
DiagCORE® Analyzer
User manual
Software version 1.0
PL-009-05/2018-EN
110002 OM
110001 AM
2 DiagCORE Analyzer User Manual
This document is to be used solely for the purpose of operating the DiagCORE Analyzer. No part of
this document may be reproduced or transmitted by any means, whether electronic or
mechanical, or for any purpose, without the express written permission of STAT-Dx Life, S.L.
Always maintain the DiagCORE Analyzer in good working order. Using the DiagCORE Analyzer in a
manner not specified by STAT-Dx Life, S.L. may impair protection provided by the equipment.
A printed version of this manual is available upon request.
STAT-Dx Life, S.L.
Baldiri Reixac 4
08028 Barcelona, Spain
Customer and technical support
If assistance is required, contact Technical Support using the contact information below.
Website: http.support.qiagen.com
When calling Technical Support about errors, please have the following information ready:
DiagCORE Analyzer serial number, type and version
Error code (if applicable)
Time point when the error occurred for the first time
Frequency of error occurrence (i.e., intermittent or persistent error)
Photo of error, if possible
Copy support package
110001 Analytical Module
110002 Operational Module

DiagCORE Analyzer User Manual 3
Contents
Customer and technical support ............................................................................. 2
Contents ...................................................................................................................... 3
Table of symbols ......................................................................................................... 6
1 Information about this user manual ............................................................ 7
1.1 Revision ................................................................................................................ 7
1.2 Intended use of the DiagCORE Analyzer ....................................................... 7
1.3 Limitations of use ................................................................................................ 7
1.4 Sections of the user manual ............................................................................. 8
2 Safety information ......................................................................................... 9
2.1 General safety precautions .............................................................................. 9
2.2 DiagCORE Analyzer transport precautions .................................................. 10
2.3 Electrical safety................................................................................................. 10
2.4 Chemical safety ............................................................................................... 10
2.5 Biological safety ............................................................................................... 11
2.6 Waste disposal .................................................................................................. 12
3 General background .................................................................................. 13
3.1 System description ........................................................................................... 13
3.2 DiagCORE Analyzer description ..................................................................... 13
3.3 DiagCORE cartridge description ................................................................... 14
4 Installing the DiagCORE Analyzer .............................................................. 16
4.1 Site requirements .............................................................................................. 16
4.2 What is included with the DiagCORE Analyzer ............................................ 17
4.3 Unpacking and installing the DiagCORE Analyzer ...................................... 18
4.4 Repackaging and shipping the DiagCORE Analyzer ................................. 22
5 Running a test and viewing results ............................................................ 23
5.1 Starting the DiagCORE Analyzer .................................................................... 23
5.2 Preparing the cartridge ................................................................................... 23
5.3 Procedure to run a test .................................................................................... 23
5.3.1 Instructions to abort a test ................................................................... 28
5.4 Viewing results ................................................................................................... 28
5.4.1 Viewing amplification curves ............................................................. 30

4 DiagCORE Analyzer User Manual
5.4.2 Viewing melting curves........................................................................ 31
5.4.3 Viewing test details .............................................................................. 32
5.4.4 Browse results from previous tests ....................................................... 33
5.4.5 Exporting results to a USB storage device ......................................... 34
5.4.6 Printing results ........................................................................................ 34
6 System reference ........................................................................................ 35
6.1 Main screen ....................................................................................................... 35
6.1.1 General status bar ................................................................................ 35
6.1.2 Module status bar ................................................................................. 36
6.1.3 Main Menu bar ..................................................................................... 37
6.1.4 Content area ........................................................................................ 38
6.2 Login screen ...................................................................................................... 38
6.3 Logging out ....................................................................................................... 40
6.4 Screensaver ....................................................................................................... 40
6.5 Options menu ................................................................................................... 40
6.6 User management ........................................................................................... 41
6.6.1 Accessing and managing the list of users ........................................ 42
6.6.2 Adding users .......................................................................................... 44
6.7 Assay management ........................................................................................ 45
6.7.1 Managing available assays ................................................................ 45
6.7.2 Importing new assays ........................................................................... 46
6.8 Configuring the DiagCORE Analyzer system ................................................ 47
6.8.1 Regional settings ................................................................................... 47
6.8.2 General settings .................................................................................... 49
6.8.3 Printer settings ....................................................................................... 51
6.8.4 Network settings .................................................................................... 51
6.8.5 HIS/LIS settings ....................................................................................... 53
6.8.6 System log .............................................................................................. 53
6.8.7 Technical log ......................................................................................... 54
6.8.8 Version information .............................................................................. 54
6.8.9 System update ...................................................................................... 54
6.8.10 System backup ..................................................................................... 55
6.9 Change passwords .......................................................................................... 56
6.10 DiagCORE Analyzer status .............................................................................. 56
6.11 Shutting down the instrument ......................................................................... 57

DiagCORE Analyzer User Manual 5
7 Maintenance ............................................................................................... 58
7.1 Maintenance tasks ........................................................................................... 58
7.2 Cleaning the DiagCORE Analyzer surface ................................................... 58
7.3 Decontaminating the DiagCORE Analyzer surface .................................... 59
7.4 Replacing the air filter ...................................................................................... 60
7.5 DiagCORE Analyzer repair .............................................................................. 60
8 Troubleshooting ........................................................................................... 61
8.1 Errors and warning messages ......................................................................... 63
9 Appendices ................................................................................................. 67
9.1 Technical specifications .................................................................................. 67
9.2 Disposal information ......................................................................................... 67
9.3 Declaration of Conformity .............................................................................. 68
9.4 Glossary .............................................................................................................. 68
9.5 Trademarks and copyright .............................................................................. 68
9.6 Copyright, disclaimer and warranty .............................................................. 68
9.7 Patent statement ............................................................................................. 69
9.8 Software License Agreement ......................................................................... 69
10 Index .......................................................................................................... 71

6 DiagCORE Analyzer User Manual
Table of symbols
The table below describes various symbols used throughout this user manual and on the DiagCORE
Analyzer instrument and assay cartridges.
Location
Description
Type plate on the back of the
instrument
CE marking for European Conformity
Type plate on the back of the
instrument
TÜV mark of the TÜV SÜD Product
Service for testing.
Type plate on the back of the
instrument
Legal manufacturer
Type plate on the back of the
instrument
Caution
HAZARD: Risk of personal injury and
material damage
Type plate on the back of the
instrument
WEEE mark for Europe
Type plate on the back of the
instrument
FCC mark of the United States Federal
Communications Commission
Type plate on the back of the
instrument
In vitro diagnostic medical device
Type plate on the back of the
instrument
Catalog number
Type plate on the back of the
instrument
Serial number

DiagCORE Analyzer User Manual 7
1 Information about this user manual
Read this user manual carefully and pay particular attention to the safety information before
operating the DiagCORE Analyzer. The instructions and safety information in this user manual must
be followed to ensure safe operation of the instrument and to maintain the instrument in a safe
condition.
1.1 Revision
This is the DiagCORE Analyzer User Manual, version 1.0. This manual is for use with the DiagCORE
Analyzer 1.0 and DiagCORE Application Software version 1.0.
1.2 Intended use of the DiagCORE Analyzer
The DiagCORE Analyzer is intended for in vitro diagnostic use and provides diagnostic results. All
analytical steps are fully automated using molecular diagnostic DiagCORE application cartridges
and real-time PCR detection.
The DiagCORE Analyzer system is intended for professional use only and is not intended for selftesting.
1.3 Limitations of use
The DiagCORE Analyzer can only be used with DiagCORE cartridges according to the
instructions contained in this user manual and in the DiagCORE cartridge instructions for use.
When connecting the DiagCORE Analyzer, use only the cables supplied with the system.
Any service or repairs should only be performed by personnel authorized by STAT-Dx Life, S.L.
The DiagCORE Analyzer should only be operated if it is on a flat, horizontal surface with no
angles or tilts.
Do not re-run a DiagCORE assay cartridge if it has already been used successfully, or if it has
been associated with an error or an incomplete run.
Allow at least 10 cm clearance on each side of the DiagCORE Analyzer to ensure adequate
ventilation.
Make sure that the DiagCORE Analyzer is positioned away from any air conditioning outlets or
heat exchangers.
Do not move the instrument while a test is running.
Do not change the system configuration during a run.
Do not use the touchscreen to lift or move the DiagCORE Analyzer.

