Summit Doppler LifeDop L250, LifeDop L150, LifeDop L150A, LifeDop L250AR, LifeDop L150R User Manual
...
User Manual for LifeDop®
L150 Series (Handheld Doppler without Display)
L250 Series (Handheld Doppler with Display)
REF
L150, L150A, L150R, L250, L250AR, L250R,
MC150, MC150R, MC250, MC250R
MAN0001-DFU • Rev. A • 11/12 Wallach Surgical Devices

Thank you for choosing LifeDop® Doppler products. We believe you have purchased the finest handheld
Doppler on the market today. Your total satisfaction is our highest priority as we strive to continually
improve our products and services. Please contact us with any suggestions. We look forward to enjoying
a long-term relationship with you!
Wallach Surgical Devices
95 Corporate Drive
Year of manufacture
located on the device.
Trumbull, CT 06611 USA
Here’s how you can reach us...
Phone: 1-800-243-2463
(203) 799-2000
Fax: (203) 799-2002
e-mail us at: customerservice@wallachsurgical.com
visit our website at: www.wallachsurgical.com
Table of Contents:
Page
Intended Use/Contraindications/Warnings............................................................................ 2
Description of Product........................................................................................................... 4
Operation and Installation ..................................................................................................... 6
Obtaining Doppler Signals ................................................................................................... 12
Maintenance and Cleaning ................................................................................................... 14
Replacing Batteries............................................................................................................... 15
Troubleshooting.................................................................................................................... 16
Clinical References............................................................................................................... 17
Accessories........................................................................................................................... 17
Specifications ....................................................................................................................... 18
Warranty and Servicing Policy............................................................................................. 21
Explanation of Symbols ....................................................................................................... 22
Please read the manual carefully and become familiar with the operation, features and maintenance of
your LifeDop prior to using the device or accessories.
(This CE mark excludes the Medichoice® models: MC150, MC150R, MC250, MC250R)
MAN0001-DFU • Rev. A • 11/12 1 Wallach Surgical Devices

Intended Use
Obstetric (2 and 3 MHz Probes)
This product will be used to detect fetal heart beats as an aid for determining fetal viability.
Vascular (4, 5 and 8 MHz Probes)
This product will be used to detect blood flow in veins and arteries for assisting in the detection of
peripheral vascular disease.
Caution
• U.S. Federal law restricts this device to sale by or on the order of a licensed practitioner.
Contraindications
Warnings
• The vascular probes (4, 5 and 8 MHz) are not for fetal use.
• The ultrasound probes are not to be used on or near the eyes.
• The device is for use only on intact skin.
• Do not plug any part of this device into a telephone or modem system.
• This device is not intended for use with HF surgical equipment.
If there are questions or concerns regarding these warnings or contraindications, please do not hesitate to
contact Wallach Surgical Devices for further clarification.
Caution
• Dropping the LifeDop, probe or accessories may cause damage to the housing or electronics.
In order to preserve, protect and improve the quality of the environment, protect human health
and utilize natural resources prudently and rationally – do not dispose of waste electrical or
electronic equipment (WEEE) as unsorted municipal waste. Contact local WEEE disposal sites.
Safety of Ultrasound
LifeDop Dopplers were designed with physician and patient safety in mind. In early design phases all
potential hazards were eliminated or reduced to As Low As Reasonably Achievable (ALARA) by
adhering to good design practices and industry wide safety standards. Ultrasound procedures should be
performed with the ALARA principle in mind when delivering ultrasound energy into the body.
MAN0001-DFU • Rev. A • 11/12 2 Wallach Surgical Devices

