adjust system without proper training.
• DO NOT operate console on an air supply of substandard or unknown
quality, either from tanks or in-house compressors.
• DO NOT use a controller outside of its planned maintenance cycle.
• DO NOT intentionally operate a system having only one functional
controller for any longer than is necessary to switch systems.
• DO NOT defeat the alarm system by turning it off, tampering with the
alarm mute button, by muffling the audible alarms, or by any other
means.
• DO NOT expose the system to any unusual environment, i.e. electric
or magnetic fields, dampness or temperature extremes.
SynCardia Systems, Inc. Page 20
Page 21

SynCardia TAH-t Instructions for Use
• DO NOT leave key in primary controller key switch during an implant.
The key may be kept on Velcro near top right side of the backup air
supply compartment.
• DO have a backup system in a state of ready standby.
• DO set backup controller parameters to the same values as the primary
controller.
• DO have a controller switch key attached to the console.
• DO keep system casters locked except for transport.
• DO have a number of spare charged air tanks on hand.
8.2 Readying Console for Clinical Use
• Two consoles should be in ready standby mode. Ensure that backup
batteries are fully charged.
• Verify that each system has been connected to AC power with the
SYSTEM POWER switch in the ON (1) position
• Confirm that each controller AC POWER/BATT CHRG lamp is on.
• Before moving system to Operating Room, moisten a clean cloth with
an antibacterial agent and wipe down all exterior surfaces of the
console.
• Do not spray any cleaning agent directly on system.
8.3 SynCardia TAH-t Startup Procedures
• Turn SYSTEM POWER switch OFF (0); disconnect system mains
power cord and move system to patient site.
• Position the rear side of the console within driveline length of the
patient’s chest.
• Lock front casters (wheels).
• Connect system power cord and turn SYSTEM POWER switch ON
(1).
SynCardia Systems, Inc. Page 21
Page 22

SynCardia TAH-t Instructions for Use
• Verify console AC POWER and CHARGE LEDs are green, indicating
system has AC power.
• Verify that both controller AC POWER/BATT CHRG indicator lights
are on.
• Connect main air supply and verify pressure is 50–110 psi (340-575
kPa) at system power interface panel gauge.
• Open primary and reserve air tank valves and verify they are fully
charged.
• Set primary and backup controllers to values listed below:
Left Drive Pressure = 0 mmHg; Right Drive Pressure = 0 mmHg
Vacuum = 0 mmHg
Heart Rate = 40 bpm
Systolic Duration = 33%
Be sure vacuum remains off until the patient’s mediastinum is closed.
• Turn computer on. Wait for WCOMDU to load. Select Patient
Monitoring Mode. Enter patient identification requested. Inhibit
WCOMDU alarms during startup.
• Be sure that LDP, RDP and VACUUM are zero. Turn primary
CONTROLLER key switch On and press controller ALARM RESET
button.
• Turn ALARM SYSTEM key switch to ON. Mute console hardware
alarms until LDP > 90 mmHg and RDP > 20 mmHg.
• Verify controller is operating normally; connect left ventricle driveline
to controller upon order by surgeon. After left ventricle is connected
and de-aired, await instructions from surgeon to start. To start left
ventricle, raise LDP to about 100 mmHg. You should see a slight
overload of the cardiopulmonary bypass waveform and WCOMDU
may show a small output.
• Connect right ventricle driveline to controller. After right ventricle is
connected and de-aired, await surgeon’s instructions to start the right
ventricle. To start right ventricle, raise RDP to 40 mmHg.
• As the perfusionist begins to slow venous return, SynCardia TAH-t
filling should increase. As filling increases, adjust drive pressures,
heart rate and systolic duration to prevent full fill and to provide full
ejection. Normal range is LDP= 170-210 mmHg, RDP= 60-100
SynCardia Systems, Inc. Page 22
Page 23