8 DiagCORE Analyzer User Manual
1.4 Sections of the user manual
This user manual provides information about the DiagCORE Analyzer in the following sections:
1. Information about this user manual
2. Safety information
3. General background
4. Installing the DiagCORE Analyzer
5. Running a test and viewing results
6. System reference
7. Maintenance
8. Troubleshooting
9. Appendices
The appendices include the following:
Technical specifications
Disposal information
Declaration of Conformity
Glossary
Trademarks and copyright
Copyright, disclaimer and warranty
Patent statement
Software License Agreement

DiagCORE Analyzer User Manual 9
2 Safety information
Before using the DiagCORE Analyzer, it is essential to read this user manual carefully and pay
particular attention to the safety information. The instructions and safety information in the user
manual must be followed to ensure safe operation of the instrument and to maintain the instrument
in a safe condition.
The following types of safety information appear throughout this manual.
WARNING
The term WARNING is used when referring to situations that could result in
personal injury to the operator or others.
Details about the circumstances are provided in a message like this one.
CAUTION
The term CAUTION is used when referring to situations that could result in
material damage to the DiagCORE Analyzer or other equipment.
Details about the circumstances are provided in a message like this one.
IMPORTANT
The term IMPORTANT is used to highlight information that is critical for the
completion of a task or optimal performance of the system.
NOTE
The term NOTE is used for information that explains or clarifies a specific
case or task.
The advice provided in this user manual is intended to supplement, not supersede, the normal
safety requirements prevailing in the user’s country.
2.1 General safety precautions
Use the DiagCORE Analyzer according to this manual. It is highly recommended to carefully read
and become acquainted with the instructions for use before using the DiagCORE Analyzer.
Follow all safety instructions printed on, or attached to, the DiagCORE Analyzer.
Improper use of the DiagCORE Analyzer or failure to comply with its proper installation and
maintenance may cause personal injuries or damage to the DiagCORE Analyzer.
The DiagCORE Analyzer must only be operated by qualified and appropriately trained
healthcare personnel.
Servicing of the DiagCORE Analyzer must only be performed by Field Service Specialists
authorized by STAT-Dx Life, S.L.
Do not use the DiagCORE Analyzer in hazardous environments for which it has not been
designed.
10 DiagCORE Analyzer User Manual
WARNING
CAUTION
Risk of personal injury and material damage
Do not open the housing of the DiagCORE Analyzer. The housing of the
DiagCORE Analyzer is designed to protect the operator and to ensure
proper operation of the DiagCORE Analyzer. Using the DiagCORE Analyzer
without the housing leads to electrical hazards and DiagCORE Analyzer
malfunction.
WARNING
CAUTION
Risk of personal injury and material damage
Use caution when the lid of the cartridge entrance port closes to avoid
personal injury, such as pinched fingers.
2.2 DiagCORE Analyzer transport precautions
WARNING
CAUTION
Risk of personal injury and material damage
The DiagCORE Analyzer is a heavy instrument. To avoid personal injury or
damage to the DiagCORE Analyzer, take care when lifting it and use
appropriate lifting methods.
2.3 Electrical safety
Observe all general safety precautions that apply to electrical instruments.
Disconnect the line power cord from the power outlet before servicing.
WARNING
Electrical hazard
Lethal voltages inside the DiagCORE Analyzer. Do not open the housing
of the DiagCORE Analyzer.
The line power cord must be connected to a line power outlet that has
a protective conductor (earth/ground).
Do not touch any switches or power cords with wet hands.
Do not use the instrument outside of the specified power conditions.
2.4 Chemical safety
Material safety data sheets for the cartridge materials are available and can be requested from
STAT-Dx Life, S.L.
Used cartridges must be disposed of in accordance with all national, state and local health and
safety regulations and laws.

DiagCORE Analyzer User Manual 11
WARNING
Hazardous chemicals
Chemicals may leak from the cartridge in the event that the cartridge
housing is damaged. Some chemicals used in DiagCORE cartridges may
be hazardous or may become hazardous. Always wear eye protection,
gloves and a lab coat.
CAUTION
Risk of damage to the DiagCORE Analyzer
Avoid spilling chemicals or other liquids into or out of the DiagCORE
Analyzer. Damage caused by liquid spillage will void the warranty.
2.5 Biological safety
The DiagCORE Analyzer and cartridges do not themselves contain biohazardous materials,
however, samples and reagents containing materials from biological sources should generally be
handled and disposed of as potentially biohazardous. Use safe laboratory procedures as outlined
in publications such as Biosafety in Microbiological and Biomedical Laboratories, from the Centers
for Disease Control and Prevention and the National Institutes of Health
(www.cdc.gov/od/ohs/biosfty/biosfty.htm).
Samples tested on the DiagCORE Analyzer may contain infectious agents. Users should be aware
of the health hazard presented by such agents and should use, store and dispose of such samples
according to the required safety regulations. Wear personal protective equipment and disposable
powder-free gloves when handling reagents or samples, and wash hands thoroughly thereafter.
Always observe safety precautions as outlined in relevant guidelines, such as the Clinical and
Laboratory Standards Institute (CLSI) Protection of Laboratory Workers from Occupationally
Acquired Infections, Approved Guidelines M29, or other appropriate documents provided by:
OSHA: Occupational Safety and Health Administration (United States of America)
ACGIH: American Conference of Government Industrial Hygienists (United States of America)
COSHH: Control of Substances Hazardous to Health (United Kingdom)
Avoid contamination of the DiagCORE Analyzer and workspace by handling samples and
DiagCORE cartridges with care. In the event of contamination (e.g., a leak from a cartridge), clean
and decontaminate the affected area and the DiagCORE Analyzer.
WARNING
Biological hazard
Use caution when loading or removing cartridges containing infectious
samples into or from the DiagCORE Analyzer. A break in the cartridge
could contaminate the DiagCORE Analyzer and the surrounding area.
All cartridges should be handled as if they contain potentially infectious
agents.

12 DiagCORE Analyzer User Manual
CAUTION
Risk of contamination
Contain and clean contamination from a broken or visibly damaged
cartridge immediately. Contents, though not infectious, can be spread
by normal activity and may contaminate further analytical results,
leading to false positives.
For instructions on cleaning and decontaminating the DiagCORE Analyzer, refer to sections 7.2
Cleaning the DiagCORE Analyzer surface and 7.3 Decontaminating the DiagCORE Analyzer
surface.
2.6 Waste disposal
Used cartridges and plasticware may contain hazardous chemicals or infectious agents. Such
waste must be collected and disposed of properly in accordance with all national, state and local
health and safety regulations and laws.

DiagCORE Analyzer User Manual 13
3 General background
3.1 System description
The DiagCORE Analyzer, in combination with DiagCORE assay cartridges, uses real-time PCR to
detect pathogen nucleic acids in human biological samples. The DiagCORE Analyzer and
cartridges are designed as a closed system that enables hands-off sample preparation followed
by detection and identification of pathogen nucleic acids. Samples are inserted into a DiagCORE
cartridge, which carries on board all reagents necessary to isolate and amplify nucleic acids from
the sample. Detected real-time amplification signals are interpreted by the integrated software
and are reported via an intuitive user interface.
3.2 DiagCORE Analyzer description
The DiagCORE Analyzer consists of an Operational Module and an Analytical Module. The
Operational Module includes elements that provide connectivity to the Analytical Module and
enable user interaction with the DiagCORE Analyzer. The Analytical Module contains the hardware
and software for sample testing and analysis.
The DiagCORE Analyzer includes the following elements:
Touchscreen for user interaction with the DiagCORE Analyzer
Barcode reader for sample, patient and cartridge identification
USB ports for assay and system upgrades, document export and printer connectivity (one in
front, three in back)
Cartridge entrance port for inserting DiagCORE cartridges into the DiagCORE Analyzer
Ethernet connector for network connectivity

14 DiagCORE Analyzer User Manual
The following images show the locations of various DiagCORE Analyzer features.
Front view of the DiagCORE Analyzer. The Operational Module is on the left, and the Analytical
Module is on the right.
Rear view of the DiagCORE Analyzer. The Operational Module is on the right, and the
Analytical Module is on the left.
3.3 DiagCORE cartridge description
The DiagCORE qPCR cartridge is a disposable plastic device that allows performance of fully
automated molecular assays. Main features of the DiagCORE qPCR cartridge include compatibility
with various sample types (e.g., fluids, swabs), hermetical containment of all pre-loaded reagents
necessary for testing, and true walk-away operation. All sample preparation and assay testing
steps are performed within the cartridge.
All reagents required for the complete execution of a test run are pre-loaded and self-contained
in the DiagCORE cartridge. The user does not need to come in contact with and/or manipulate
any reagents. During the test, reagents are handled in the Analytical Module by pneumatically
operated microfluidics and make no direct contact with the DiagCORE Analyzer actuators. The

DiagCORE Analyzer User Manual 15
DiagCORE Analyzer houses air filters for both incoming and outgoing air, further safeguarding the
environment. After testing, the cartridge stays hermetically closed at all times, greatly enhancing
its safe disposal.
Within the cartridge, multiple steps are automatically performed in sequence using pneumatic
pressure to transfer samples and fluids via the transfer chamber to their intended destinations. After
the cartridge is introduced into the DiagCORE Analyzer, the following assay steps occur
automatically:
Resuspension of internal control
Cell lysis using mechanical and/or chemical means
Membrane-based nucleic acid purification
Mixing of the purified nucleic acid with lyophilized master mix reagents
Transfer of defined aliquots of eluate/master mix to different reaction chambers
Performance of real-time, multiplex PCR testing within each reaction chamber. An increase in
fluorescence, indicating presence of the target analyte, is detected directly within each
reaction chamber.
The general layout of the cartridge and its features are illustrated below.
DiagCORE cartridge.