The following official statements from the American Institute of Ultrasound Medicine (AIUM) are provided for
your general information regarding the safe use of ultrasound.
Clinical Safety
Approved March 1997, October 1982
Diagnostic ultrasound has been in use since the late 1950s. Given its known benefits and recognized
efficacy for medical diagnosis, including use during human pregnancy, the American Institute of
Ultrasound in Medicine herein addresses the clinical safety of such use:
There are no confirmed biological effects on patients or instrument operators caused by exposures from
present diagnostic ultrasound instruments. Although the possibility exists that such biological effects may
be identified in the future, current data indicate that the benefits to patients of the prudent use of
diagnostic ultrasound outweigh the risks, if any, that may be present.
Prudent Use
Approved May 1999
The AIUM advocates the responsible use of diagnostic ultrasound. The AIUM strongly discourages the
non-medical use of ultrasound for psychosocial or entertainment purposes. The use of either twodimensional (2D) or three-dimensional (3D) ultrasound to only view the fetus, obtain a picture of the
fetus or determine the fetal gender without a medical indication is inappropriate and contrary to
responsible medical practice. Although there are no confirmed biological effects on patients caused by
exposures from present diagnostic ultrasound instruments, the possibility exists that such biological
effects may be identified in the future. Thus ultrasound should be used in a prudent manner to provide
medical benefit to the patient.
Safety in Training and Research
Approved March 1997, March 1983
Diagnostic ultrasound has been in use since the late 1950s. There are no confirmed adverse biological
effects on patients resulting from this usage. Although no hazard has been identified that would preclude
the prudent and conservative use of diagnostic ultrasound in education and research, experience from
normal diagnostic practice may or may not be relevant to extended exposure times and altered exposure
conditions. It is therefore considered appropriate to make the following recommendation:
In those special situations in which examinations are to be carried out for purposes other than direct
medical benefit to the individual being examined, the subject should be informed of the anticipated
exposure conditions, and of how these compare with conditions for normal diagnostic practice.
MAN0001-DFU • Rev. A • 11/12 3 Wallach Surgical Devices

Description of Product
The LifeDop Doppler is factory configurable to include many different features and product
enhancements. Along with user interchangeable ultrasound transducers, the LifeDop Doppler device is
well suited to meet your specific needs.
Main Unit
The main handheld unit is ergonomically designed to fit the palm of your hand with comfort and allow
easy access to each control feature. Each unit is individually tested and inspected to ensure the highest
quality standards.
SSQ (available in all models) – Superior Sound Quality. Every LifeDop Doppler is designed with a state
of the art sound system that produces excellent sound quality and long-term reliability.
Recharge (available in model numbers with “R”) – We offer the ease of use of a rechargeable system or
the attractive cost of a non-rechargeable unit. Either way, the LifeDop Doppler battery system has been
designed with your long-term battery life needs in mind.
LCD Display (L250 Series models) – The LCD display (optional) allows you to view the fetal heart rate
in larger easy to read digits, monitor battery life and battery recharging, observe signal strength
indicators, Play/Record functions, and multiple diagnostic indicators that ensure your unit is functioning
at peak performance levels.
Units without LCD display incorporate bright, easy to read LED indicators that also allow you to monitor
battery life, battery recharging and Play/Record functions as appropriate to your unit.
Record (available in model numbers with “A”) – This unique built-in enhancement allows you to record
Doppler sounds during an examination and refer to these sounds for later evaluation, replay for parents or
colleagues, or download the audio to a PC for permanent storage or e-mail. NOTE: Downloading is
“allowed” in those models with “AR”.
MAN0001-DFU • Rev. A • 11/12 4 Wallach Surgical Devices

