Swiss Bionic Solutions Omnium1 iMRS one 2.0 Operating Manual

intelligent Lifestyle
Operating Manual
2.0

2
Important Notes for Getting Started
We congratulate you on your purchase of the iMRS one magnetic resonance stimulation system.
In combination with the Omnium1 control device, the iMRS one represents the latest development and application standard in the eld of magnetic resonance stimulation systems for home use.
The iMRS one is a system for use at home. In medical use, it can also play
an accompanying and supportive role for a number of conditions.
The iMRS one complies with the following guidelines and standards:
• Medical Product Guidelines (MPG)
• IEC 60601-1 Electrical Safety
• IEC 60601-1-2 Tolerance of Electromagnetic Fields
This operating manual is a component of the scope of delivery.
It should be kept close at hand and remain with the system when sold.
Copyright
Copyright © 2019 Swiss Bionic Solutions Holding GmbH
All rights reserved.
No part of this manual, including the products and software described
herein, may be reproduced, transferred, transcribed, stored in a retrieval
system or translated into another language, without the implicit written
permission of Swiss Bionic Solutions Holding GmbH. Documentation
stored by the purchaser for backup purposes is excluded from this condition. This condition shall not apply for software that has been licensed
under the General Public License (GPL) or other free open source licensing systems.
Omnium1, the Omnium1 logo and the iMRS Logo are trademarks of Swiss
Bionic Solutions Holding GmbH. All other trademarks are the property of
their corresponding owners. The details of the content of this manual
may deviate from the product or the associated software. All information
in this document may be changed without prior notication.

3
Table of Contents
1. Safety Instructions: Where You should be Careful ................................ 4
1.1 Contra-indications ................................................................................. 9
1.2 Side Effects ............................................................................................ 9
2. Intended Use ......................................................................................... 10
3. Possible Applications ............................................................................ 10
4. Scope of Delivery ................................................................................... 11
4.1 Control Unit ......................................................................................... 12
5. Installation ............................................................................................. 13
5.1 Installing / removing the battery.........................................................13
6. Activation ............................................................................................... 14
7. Saving and Loading Pre-set Parameters .............................................. 15
7.1 Saving ................................................................................................... 15
7.2 Loading ................................................................................................ 16
8. Starting an Application.......................................................................... 17
9. Quick Start Programs ............................................................................ 18
10. Settings ................................................................................................ 18
10.1 About ................................................................................................. 18
10.2 Factory Settings ................................................................................. 19
10.3 Users .................................................................................................. 19
11. Updating .............................................................................................. 19
12. Applicators ........................................................................................... 19
12.1 OmniMat ............................................................................................ 19
12.2 OmniPad ............................................................................................ 20
12.3 OmniSpot ........................................................................................... 20
13. Cleaning and Maintenance ................................................................. 21
14. Maintenance & Error Message ........................................................... 22
15. Identication & Technical Data .......................................................... 23
15.1 Identication ..................................................................................... 23
15.2 Technical Data ................................................................................... 23
16. Warranty .............................................................................................. 25