16 DiagCORE Analyzer User Manual
4 Installing the DiagCORE Analyzer
4.1 Site requirements
Select a flat, dry and clean workbench space for the DiagCORE Analyzer. Ensure that the space is
free of excessive drafts, moisture and dust, as well as protected from direct sunlight, large
temperature fluctuations, heat sources, vibration and electrical interference. Refer to section 9.1
Technical specifications of the appendix for the weight and dimensions of the DiagCORE Analyzer
and the correct operating conditions (temperature and humidity). The DiagCORE Analyzer should
have sufficient clearance on all sides to enable proper ventilation, and to allow unimpeded access
to the cartridge entrance port, the back of the DiagCORE Analyzer, the ON/OFF button, the
barcode reader and the touchscreen.
CAUTION
Impeded ventilation
To ensure proper ventilation, maintain a minimum clearance of 10 cm at
the rear of the DiagCORE Analyzer and do not block airflow under the
unit.
Slits and openings that ensure instrument ventilation must not be
covered.
CAUTION
Electromagnetic interference
Do not place or use the DiagCORE Analyzer in close proximity of sources
of strong electromagnetic radiation (e.g., unshielded intentional RF
sources), as these can interfere with proper operation.
NOTE
Before installing and using the DiagCORE Analyzer, refer to section 9.1
Technical specifications of the appendix to become familiar with the
DiagCORE Analyzer operating conditions.

DiagCORE Analyzer User Manual 17
4.2 What is included with the DiagCORE Analyzer
The DiagCORE Analyzer is delivered in two separate boxes and includes all the necessary
components for setting up and operating the system. The contents of the boxes are described
below.
Box 1 contents:
1x Analytical Module
1x USB storage device containing the user manual and quickstart guide in multiple languages
1x Power cord
1x Analytical/Analytical Module bridge
1x Termination bridge
1x Analytical-Operational Module assembly tool
1x Screen suede
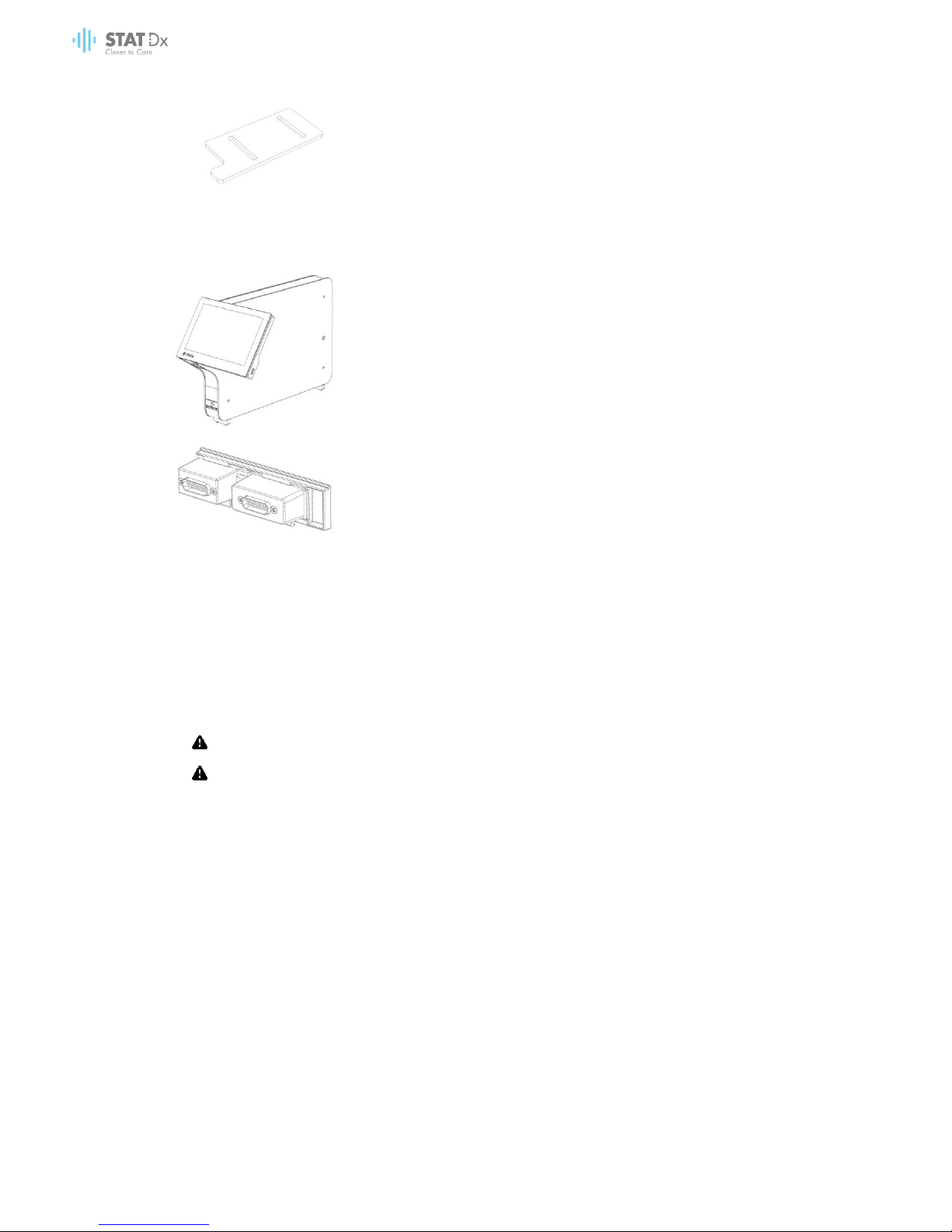
18 DiagCORE Analyzer User Manual
1x Protective cover removal tool
Box 2 contents:
1x Operational Module
1x Analytical/Operational Module bridge
4.3 Unpacking and installing the DiagCORE Analyzer
Carefully unpack the DiagCORE Analyzer according to the following steps:
1. Remove the Analytical Module from its box and place it on a level surface. Remove the foam
pieces attached to the Analytical Module.
WARNING
CAUTION
Risk of personal injury and material damage
The DiagCORE Analyzer is a heavy instrument. To avoid personal injury
or damage to the DiagCORE Analyzer, take care when lifting and use
appropriate lifting methods.
The analyzer must be lifted and handled by taking it from the base with two hands, as shown in the
figure below.

DiagCORE Analyzer User Manual 19
2. Remove the protective covers from the side of the Analytical Module using the protective cover
removal tool delivered with the DiagCORE Analyzer.
3. Remove the Operational Module from its box and attach it to the left side of the Analytical
Module. Tighten the screws using the Analytical-Operational Module assembly tool delivered
with the DiagCORE Analyzer.
CAUTION
Risk of mechanical damage
Do not leave the Operational Module without support or resting on the
screen, as this may damage the touchscreen.

20 DiagCORE Analyzer User Manual
4. Reattach the protective covers on the side of the Analytical Module.
5. Connect the Analytical/Operational Module bridge at the back of the DiagCORE Analyzer to
link the Operational and Analytical Modules together.
6. Connect the termination bridge at the back of the Analytical Module.

DiagCORE Analyzer User Manual 21
7. Connect the power cord that was delivered with the DiagCORE Analyzer to the back of the
Analytical Module.
8. Connect the power cord to a power outlet.
9. Turn the power switch on the back of the Analytical Module to the “I” position. Check that the
status indicators of the Analytical and Operational Modules are blue.
NOTE
If a status indicator is red, there is a malfunction in the Analytical Module.
Contact Technical Support using the contact information in section 8
Troubleshooting for assistance.
NOTE
The instrument shall not be positioned so that it is difficult to operate the
disconnecting power switch
10. The DiagCORE Analyzer is ready to be configured for its intended setup. Refer to section 6.8
Configuring the DiagCORE Analyzer system to configure the system parameters, set the system
time and date and configure the network connection.

22 DiagCORE Analyzer User Manual
4.4 Repackaging and shipping the DiagCORE Analyzer
When repackaging the DiagCORE Analyzer for shipping, the original packaging materials must be
used. If the original packaging materials are not available, contact Technical Support. Ensure that
the instrument has been properly prepared (see section 7.2 Cleaning the DiagCORE Analyzer
surface) prior to packing and that it poses no biological or chemical hazard. To repackage the
instrument:
1. Ensure that the instrument is off.
2. Disconnect the power cord from the power outlet.
3. Disconnect the power cord from the back of the Analytical Module.
4. Disconnect the termination bridge at the back of the Analytical Module.
5. Disconnect the Analytical/Operational Module bridge linking the Operational and Analytical
Modules at the back of the DiagCORE Analyzer.
6. Remove the protective covers on the side of the Analytical Module using the protective cover
removal tool.
7. Use the Analytical-Operational Module assembly tool delivered to loosen the two screws
holding the Operational Module to the Analytical Module. Package the Operational Module in
its box.
8. Reposition the protective covers on the side of the Analytical Module. Package the Analytical
Module with its foam pieces into its box.