Probes
LifeDop Doppler ultrasound transducers were designed to meet your specific applications needs. Each
probe has been ergonomically designed for comfort while providing excellent maneuverability for
locating the fetus or vascular target.
Each probe is carefully measured and tested to ensure it meets exacting performance standards.
2 MHz - Late term obstetrical examination. This probe frequency is typically used during the last
trimester for deep fetal positions associated with larger women. Waterproof versions of the 2 MHz probe
are available.
3 MHz – Early and general-purpose obstetrical examination. This probe frequency is a general use model
ideal for most stages of fetal examination. Fetal heart sounds can be heard as early as 12 weeks and
sometimes sooner depending on the position and size of the fetus.
4 MHz Broad – This unique peripheral vascular probe is ideal for quickly locating brachial, radial and
ankle arteries in the performance of Ankle/Brachial Index testing. The broad beam of the 4 MHz probe
allows the user to place the probe over the general location of the artery and with very little movement
find the vessel for fast blood pressure measurements.
5 MHz – This standard “pencil” style probe is an excellent vascular tool for locating deep specific vessels
in the peripheral vascular system. The narrow grip and small face of the probe make it ideal for
maneuvering for maximizing the signal.
8 MHz – This standard “pencil” style probe is an excellent vascular tool for locating shallow specific
vessels in the peripheral vascular system. The narrow grip and small face of the probe make it ideal for
maneuvering for maximizing the signal.
MAN0001-DFU • Rev. A • 11/12 5 Wallach Surgical Devices
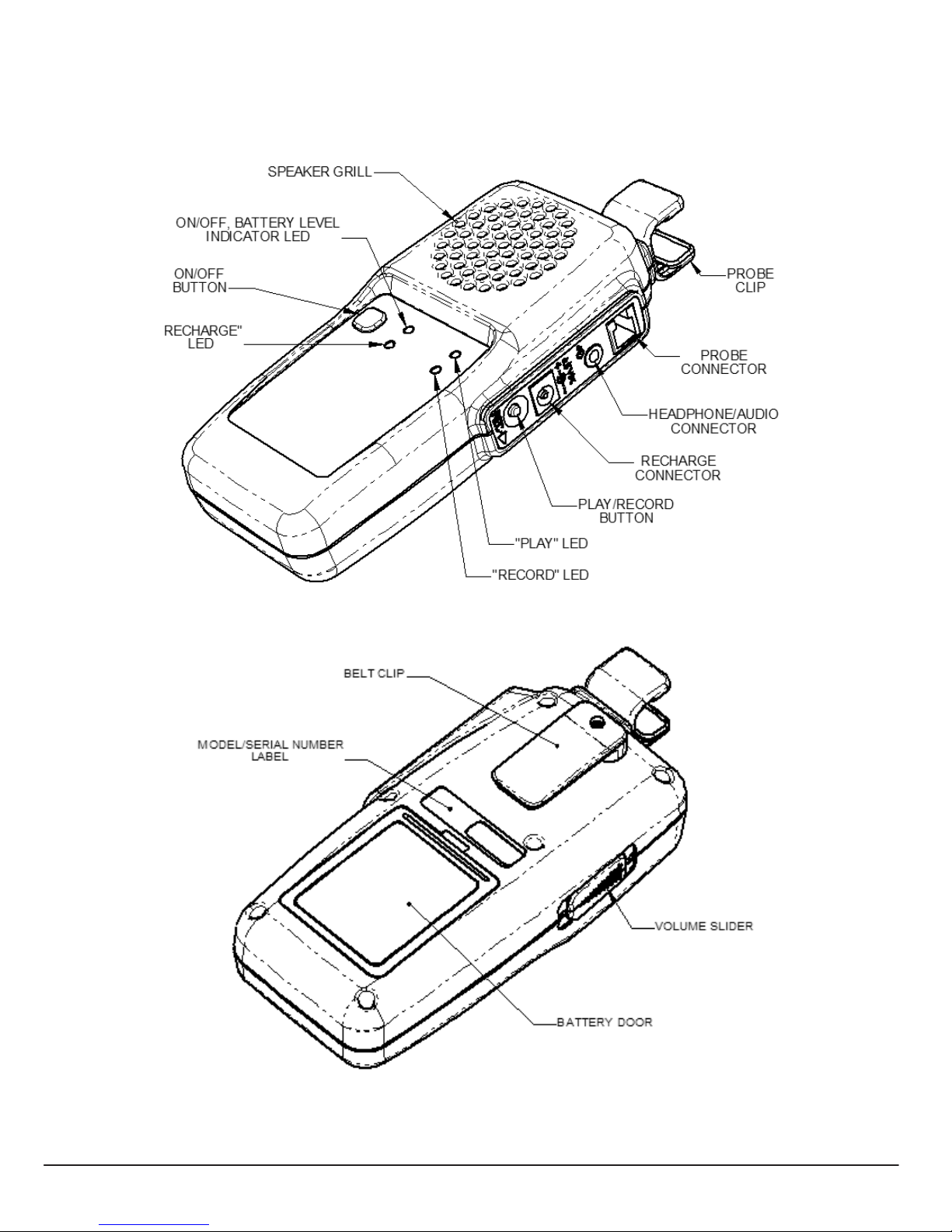
Operation and Installation
MAN0001-DFU • Rev. A • 11/12 6 Wallach Surgical Devices
 Loading...
Loading...