4
1. Safety Instructions: Where You
should be Careful
If you feel dizzy, be careful when standing up!
There are no negative reports on the application of Magnetic-Resonance-Stimulation anywhere in the world. However, for reasons of
safety, we recommend that people with orthostatic problems (dizziness
when getting up) get up very slowly and carefully after application.
Avoid humidity:
This device may not be positioned in a damp or wet room!
The strength of the eld of magnetic resonance stimulation system also
corresponds to no more than 120 μT at its highest intensity setting.
Thereby, it is well under the values of popular electrical household appliances and well under the recommended threshold of 400 μT for harmlessness according to DIN 0848.
The fact that these values are in the range of the Earth’s owns natural
magnetic elds and the frequencies in the ionosphere (Schumann resonances) exclude all possible danger.
Note about electromagnetic tolerance (EMC)
Medical, electric devices are subject to special precautions in terms
of EMC and must be installed and placed in operation in accordance
with the EMC instructions in the included documents. Electro-medical
devices are particular susceptible to the radio frequencies of portable
and mobile communications equipment, such as cell phone phones and
walkie-talkies.
The manufacturer only guarantees the compliance of the device
with the EMC requirement when used with the accessories listed
in 14.2. The use of other accessories may lead to increased emissions of electromagnetic interference or to reduced resistance
to such interference. The accessories listed may only be used
together with an Omnium1 control system from Swiss Bionic
Solutions Schweiz GmbH.
The device may not be operated in combination with other devices, nor
be placed in a stack of, or located in the proximity, to such other devices.
5
However, if such an arrangement is necessary, the operation of the
device must be checked to ensure that it will operate as intended when
stored in this manner.
The expansion of the magnetic eld from the applicators will have largely
abated at a distance of about 1.5 meters. People who are not receiving
treatment should remain outside the indicated range during the course
of an application.
We are legally obliged, in accordance with the EMC regulations for
medical products, to provide you with the following information.
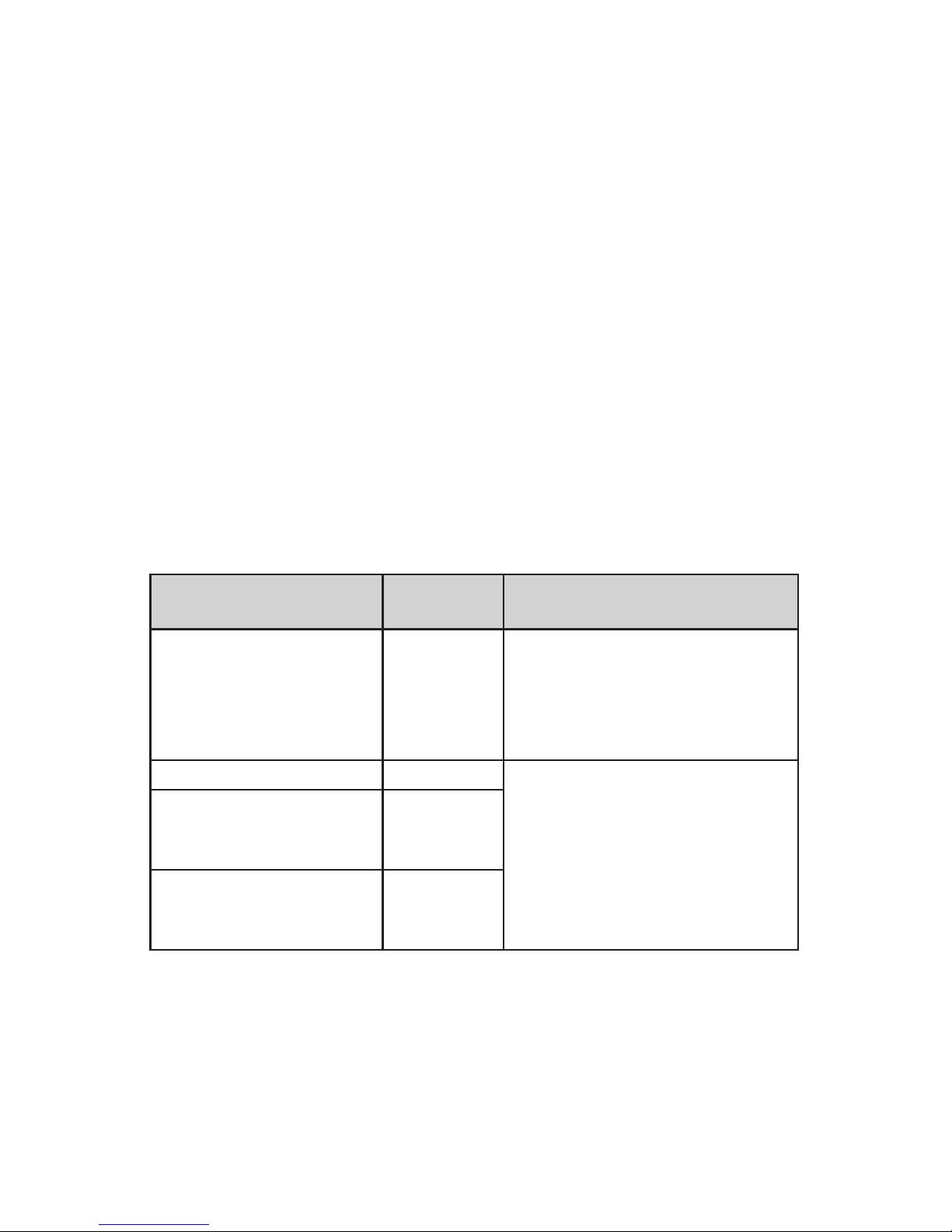
Guidelines and Manufacturer‘s Declaration: Electromagnetic Interference Emissions
The iMRS one is intended for operation in an ELECTROMAGNETIC ENVIRONMENT
as shown below. The customer or user of the iMRS one should ensure that it is
operated in such an environment.
Interference emission mea-
surements
Compliance Electromagnec Environment Gui-
deline
RF emissions acc. to CISPR 11 Group 1 The iMRS one only uses RF energy for
its internal OPERATION. Its RF emis-
sion is therefore very low and it is
unlikely to interfere with neighboring
electronic devices.
RF emissions acc. to CISPR 11 B The iMRS one is suitable for use in
all establishments including those
in residenal, and similar areas that
are directly connected to the PUBLIC
POWER GRID that also supplies buildings used for residenal purposes.
Emission of harmonic
frequencies according to IEC
61000-3-2
A
Emission of voltage uctuaons or icker according to
IEC 61000-3-3
In compliance