DiagCORE Analyzer User Manual 23
5 Running a test and viewing results
5.1 Starting the DiagCORE Analyzer
Press the ON/OFF button on the DiagCORE Analyzer front to start the unit.
NOTE
The power switch at the back of the Analytical Module must be set in the
“I” position. The Operational and Analytical Module indicators turn blue in
this case.
Wait until the main screen appears and the Analytical and Operational Module status indicators
turn green and stop blinking.
NOTE
The Login screen will appear if User Access Control is activated. Refer to
section 6.6 User management for further details.
5.2 Preparing the cartridge
Remove the cartridge from its packaging. For details about adding the sample to the cartridge
and for information specific to the assay to be run, refer to the instructions for use of the specific
assay (e.g., Respiratory Panel Assay). Always ensure that both sample lids are firmly closed after
adding a sample to the cartridge.
5.3 Procedure to run a test
All operators should wear appropriate personal protective equipment, such as gloves, when
touching the DiagCORE Analyzer touchscreen.
1. Press the Run Test button at the top right corner of the main screen.

24 DiagCORE Analyzer User Manual
2. When prompted, scan the sample ID barcode using the barcode reader that is integrated into
the Operational Module.
Prompt to scan the sample ID barcode.
NOTE
Depending on the DiagCORE Analyzer configuration, it may also be
possible to enter the sample ID using the virtual keyboard of the
touchscreen. Please refer to section 6.8.2 for further details.
NOTE
Depending on the chosen system configuration, entering patient ID may
also be required at this point. Please refer to section 6.8.2 for further details.
3. When prompted, scan the barcode of the cartridge to be used. The DiagCORE Analyzer
automatically recognizes the assay to be run, based on the cartridge barcode.
Prompt to scan the DiagCORE assay cartridge barcode.
NOTE
The DiagCORE Analyzer will not accept cartridges with elapsed expiration
date, previously used cartridges or cartridges for assays that are not
installed on the unit. An error message will be shown in these cases. Refer
to section 8.1 Errors and warning messages for further details.
Refer to section 6.7.2 Importing new assays for instructions on importing
and adding assays to the DiagCORE Analyzer.

DiagCORE Analyzer User Manual 25
4. If required, select the appropriate sample type from the list.
Prompt to select the sample type.
5. The Confirm screen will appear. Review the data entered and make any necessary changes
by pressing the relevant fields on the touchscreen and editing the information.
Confirm screen.
6. Press Confirm when all the displayed data are correct. If needed, press on the appropriate
field to edit its content, or press Cancel to cancel the test.
7. Ensure that both sample lids of the cartridge are firmly closed. When the cartridge entrance
port on the top of the DiagCORE Analyzer automatically opens, insert the cartridge with the
barcode facing to the left and the reaction chambers facing down.

26 DiagCORE Analyzer User Manual
There is no need to push the cartridge into the DiagCORE Analyzer. Position it correctly into the
cartridge entrance port, and the DiagCORE Analyzer will automatically move the cartridge into
the Analytical Module.
Prompt to insert the DiagCORE assay cartridge.
Upon detecting the cartridge, the DiagCORE Analyzer will automatically close the lid of the
cartridge entrance port and start the test run. No further action from the operator is required to
start the run.
NOTE
The DiagCORE Analyzer will not accept a cartridge other than the one
used and scanned during the test setup. If a cartridge other than the
one scanned is inserted, an error will be generated and the cartridge will
be automatically ejected.
NOTE
Up to this point, it is possible to cancel the test run by pushing the Cancel
button in the bottom right corner of the screen.
NOTE
Depending on the system configuration, the operator may be required
to re-type their user password to start the test run.
8. While the test is running, the time remaining in the run is displayed on the screen.
Test execution screen and remaining run time.

DiagCORE Analyzer User Manual 27
9. After the test run is completed, press Eject to remove the cartridge and dispose of it as
biohazardous waste in accordance with all national, state and local health and safety
regulations and laws.
Prompt to eject the used cartridge after running a test.
The cartridge should be removed when the cartridge entrance port opens and ejects the
cartridge. If the cartridge is not removed after a certain time, it will be automatically moved
back into the DiagCORE Analyzer and the lid of the cartridge entrance port will close. If this
occurs, press Eject to open the lid of the cartridge entrance port again and then remove the
cartridge.
NOTE
Used cartridges must be discarded.
It is not possible to re-use cartridges for tests for which the execution was
started but then subsequently aborted, or for which an error was
detected.
The Results Summary screen will appear. Refer to section 5.4 Viewing results for further details.
Results Summary screen.

28 DiagCORE Analyzer User Manual
5.3.1 Instructions to abort a test
If a test run is already in progress, pressing Abort will stop the execution of the test.
Aborting a running test.
After aborting a test, the cartridge can no longer be processed and cannot be re-used. After
pressing Abort, a dialog will appear prompting the operator to confirm that the test should be
cancelled.
Prompt to confirm (Yes) or cancel (No) abort of a running test.
NOTE
Used cartridges must be discarded.
It is not possible to re-use cartridges for tests for which the execution
was started but then subsequently aborted, or for which an error was
detected.
5.4 Viewing results
The DiagCORE Analyzer automatically interprets and saves test results. After ejecting the cartridge,
the Results Summary screen is automatically displayed.

DiagCORE Analyzer User Manual 29
Example screen showing Test Data on the left panel and Test Results Summary in the main
panel.
NOTE
Please Refer to assay-specific Instructions for Use for instructions on how to
interpret assay results
The main part of the screen provides the following two lists and uses color-coding and symbols to
indicate the results:
The first list includes all pathogens detected and identified in the sample, preceded by a
sign and are colored red.
The second list includes all pathogens tested in the sample. Pathogens detected and identified
in the sample are preceded by a sign and are colored red. Pathogens that were tested but
not detected are preceded by a sign and are colored green.
Note that the pathogens detected and identified in the sample are shown in both lists.
In case the test failed to complete successfully, a message will indicate “Failed” followed by the
specific error code.
The following Test Data is shown on the left side of the screen:
Sample ID
Patient ID (if available)
Assay Type
Sample Type
Further data about the assay is available, depending on the operator’s access rights, through the
tabs at the bottom of the screen (e.g., amplification plots, melting curves and test details).
Assay data can be exported by pressing Save Report in the bottom bar of the screen.
A report can be sent to the printer by pressing Print Report in the bottom bar of the screen.

30 DiagCORE Analyzer User Manual
5.4.1 Viewing amplification curves
To view the test amplification curves, press the Amplification Curves tab.
Amplification Curves screen showing the Pathogens tab.
Details about the tested pathogens and controls are shown on the left, and the amplification
curves are shown in the center.
NOTE
If User Access Control is enabled (refer to section 6.6 User management)
the Amplification Curves screen is only available for selected operators.
Press the Pathogens tab on the left side to display the plots corresponding to the tested pathogens.
Press on the pathogen name to select which pathogens are shown in the amplification plot. It is
possible to select single, multiple or no pathogens. Each pathogen in the selected list will be
assigned a color corresponding to the amplification curve associated with this pathogen.
Unselected pathogens will be listed in grey.
The corresponding CT and endpoint fluorescence values are shown below each pathogen name.
Press the Controls tab on the left side to view the controls and select which controls are shown in
the amplification plot. Press the circle next to the control name to select or deselect it.
Amplification Curves screen showing the Controls tab.

DiagCORE Analyzer User Manual 31
The amplification plot displays the data curve for the selected pathogens or controls. To alternate
between logarithmic or linear scale for the Y-axis, press the Lin or Log button at the bottom left
corner of the plot.
The range of the X-axis and Y-axis can be adjusted using the gray pickers on each axis. Press and
hold a picker and then move it to the desired location on the axis. Move a picker to the axis
origin to return to the default values.
5.4.2 Viewing melting curves
To view the test melting curves, press the Melting Curves tab. Details about the tested pathogens
and controls are shown on the left, and the melting curves are shown in the center.
NOTE
The Melting Curves tab is only available for assays implementing melting
analysis.
If User Access Control is enabled (refer to section 6.6 User management),
this screen is only available for selected operators.
Press the Pathogens tab on the left side to display the tested pathogens. Press the circle next to the
pathogen name to select which pathogen melting curves are shown. It is possible to select single,
multiple or no pathogens. Each pathogen in the selected list will be assigned a color corresponding
to the melting curve associated with this pathogen. Unselected pathogens will be listed in gray.
The melting temperature is shown below each pathogen name.
Press the Controls tab on the left side to view the controls and select which controls are shown in
the melting plot. Press the circle next to the control name to select or deselect it.
Controls that passed the analysis are shown in green and are labeled Passed Controls, while those
that failed are shown in red and are labeled Failed Controls.
The range of the X-axis and Y-axis can be adjusted using the gray pickers on each axis. Press and
hold a picker and then move it to the desired location on the axis. Move a picker to the axis
origin to return to the default values.

32 DiagCORE Analyzer User Manual
5.4.3 Viewing test details
Press Test Details to review the results in more detail. Scroll down to see the complete report.
The following test details are shown in the center of the screen:
User ID
Cartridge serial number
Cartridge expiration date
Analytical Module serial number
Test status (completed, failed, canceled by user)
Error code
Test start date and time
Test execution time
Assay name
Test Result for every analyte: Positive, Negative or Failed
List of analytes tested in the assay, with C
T
and endpoint fluorescence
List of controls, with C
T
and endpoint fluorescence
Example screen showing Test Data on the left panel and Test Details in the main panel.