SynCardia TAH-t Instructions for Use
mmHg, % Systole=50-60. Be vigilant during the weaning process, you
may need to make rapid adjustments. Observe WCOMDU waveforms
for signs of flow obstruction and other cardiac output information.
• After chest is closed, vacuum may be started (normal range is
approximately 10 mmHg). Do not exceed 30 mmHg vacuum.
• Remove key from the primary controller key switch before moving
patient.
• Pneumatic drive ejection pressures should be set to achieve full
ejection. Pressure tracings on the monitoring computer can be viewed
to assure the right drive pressure is set to overcome the pulmonary
systolic pressure, and the left drive pressure is set to overcome the
aortic systolic pressure.
• The SynCardia TAH-t rate should be set to achieve a stroke volume
between 50 and 65 milliliters on the monitoring computer. SynCardia
TAH-t beat rates should be between 100 and 130 beats per minute.
SynCardia Systems, Inc. Page 23
Page 24

SynCardia TAH-t Instructions for Use
9.0 Explantation Procedures
Explantation of the device should be handled like any other redo cardiac procedure.
Great care should be taken in the separation of the sternum from the device, the great
vessel connector, and the drivelines. Explantation may be easier if the device is
covered with a Gortex membrane.
Cardiopulmonary bypass is initiated with dual caval cannulation with tourniquets, the
aorta is cross-clamped, and the SynCardia TAH-t is turned off. The artificial
ventricles are separated from the atrial inflow cannula. The great vessels outflow
connectors are amputated at the level of the connector/great vessel anastomosis. The
artificial ventricles are transected at the base to the driveline conduit connection, and
the SynCardia TAH-t is removed from the operating field. The driveline conduits are
pulled through the skin. The remaining atria inflow connectors are still in the
remaining portion of ventricular muscle where they were initially sutured. They are
removed by transecting the AV groove throughout. The remaining atria and great
vessels can now be trimmed to accept the donor heart.
SynCardia Systems, Inc. Page 24
Page 25

SynCardia TAH-t Instructions for Use
10.0 System Components
The SynCardia TAH-t system is comprised of the following:
• Implant Kit - Part # 500101 (Sterile)
Contains left artificial ventricle, right artificial ventricle, 2 inflow connectors, 2
outflow connectors, and an ancillary pack with drivelines, inflow pressure test
plug, outflow pressure test plug, locking ties, and 2 de-airing needles (all
sterile). All sterile components are packaged in double aseptic transfer
packages.
• Surgical Spares Kit - Part # 500177 (Sterile)
Contains inflow connector, outflow connector, drivelines, inflow pressure test
plug, outflow pressure test plug, and locking ties.
• Circulatory Support System (External Console) – Part # 400207 (Non-sterile)
• Air Tank - Part # 390004 (Non-sterile)
There should be two complete implant kits, one surgical spares kit, two
circulatory support systems and eight air tanks.
SynCardia Systems, Inc. Page 25
Page 26

SynCardia TAH-t Instructions for Use
Appendix A
Patient Selection and Management
Management and coordination of successful SynCardia TAH-t support requires a
multidisciplinary team that has experience with circulatory support systems. Teams can
include surgeons, cardiologists, heart transplant coordinators, perfusionists, engineers, nurses,
cardiac rehabilitation therapists and coagulation specialists. The following reports the
experience and recommendation of the largest enrolling clinical site, University Medical
Center, Tucson, Arizona.
Patient Selection
Successful bridge to transplant with the SynCardia TAH-t involves selecting patients who are
transplant eligible and who additionally are assessed in two main areas: 1) evaluation of fit of
the SynCardia TAH-t in the patient’s chest, and 2) evaluation of the potential for reversal of
any end organ dysfunction.
Once the SynCardia TAH-t is implanted, and there are no fit issues, flow is maximized
through the TAH-t. The controller nominal settings are: left drive pressure of 180-220
mmHg, right drive pressure of 50-70mmHg, device rate of 110-130 BPM, percent systole of
50-55%, and diastolic vacuum of 8-12 mmHg. With these settings an average device output
of 6.5-7.5 LPM should be achieved, with a CVP of 8-12 mmHg.
The SynCardia TAH-t is specified for patients with body surface areas of at least 1.7 m². At a
cardiac index of 2.5 l/min/m², the calculated flow would be 4.25 liters/min. This is the flow
used to simulate hypotensive conditions tested during product reliability testing. The TAH-t
console is pre-set with an alarm to indicate flows <3.5 l/min.
With normalized hemodynamics, device outputs remain relatively constant, changing as the
CVP fluctuates. This “Starling like response”, (where an increase in CVP fills the SynCardia
TAH-t with more volume, which is ejected on the next beat, increasing device output),
requires no controller adjustments. Constant device output and high flow under normal CVP
provides washing of the artificial ventricles.
Anticoagulation therapy
The level of anticoagulation will vary depending on the patient’s coagulation status. In
general, the patients require systemic anticoagulation, similar to that used for patients with
mechanical valves. The following guidelines are recommended based on the experience of
the largest enrolling clinical site, University Medical Center.
Pre-operative baseline
Obtain results of PT, PTT, bleeding time, TEG, platelet count, platelet aggregation studies
and fibrinogen.
Intra-operative period
Heparinize for CBP per usual routine. Protamine may be used for reversal per usual routine.
SynCardia Systems, Inc. Page 26
Page 27