6
Interference
Immunity Tests
IEC 60601 Test
Level
Compliance Level Electromagnetic Environ-
ment - Guidelines
Discharge of
static electricity
(ESD)
Acc. to IEC 610004-2
± 6 kV contact
discharge
± 8 kV air discharge
± 6 kV contact
discharge
± 8 kV air discharge
Floors should be made
of wood or concrete or
covered in ceramic tiles. If
the oor is covered with
synthetic material, the
relative air humidity must
be at least 30 %.
Fast-transient
interference test/
Bursts acc. to IEC
61000-4-4
± 2 kV for power
supply lines
± 1 kV for input
and output lines
± 1 kV for power
supply lines
Not applicable
The quality of the supply
voltage should correspond
to that in typical business
or hospital surroundings.
Surges acc. to IEC
61000-4-5
± 1 KV voltage
outer conductor-outer
conductor
± 2 kV voltage
outer conductor
- ground
± 1 KV voltage
outer conductor-outer conductor
Not applicable
The quality of the supply
voltage should correspond
to that in typical business
or hospital surroundings.
Voltage dips,
short interruptions and
uctuations in the
supply voltage
acc. to IEC 610004-11
< 5 % Ut
< (> 95 % dip in
Ut) for 1/2 period
40 % Ut (60 %
dip in Ut) for 5
periods
70 % Ut (30 %
dip in Ut) for 25
periods
< 5 % Ut (> 95
% dip in Ut) for
5 sec
0 % Ut
< (> 95 % dip in
Ut) for 1/2 period
40 % Ut (60 %
dip in Ut) for 5
periods
70 % Ut (30 %
dip in Ut) for 25
periods
0 % Ut (> 95 %
dip in Ut) for 5
sec
The quality of the supply
voltage should correspond
to that in typical business
or hospital surroundings. If
the iMRS one user requires
continuous operation,
even when interruptions
in the power supply occur,
supplying the iMRS one
from an uninterruptible
power supply or a battery
is recommended.
Magnetic eld
at a supply
frequency (50/60
Hz) acc. to IEC
61000-4-8
3 A/m 3 A/m Magnetic elds at power
grid frequency should correspond to values typical
for a business or hospital
environment.
Comment: Ut is the AC power voltage before the application of the test level.