DiagCORE Analyzer User Manual 33
5.4.4 Browse results from previous tests
To view results from previous tests that are stored in the results repository, press View Results from
the Main Menu bar.
Example of View Results list.
The following information is available for every executed test:
Sample ID
Assay name
Operator ID
Analytical Module on which the test was executed
Date and time when the test was finished
Outcome of the test
NOTE
When User Access Control is enabled (refer to section 6.6 User
management), the data for which the user has no access rights will be
hidden with asterisks.
Select one or more test results by pressing the circle to left of the sample ID. A checkmark will
appear next to selected results. Unselect test results by pressing this checkmark. The entire list of
results can be selected by pressing the button in the top row.
Example of selecting test results in the View Results list.

34 DiagCORE Analyzer User Manual
Press anywhere in the test row to view the result for a particular test.
Press a column headline (e.g., Sample ID) to sort the list in ascending or descending order
according to that parameter. The list can be sorted according to only one column at a time.
The Result column shows the outcome of each test:
Name
Button
Description
Positive
pos
At least one pathogen is positive.
Negative
neg
No analytes were detected.
Failed
fail
The test failed, either because an error occurred or the test was
cancelled by the user.
Successful
suc
The test is either positive or negative, but the user does not have the
access rights to view the test results.
Press Print Report to print the report(s) for the selected result(s).
Press Save Report to save the report(s) for the selected result(s) in PDF format to an external USB
storage device.
Select the report type: List of Tests or Test Reports.
Press the Search button to search the test results by Sample ID, Assay and Operator ID. Enter the
search string using the virtual keyboard, and press Enter to start the search. Only the records
containing the search text will be displayed in the search results.
If the results list has been filtered, the search will only apply to the filtered list.
Press and hold a column headline to apply a filter based on that parameter. For some parameters,
such as Sample ID, the virtual keyboard will appear so the search string for the filter can be entered.
For other parameters, such as Assay, a dialog will open with a list of assays stored in the repository.
Select one or more assays to filter only the tests that were performed with the selected assays.
The symbol to the left of a column headline indicates that the column’s filter is active.
A filter can be removed by pressing the Remove Filter button in the submenu bar.
5.4.5 Exporting results to a USB storage device
From any tab of the Test Results screen, select Save Report to export and save a copy of the test
results in PDF format to a USB storage device. The USB port is located on the front of the instrument.
5.4.6 Printing results
Select Print Report to send a copy of the test results to the system printer.

DiagCORE Analyzer User Manual 35
6 System reference
This chapter provides a description of all features and options available in the DiagCORE Analyzer
that enable customization of the DiagCORE Analyzer settings.
6.1 Main screen
In the Main screen, it is possible to view the status of the Analytical Modules and navigate to
different sections (Log In, Run Test, View Results, Options, Log Out) of the user interface.
Main screen of the DiagCORE Analyzer touchscreen.
The Main screen includes the following elements:
General status bar
Module status bar
Main Menu bar
Content area
Tab Menu bar (optionally shown, depends on screen)
Submenu bar and Instructions bar (optionally shown, depends on screen)
6.1.1 General status bar
The General status bar provides information about the status of the system. The User ID of the
logged-in user appears on the left side. The title of the screen appears in the middle, and the system
date and time appear on the right.
General status bar.
General status
Main Menu bar
Module status
bar
Content area
Submenu bar

36 DiagCORE Analyzer User Manual
6.1.2 Module status bar
The Module status bar displays the status of each Analytical Module (1–4) available in the system
in corresponding status boxes. The boxes display “Not Installed” if no Analytical Module is available
for that position.
Module status bar.
Click on the box corresponding to a particular Analytical Module to access more detailed
information.
The following module states may be displayed in a status box of the Module status bar:
State
Description
Not installed
No Analytical Module is installed at that position.
Calibration pending
A calibration action is needed.
Maintenance pending
A maintenance action is pending.
Excluded
The Analytical Module has been excluded by the user via user
settings.
Error
The Analytical Module reported a serious error. The Analytical Module
is out of order.
Initializing
The Analytical Module is starting up and is performing the self-test.
Available
The Analytical Module is available for a new test. There is no test
running in this Analytical Module, no cartridge is inserted and the lid
of the cartridge entrance port is closed.
Test running
User administrator is currently running the Resp_3018_19c test on
Analytical Module 1. There are 32 minutes and 14 seconds remaining
to complete the test.

DiagCORE Analyzer User Manual 37
State
Description
Test completed
User administrator has run the Respiratory Panel test on Analytical
Module 1.
The progress bar in the box will show the test status:
TEST COMPLETED: the test was completed successfully.
TEST FAILED: the test was completed, but an error occurred.
TEST CANCELLED: the user canceled the test.
Once the cartridge has been removed and the lid of the cartridge
entrance port has closed, the Analytical Module will be available
again.
Eject cartridge
The Analytical Module contains a cartridge and the lid of the
cartridge entrance port is closed, but no test is currently running. This
can occur in the following situations:
The cartridge was not removed after an ejection due to a canceled
test.
The system was powered off with a cartridge inside the Analytical
Module.
6.1.3 Main Menu bar
The following options are available to the user through the Main Menu bar:
Name
Button
Description
Run Test
Starts the run test sequence (see section 5.3 Procedure to run a test).
The DiagCORE Application Software automatically selects an
Analytical Module from the ones available and starts the test
preparation sequence.
View Results
Opens the View Results screen (see section 5.4 Viewing results).
Options
Displays the Options submenu (see section 6.5 Options menu).
Log Out
Logs the user out (only active when User Access Control is enabled).

38 DiagCORE Analyzer User Manual
6.1.4 Content area
The information displayed in the main content area varies according to the state of the user
interface. Results, summaries, configurations and settings are displayed in this area upon entering
different modes and selecting items from the menus described below.
Depending on the content, further options may be available through the Tab menu bar and
Options menu.
The Options submenu is accessed by pressing the Options button.
6.2 Login screen
When User Access Control is enabled (refer to section 6.6 User management), users must identify
themselves by logging in to access the DiagCORE Analyzer functions.
The content area of the login screen includes a text box for entering the User ID. If the option “Show
previous user logins” is selected, a list of the previous five users that logged in successfully will also
be displayed.

DiagCORE Analyzer User Manual 39
Enter the user name either by clicking on one of the names available in the list or by clicking on the
User ID text box and entering the name using the virtual keyboard. Once the user name is entered,
confirm by pressing the check mark on the virtual keyboard.
Login screen.
Virtual keyboard of touchscreen.
If the option “Require password” is selected (refer to section 6.6 User management), a password
text box and the virtual keyboard for entering the password will be shown. If no password is
required, the password text box will be grayed out.
If a user forgets his or her password, the system administrator can reset it.
For security reasons, if a password is entered incorrectly three times, the system will lock for one
minute before the user can try to log in again.
40 DiagCORE Analyzer User Manual
6.3 Logging out
When User Access Control is enabled (refer to section 6.6 User management), users can log out at
any time using the Log Out option in the Main Menu. See section 6.1.3 Main Menu bar for more
information.
Users will be automatically logged out when the time of the automatic log-off expires. This time can
be configured in the General settings of the Options menu (see section 6.8.2 General settings).
6.4 Screensaver
The DiagCORE screensaver is shown after there has been no user interaction for a pre-defined
period of time. This time can be configured in the Options menu (see section 6.5 Options menu).
The screen saver shows the availability of Analytical Modules and the remaining test after test
completion.
Screensaver showing one available Analytical Module.
6.5 Options menu
The Options menu is accessible from the Main Menu Bar. The menu contains the elements listed
below. Options that are not available will be grayed out.
Name
Button
Description
Assay
Management
Available for users with rights to manage assays.
User
Management
Available for users with rights to manage users and user
profiles.
System
Configuration
Available for users with the rights to configure the system.
Change
Password
Available if user access control is enabled.

DiagCORE Analyzer User Manual 41
6.6 User management
The DiagCORE Application Software is flexible in order to support different use scenarios.
Concerning the management of users and rights, the following modes are available:
“Single User” mode: User Access Control is disabled and no control of the users that log in to the
DiagCORE Analyzer is performed. All DiagCORE Analyzer functions and features will be
available without any restrictions to all users.
“Multi-User” mode: User Access Control is enabled and users must log in before performing any
action on the DiagCORE Analyzer. The actions they are allowed to perform are limited and
defined according to their user profiles.
NOTE
The user management option is available only to users with
“Administrator” or “Laboratory Supervisor” profiles.
NOTE
User Access Control can be enabled and disabled in the General settings
under System Configuration in the Options menu.
The user management option permits users with “Administrator” and “Laboratory Supervisor”
profiles to add new users to the system, define their rights and user profiles, and to activate or
inactivate users.
The following user profiles are available in the DiagCORE Analyzer:
User Profile
Rights
Example
Administrator
Full
Instrumentation/IT responsibility
Laboratory
Supervisor
Add and delete new users
Introduce and delete new assays in
the assay collection
Running assays and viewing results
from all users
Laboratory head
Advanced User
Running assays
Viewing detailed results of own user
tests (amplification plots, etc.)
Microbiologist, laboratory technician
Basic User
Running assays
Viewing non-detailed results of own
user tests (positive/negative results)
Healthcare provider (e.g., nurse,
doctor, general practitioner, etc.)