SynCardia TAH-t Instructions for Use
Post-operative period (immediate)
Start Dipyridamole at 100 mg -250 mg PO or NG every 6 hours. The dose is adjusted to
balance platelet aggregation factors: keep collagen factor positive; keep ADP, epinephrine,
arachnadonic acid factors negative. If all factors are positive, the dose of dipyridamole should
be maximized. If only one factor other than collagen is positive, dipyridamole or ASA is
increased until only collagen is positive. Platelet aggregation studies are checked twice per
week.
Start ASA when platelet aggregation shows any factor other than collagen is positive, usually
within 24 hours post-operative. The ASA dose is started between 81-650 mg PO per day. If
all platelet aggregation factors are positive and the dipyridamole is already started, 325 mg of
ASA is used per day to start. If only one factor, other than collagen, is positive, ASA is
started at 81 mg per day. The dipyridamole is adjusted according to the results of Platelet
Factor 4 and Beta Thromboglobulin. If these tests are elevated, the platelets are very active
and the dipyridamole needs to be increased. ASA is adjusted with the platelet aggregation
and bleeding time studies. The bleeding time is kept between 10 - 20 min if possible.
If the collagen is negative, too much dipyridamole or ASA is being given; daily dosages of
one or both are decreased to prevent bleeding.
Pentoxifylline 400 mg is started PO every 8 hours in the early post-operative period (2-3
days). Pentoxifylline may be increased if fibrinogen increased above normal.
Post-operative (chest tubes pulled)
Start IV Heparin at 25,000 units in 250 cc of D5W at 500-1000 units per hour, when chest
tubes are discontinued. IV Heparin is continued to maintain PTT at 50-55 sec for 2 weeks,
then converted to Coumadin to keep INR 2.5-3.5 or PT 18-22 sec, then IV Heparin is stopped.
Exit Site Management
Take care to keep driveline exit sites clean and dry. Infections should be treated according to
hospital protocol.
SynCardia Systems, Inc. Page 27
Page 28

SynCardia TAH-t Instructions for Use
Appendix B
Outline of Training Program
Operation of this device should only be undertaken by personnel trained in accordance with
the SynCardia Training Program. The training will include the following topics:
1) Indications and Contraindications
2) System Overview
3) Implant Procedures
4) Operation of the console
5) Explant Procedures
6) Patient Management
7) Summary of Clinical Studies
8) Animal Procedure – a minimum of one implant needs to be performed.
9) Practical Experience - Physicians will be required to minimally view
one live implant procedure or have their first procedure proctored.
SynCardia will maintain centers of excellence where surgeons may
view implantations. Further, proctors will be made available by
SynCardia for surgical teams during their first case.
SynCardia Systems, Inc. Page 28
Page 29

SynCardia TAH-t Instructions for Use
Appendix C
Materials Matrix
The SynCardia TAH-t ventricle components are manufactured from the raw materials as
defined in the matrix below. The artificial ventricles have met the test requirements of ISO
10993, Biological Evaluation of Medical Devices.
SynCardia TAH-t Patient Contacting Materials Matrix
Component Material
Ventricle and diaphragm Segmented polyurethane
Nylon
Inflow connector Segmented polyurethane
Polyester fabric
Outflow connector Segmented polyurethane
Polyethylene material
Valves
Drivelines Polyvinyl chloride tubing
©2005-2010 Syncardia Systems, Inc.
Titanium and pyrolitic carbon
(Medtronic Hall Heart Valves)
SynCardia Systems, Inc. Page 29
 Loading...
Loading...