7
Guidelines and Manufacturer’s Declaration:
Electromagnetic Stability Interference
The iMRS one is intended for operation in an ELECTROMAGNETIC ENVIRONMENT
as indicated below. The customer or iMRS user should ensure that it is operated
in such an environment.
Interference
Immunity Tests
IEC 60601
Test Level
Compliance
Level
Electromagnetic Environment - Guidelines
Conducted RF
interference
acc. to IEC
610004-4-6
Radiated RF
interference
acc. to IEC
610004-4-3
3 V effective value
150 kHz up
to 80 MHz
3 V/m
80 MHz up
to 2.5 GHz
3V Portable and mobile radio devices, includ-
ing their cables, should not be used at
a distance closer to the iMRS one than
recommended, which has been calculated
according to relevant equation for the transmission frequency.
Recommended safe distance:
for 80 MHz up to 800 MHz
for 800 MHz up to 2.5 GHz
Where P is the rated power of the transmitter in watts (W) according to the information
from the transmitter manufacturer and d is
the recommended safe distance in meters
(m). The eld strength of stationary radio
transmitters should be investigated locally
for all frequencies lower than the compliance level
6
Interference is possible in proximity
to devices that bear the following
symbol.
NOTE 1 The higher frequency range applies at 80 MHz and 800 MHz.
NOTE 2 These guidelines may not apply in all cases. The expansion of electromagnetic
quantities will be affected by absorption and reection from buildings, objects and
people.
The eld strength of stationary transmitters, such as: the base stations of cordless
telephones and land mobile radio systems, amateur radio stations, AM/FM radio and
television transmitters; cannot be determined in advance with theoretic precision. A
study of the electromagnetic phenomena of the location should be considered in order
to determine the nature of the ELECTROMAGNETIC SURROUNDINGS in terms of sta-
tionary transmitters. If the eld strength measured at the location where the iMRS one
will be used exceeds the COMPLIANCE LEVEL mentioned above, the iMRS one should
be checked to verify its OPERATION in the manner intended. If unusual performance
characteristics are observed, additional measures may necessary, such as changing the
orientation or choosing a different location for the iMRS one.
The eld strength should be less than 3V/m over the frequency range 150 kHz to 80
MHz.
6)
National footnote: User here is meant in the sense of RESPONSIBLE ORGANISATION.
d = 1,2√P
d = 1,2√P
d = 2,3√P

8
Recommended Safe Distances between Portable or Mobile RF Telecommunication Devices and the iMRS one
The iMRS one is intended for operation in an ELECTROMAGNETIC ENVIRONMENT
in which radio frequency interference is controlled. The customer or the iMRS
one user can help with the avoidance of electromagnetic interference by main-
taining the minimum distance between portable and mobile radio frequency
telecommunication devices (transmitters) and the iMRS one, depending on the
output power of the communication device as indicated below.
Nominal rating of
the transmitter W
Safe distance (m) dependent on transmitter frequency
150 kHz to 80 MHz 80 MHz to 800 MHz 800 MHz to 2,5 GHz
0,01 0,12 0,12 0,23
0,1 0,38 0,38 0,73
1 1,2 1,2 2,3
10 3,8 3,8 7,3
100 12 12 23
For transmitters whose maximum nominal output is not indicated in the table
above, the recommended safe distance of d in meters (m) should be determined
from the equation associated with the particular column, where P is the maximum nominal output of the transmitter in watts (W) according to information
from the manufacturer.
NOTE 1: The higher frequency range applies at 80 MHz and 800 MHz.
NOTE 2: These guidelines may not apply in all cases. The expansion of electro-
magnetic quantities will be affected by absorption and reection from buildings,
objects and people.

9
1. 1. Contraindications
Use of the iMRS one system is contraindicated for the following conditions:
• Pregnancy
• Epilepsy
• Electronic implants such as pace makers or insulin pumps
(with the exception of approval by the consulting physician)
The iMRS one system may only be used with the approval of a health care
practitioner and under medical supervision under the following conditions:
• Presence of tumors
• Serious cardiac arrhythmia
• Acute attacks of hyperthyroidism
• Extreme sensitivity to electromagnetic radiation
In principle:
Magnetic resonance stimulation does not replace medical therapy. Always
consult your doctor rst about any unfamiliar complaints.
1. 2. Side Effects
In therapeutic treatment of chronic cases, a so-called initial worsening (healing
reaction) arises in approximately 10% of the patients treated in the rst days or
weeks of application, such as through an increase in the symptoms. This should
be frequently expected after prolonged medication, which should be interpreted
as a side effect of the medicinally induced regulatory habit and of the transfer
process to the activation of self-regulation.
A light itching on the body or a warm feeling may be felt in the prophylactic use.
In exactly the same manner, bruises, cramps, strains, wounds and problems with
the bones, joints, teeth or jaw may make themselves known as light pain as a
consequence of the activation of the circulatory system. In all cases in which previously unnoticed physical reactions become noticeable as an accompaniment
of the application, consultation with a doctor or therapist with experience in the
application of magnetic resonance stimulation is recommended for purposes of
safe clarication.
 Loading...
Loading...