42 DiagCORE Analyzer User Manual
6.6.1 Accessing and managing the list of users
Follow the steps below to access and manage the system users:
1. Press the Options button and then the User Management button to configure users. The User
Management screen appears in the content area of the display.
The user management menu.
2. Select the user to manage from the list in the left column of the content area.
Selecting and managing users.
3. Select and edit the following options as needed:
User Name: allows changing the user name
Password: allows changing the password for that user
User Active (yes/no): allows changing whether the user is active or not. Inactive users are
not allowed to log in or perform any action on the system

DiagCORE Analyzer User Manual 43
Assign User Profile: allows assigning a different user profile for that user (e.g., Administrator,
Laboratory Supervisor, Advanced User, Basic User). Select the appropriate user profile from
the list on the right of the content area.
Assigning user profiles to users.
Assign Assays: allows defining the assays from the assay database that the user is permitted
to run. Select the assays from the list on the right of the content area.
Assigning assays to users.

44 DiagCORE Analyzer User Manual
Assay Statistics: shows the number of times an assay was run by the selected user.
Viewing assay statistics.
4. Press Save and confirm to save the changes. Alternatively, press Cancel and confirm to discard
the changes.
6.6.2 Adding users
Follow the steps below to add new users to the DiagCORE Analyzer:
1. Press the Options button and then the User Management button to configure users. The User
Management screen appears in the content area of the display.
Adding a new user.
2. Press Add User at the bottom left of the screen to add a new user to the system.
3. Use the virtual keyboard to enter the User Name and Password for the new user.
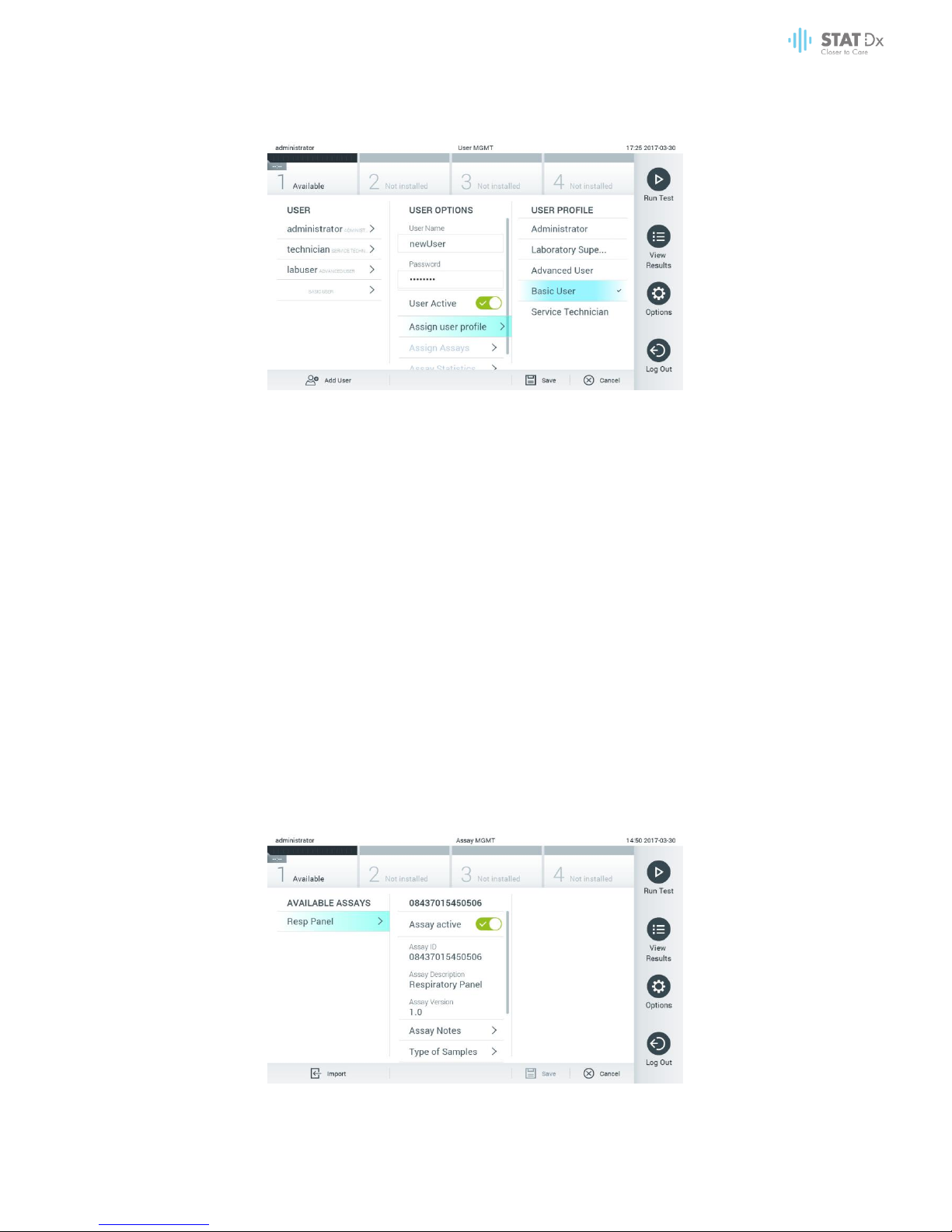
DiagCORE Analyzer User Manual 45
4. Press Assign User Profile and assign the appropriate user profile the new user from the list on the
right of the content area.
Assigning a user profile to a user.
5. Press Assign Assays and select the assays that the user is allowed to run from the displayed
assay list.
6. Press Save and confirm to save and store the new information. The new user has been set up
and is immediately allowed to log in to the DiagCORE Analyzer.
6.7 Assay management
From the Assay Management menu, it is possible to manage assays and access assay-related
information and statistics.
NOTE
The assay management option is available only to users with
“Administrator” or “Laboratory Supervisor” profiles.
6.7.1 Managing available assays
1. Press the Options button and then the Assay Management button to access the Assay
Management screen. The available assays are listed in the first column of the content area.
Managing available assays.
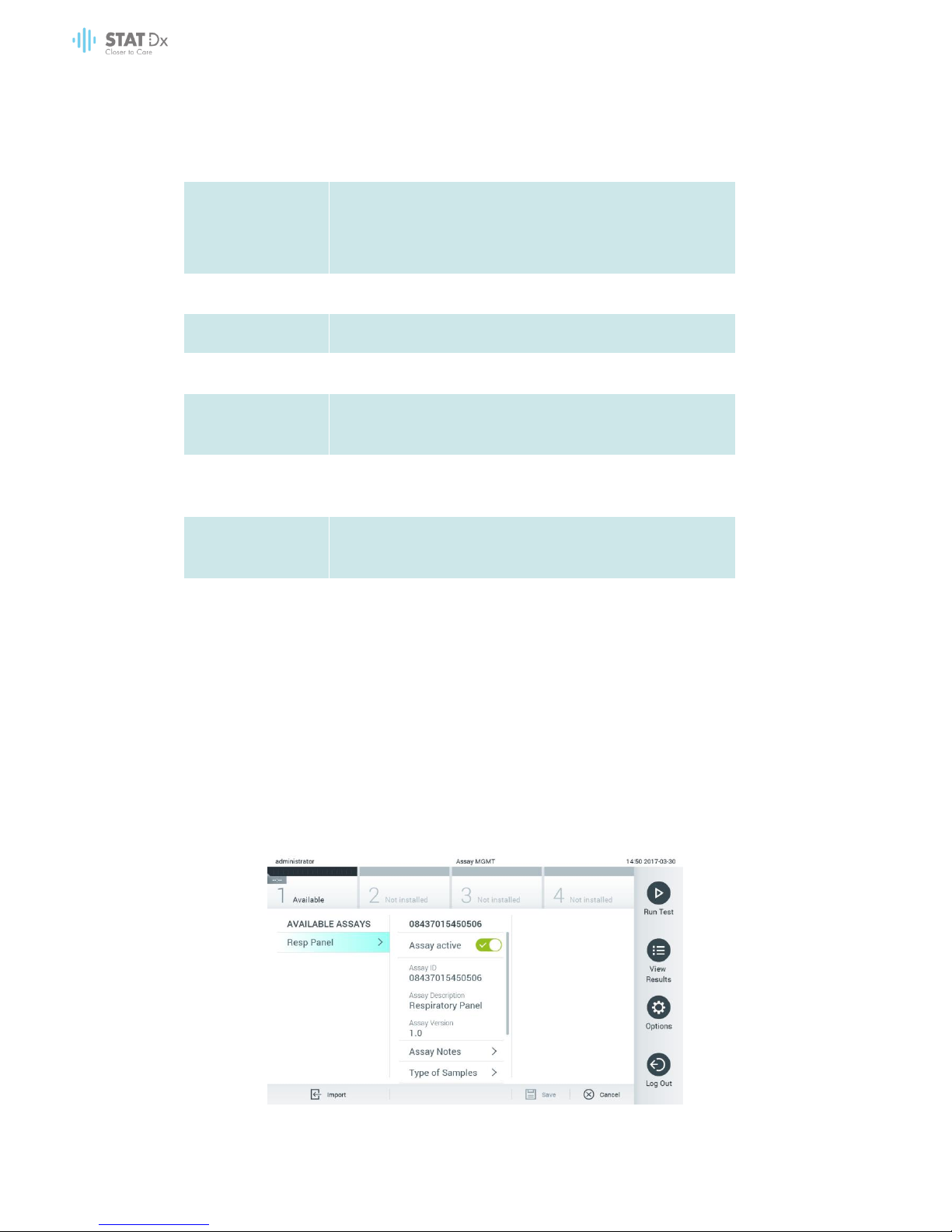
46 DiagCORE Analyzer User Manual
2. Press the name of the assay to manage in the left column of the content area.
3. Select one of the following options:
Option
Description
Assay Active
This button allows setting an assay to active or inactive.
It is only possible to test cartridges for a particular assay if the
assay is active.
Assay ID
Provides the assay identification number.
Assay Version
Provides the assay version.
Assay Notes
Provides additional information about the assay.
Type of Samples
Provides a list of the various sample types supported by the
assay.
List of Analytes
Provides a list of analytes that are detected and identified by
the assay.
List of Controls
Provides the lists of control analytes that are implemented in
the assay.
Assay Statistics
Provides the number of tests ever run by the DiagCORE
Analyzer for the selected assay, as well as the number of
positive, negative, failed and aborted tests.
6.7.2 Importing new assays
1. Insert the USB storage device with assay file to import into the USB port of the DiagCORE
Analyzer.
2. To import the new assay(s) to the DiagCORE Analyzer, press the Options button and then the
Assay Management button. The Assay Management screen appears in the content area of the
display.

DiagCORE Analyzer User Manual 47
3. Press the Import icon at the bottom left of the screen.
4. Select the file from the USB storage device corresponding to the assay to be imported. To be
recognized by the system, the assay definition file must be contained in the root folder.
5. A dialog will appear to confirm upload of the file.
6.8 Configuring the DiagCORE Analyzer system
In the System Configuration menu, it is possible to manage the DiagCORE Analyzer system and
define region-specific parameters.
6.8.1 Regional settings
1. Press the Options button and then the System Configuration button.
2. Select Regional from the Settings list in the left column. Select and define the following settings
as needed:
Setting
Description
Date
Defines the system date (year, month, day)
Time
Defines the system time (hours, minutes)
Date format
Defines the date format. The following options are available:
DD-MM-YYYY
DD-MM-YY
MM-DD-YYYY
YYYY-MM-DD (default)
YY-MM-DD
Date separator
Defines the date separator. The following options are available:
.
- (default)
/
Time format
Defines the time format. The following options are available:
24 hours (HH:mm:ss) (default)
12 hours (HH:mm:ss AM/PM)
Language
English (default)

48 DiagCORE Analyzer User Manual
Setting the system date.
Setting the system date format.
Setting the system date separator.

DiagCORE Analyzer User Manual 49
Setting the system time format.
6.8.2 General settings
1. Press the Options button and then the System Configuration button.
2. Select General from the Settings list in the left column. Select and define the following options
as needed:
Setting
Description
User Access Control
Enables the User Access Control, which requires all users to log in to
the system and limits users to only perform the actions allowed by
their user profile.
When this option is not enabled, it is not possible to distinguish
between users. All features will be available as if they were run by
the Administrator profile.
This option is enabled by default.
Automatic log-off
time
Only active if User Access Control is enabled. This setting defines
the time interval after which a user is automatically logged out of
the system because the DiagCORE Analyzer receives no more user
input. The allowed range is from 5 minutes up to 99:59 hours.
Default: 30 minutes.
User input such as a cursor movement, cursor clicks, pressing a key
on an external keyboard or a touch on the touchscreen resets the
automatic log-off time.
If a user has entered data (for example, in the Run Test screen)
when the automatic log-off occurs, these data will be lost.
Require password
before executing
assay
Only active if User Access Control is enabled. With this field
activated, all users will be required to enter a password before
executing an assay, after pressing the confirm button.

50 DiagCORE Analyzer User Manual
Setting
Description
Use patient ID
With Use Patient ID activated, the DiagCORE Application Software
will provide the option for users to enter a Patient ID or scan a
Patient ID when preparing to run a test (see section 5.3 Procedure
to run a test).
Prefer patient ID
barcode
Determines if users will be prompted to scan the Patient ID using
the barcode reader first.
Default: Disabled.
Patient ID mandatory
Only active if Use Patient ID is enabled. When activated, users will
be required to enter a patient ID before executing an assay. When
not activated, users can leave the patient ID data field empty.
Default: Disabled.
Sample ID mandatory
When activated, users will be required to enter a Sample ID before
executing an assay. When not activated, users can leave the
Sample ID data field empty, and a unique Sample ID will be
automatically generated by the DiagCORE Analyzer.
Default: Disabled.
Prefer sample ID
barcode
Determines if users are prompted to scan the Sample ID using the
barcode reader first.
Default: Disabled.
Exclude modules
Allows the possibility to exclude specified Analytical Modules from
running tests. This may be useful in the event that a module is
suspected of failure.
Default: Disabled.
Number of results per
page
This setting defines the number of results shown per page in the
View Results list screen.
Show previously
logged-in user IDs
Only active if User Access Control is enabled. When this setting is
enabled, the list of previously logged-in users will be displayed on
the login screen.
Default: Enabled.
Require password to
log in
Only active if User Access Control is enabled. When this setting is
enabled, all users must enter their password to log in. When
disabled, only the User ID will be required to log in.
Default: Enabled.

DiagCORE Analyzer User Manual 51
Setting
Description
Restore factory
default
Enables resetting the system back to all factory default settings.
6.8.3 Printer settings
The Printer settings option enables selection of the system printer. The DiagCORE Analyzer allows
use of networked printers or printers connected to the Operational Module via the USB ports on the
back of the instrument.
1. Press the Options button and then the System Configuration button.
2. Select Printer from the settings list in the left column.
3. Select a printer from the list of available printers.
Selecting a system printer.
6.8.4 Network settings
The Network option enables connecting the DiagCORE Analyzer to a network, accessing
networked printers, and provides connectivity to the HIS/LIS (available in future software releases).
Contact the network administrator for details on how to configure the following fields.
Follow these steps to define the network settings:
1. Press the Options button and then the System Configuration button.
2. Select Network from the settings list in the left column.
3. Select and define the options below according to instructions from the network administrator:

52 DiagCORE Analyzer User Manual
Option
Description
Enable IPv6
Enables the use of the IP v6 protocol. The submenu IPv6 Settings is
only active if “Enable IPv6” is enabled.
Obtain IPv6 address
automatically
Allows the unit to acquire the IPv6 address from the network using
DHCP.
IPv6 Address
Defines the manually configured IPv6 address of the Operational
Module. This option is only active if “Obtain IPv6 address
automatically” is disabled.
Subnet Prefix Length
Defines the IPv6 subnet prefix length. This option is only active if
“Obtain IPv6 address automatically” is disabled.
Enable IPv4
Enables the use of the IP v4 protocol. The submenu IPv4 Settings is
only active if “Enable IPv4” is enabled.
Obtain IPv4 address
automatically
Allows the unit to acquire the IPv4 address from the network using
DHCP.
IPv4 Address
Defines the manually configured IPv4 address of the Operational
Module. This option is only active if “Obtain IPv4 address
automatically” is disabled.
Subnet Mask
Defines the IPv4 subnet prefix length. This option is only active if
“Obtain IPv4 address automatically” is disabled.
Default Gateway
Defines the IPv6 or IPv4 default gateway, depending on which is
enabled. This option is only active if either “Obtain IPv6 address
automatically” or “Obtain IPv4 address automatically” is disabled.
Obtain DNS address
automatically
Allows the unit to acquire the DNS configuration from the network
using DHCP.
Preferred DNS
Server
Defines the primary DNS server. This option is only active if “Obtain
DNS address automatically” is disabled.
Alternate DNS
Server
Defines the secondary DNS server. This option is only active if “Obtain
DNS address automatically” is disabled.

DiagCORE Analyzer User Manual 53
Configuring the system network settings.
6.8.5 HIS/LIS settings
The HIS/LIS functionality is not available in the current version of the software. It will be supported in
future releases.
6.8.6 System log
The system log records general information about the use of the Operational and Analytical
Modules, such as adding or removing users, adding or removing assays, logins, logouts, starts of
tests, etc. Press the Options button and then the System Configuration button followed by System
Log to access the system log information. The System Log Capacity is shown in the center of the
screen followed by the log content. Press Export Log File to export the content.
Accessing the system log.

54 DiagCORE Analyzer User Manual
6.8.7 Technical log
The technical log records detailed information about the execution of tests on the Analytical
Module(s). This information is used by Technical Support for troubleshooting. Press the Options
button and then the System Configuration button followed by Technical Log to access the
technical log information. The number of files available in the technical log is configurable. Each
log file is identified with date and time of creation. Press Export Log File to export the content.
Technical logs may be requested by Technical Support.
Accessing the technical log.
6.8.8 Version information
Press the Options button and then the System Configuration button followed by Version Info to view
the DiagCORE Application Software version, the serial numbers and the firmware versions for the
installed Analytical Modules.
6.8.9 System update
To update the DiagCORE Analyzer system, press the Options button and then the System
Configuration button followed by System Update. Select the appropriate .dup file stored on a USB

DiagCORE Analyzer User Manual 55
storage device to update the system to a newer version. A message will appear recommending
to perform a system backup first (refer to 6.8.10 System backup).
Performing a system update.
After the update, the user may be requested to shut down the DiagCORE Analyzer and start it
again.
6.8.10 System backup
To back up the DiagCORE Analyzer system, press the Options button and then the System
Configuration button followed by System Backup. Insert a USB storage device into the front USB
port.
Performing a system backup.
Press the Make Backup button. A file with the extension .dbk will be generated with a default file
name.
To restore a backup, press the Restore Backup button and select the appropriate backup file with
a .dbk extension from the connected USB storage device. A message will appear recommending
to make a backup before restoring.

56 DiagCORE Analyzer User Manual
6.9 Change passwords
To change a user password, press the Options button and then Change Password. First enter the
current password in the text field, and then enter the new password into the New Password field.
Type the new password again in the Confirm Password field.
After three failed attempts to enter a password, the password entry field will be deactivated for
one minute, and a dialog will appear with the message “Password failed, please wait 1 minute to
try it again”.
First step of the password change process.
Entering and confirming the new password.
6.10 DiagCORE Analyzer status
The status of the Operational and Analytical Modules is indicated by the color of the status
indicators (LEDs) at the front of the DiagCORE Analyzer.

DiagCORE Analyzer User Manual 57
The Operational Module can display any of the following status colors:
Status light
Description
Off
DiagCORE Analyzer is off
Blue
DiagCORE Analyzer is in standby
mode
Green
DiagCORE Analyzer is running
The Analytical Module can display any of the following status colors:
Status light
Description
Off
DiagCORE Analyzer is off
Blue
DiagCORE Analyzer is in standby
mode
Green (blinking)
DiagCORE Analyzer is initializing
Green
Analytical module is running
6.11 Shutting down the instrument
The DiagCORE Analyzer is designed to operate continuously. If the unit will not be used for a short
time (less than a day), we recommend placing the DiagCORE Analyzer in standby mode by
pressing the ON/OFF button on the front of the instrument. To shut down the DiagCORE Analyzer for
a longer time period, turn off the main switch on the back of the instrument.
If someone attempts to put the DiagCORE Analyzer in stand-by mode while the Analytical Module
is running a test, a dialog will appear indicating that shutdown is currently not possible. Allow the
instrument to finish running the test(s) and try shutting it down afterwards.

58 DiagCORE Analyzer User Manual
7 Maintenance
This chapter describes the maintenance tasks required for the DiagCORE Analyzer.
7.1 Maintenance tasks
The table below provides a list of maintenance tasks to be performed on the DiagCORE Analyzer.
Description
Frequency
Cleaning or Decontaminating
the DiagCORE Analyzer
surface
To be performed in case liquids, chemicals or biological
specimens (potentially infectious) are spilled on the
DiagCORE Analyzer surface.
Exchange of air filter
To be performed annually.
7.2 Cleaning the DiagCORE Analyzer surface
WARNING
CAUTION
Risk of personal injury and material damage
Wear protective glasses, a lab coat and gloves when cleaning the
instrument to avoid biological and chemical hazards.
WARNING
CAUTION
Risk of personal injury and material damage
Disconnect the DiagCORE Analyzer from the power outlet before
cleaning.
CAUTION
Risk of damage to the DiagCORE Analyzer
Avoid spilling chemicals or other liquids into the DiagCORE Analyzer.
Damage caused by liquid spillage will void the warranty.
CAUTION
Risk of damage to the DiagCORE Analyzer
Avoid spilling liquids on or wetting the touchscreen. To clean the
touchscreen, use the screen suede provided with the DiagCORE
Analyzer.
Use the following to clean the DiagCORE Analyzer surface:
Mild detergent
Paper towels
Distilled water
Follow the steps below to clean the DiagCORE Analyzer surface:
1. Wear laboratory gloves, coat and protective glasses.

DiagCORE Analyzer User Manual 59
2. Wet a paper towel in mild detergent and wipe down the DiagCORE Analyzer surface, as well
as the surrounding workbench area. Take care not to wet the touchscreen. To clean the
touchscreen, use the screen suede provided with the DiagCORE Analyzer.
3. Repeat step 2 three times with fresh paper towels.
4. Wet a paper tower in distilled water and wipe down the DiagCORE Analyzer surface to rinse
away detergent remains. Repeat two times.
5. Dry the DiagCORE Analyzer surface with a fresh paper towel.
7.3 Decontaminating the DiagCORE Analyzer surface
WARNING
CAUTION
Risk of personal injury and material damage
Wear protective glasses, a lab coat and gloves when cleaning the
instrument to avoid biological and chemical hazards.
Bleach is irritating to eyes and skin and may release dangerous gases
(chlorine). Wear adequate personal protection equipment.
WARNING
CAUTION
Risk of personal injury and material damage
Disconnect the DiagCORE Analyzer from the power outlet before
cleaning.
CAUTION
Risk of damage to the DiagCORE Analyzer
Avoid spilling chemicals or other liquids into the DiagCORE Analyzer.
Damage caused by liquid spillage will void the warranty.
CAUTION
Risk of damage to the DiagCORE Analyzer
Avoid spilling liquids on or wetting the touchscreen. To clean the
touchscreen, use the screen suede provided with the DiagCORE
Analyzer.
Use the following to decontaminate the DiagCORE Analyzer surface:
10% bleach solution
Paper towels
Distilled water
Follow the steps below to decontaminate the DiagCORE Analyzer surface:
1. Wear laboratory gloves, coat and protective glasses.
2. Wet a paper towel in the bleach solution and wipe down the DiagCORE Analyzer surface, as
well as the surrounding workbench area. Take care not to wet the touchscreen. Wait at least
for three minutes to allow the bleach solution to react with the contaminants.
3. Change into a new pair of gloves.
4. Repeat steps 2-3 two additional times with fresh paper towels.

60 DiagCORE Analyzer User Manual
5. Wet a paper tower in distilled water and wipe down the DiagCORE Analyzer surface to rinse
away any bleach solution. Repeat twice.
6. Dry the DiagCORE Analyzer surface with a fresh paper towel.
7.4 Replacing the air filter
The air filter must be exchanged every year to ensure the appropriate airflow rate inside the unit.
The air filter is located below the instrument and can be accessed by the user at the front of the
instrument.
Only air filters from STAT-Dx Life, S.L.must be used as replacement.
Follow these steps to exchange the air filter:
1. Set the DiagCORE Analyzer in standby mode by pressing the front ON/OFF button.
2. Place a hand below the air filter drawer at the front of the DiagCORE Analyzer and use fingers
to push slightly up.
3. Pull the air filter back until the filter drawer is completely removed. Dispose of it.
4. Remove the new air filter drawer from its protective bag.
5. Insert the new air filter drawer into the DiagCORE Analyzer. The unit is now ready for use.
CAUTION
Risk of damage to the DiagCORE Analyzer
Only use original parts from STAT-Dx Life, S.L. Use of non-authorized parts
may result in damage to the unit and will void the warranty.
7.5 DiagCORE Analyzer repair
The DiagCORE Analyzer can only be repaired by field service technicians authorized by STAT-Dx
Life, S.L. If the DiagCORE Analyzer is not working as expected, contact Technical Support using the
contact information at the beginning of this manual.
WARNING
CAUTION
Risk of personal injury and material damage
Do not open the DiagCORE Analyzer housing. Do not attempt to repair
or modify the DiagCORE Analyzer.
Opening the housing or modifying the DiagCORE Analyzer
inappropriately may result in user injury and DiagCORE Analyzer
damage and will void the warranty.

DiagCORE Analyzer User Manual 61
8 Troubleshooting
This section provides information on some issues that may occur with the DiagCORE Analyzer, along
with possible causes and solutions. The information is specific to the instrument. For troubleshooting
relevant to a DiagCORE cartridge, see the instructions for use of the respective cartridge.
If further assistance is required, contact Technical Support using the contact information below.
Website: http.support.qiagen.com
When contacting Technical Support about an error with the DiagCORE Analyzer, note the steps
leading up to the error and any information appearing in any dialog boxes. This will help us solve
the problem.
When calling Technical Support about errors, please have the following information ready:
Instrument serial number, type and version
Error code (if applicable)
Timepoint when the error occurred for the first time
Frequency of error occurrence (i.e., intermittent or persistent error)
Photo of error, if possible
Copy of support package
Problem
Possible Cause
Solution
The DiagCORE Analyzer
does not start.
The DiagCORE Analyzer is not
connected to the power outlet.
The power switch at the back
of the DiagCORE Analyzer is
not turned on.
The DiagCORE Analyzer is in
standby mode.
Check that the DiagCORE
Analyzer is connected to the
power mains.
Turn on the power switch at
the back of the DiagCORE
Analyzer.
Press the ON/OFF button to
take the DiagCORE Analyzer
out of standby mode.
Analytical Module not
detected.
Analytical/Operational Module
bridge is not properly
connected.
Check that the bridge
between the Operational
Module and the Analytical
Module is properly
connected.
The Analytical Module status
indicator is red.
Hardware failure.
Call Technical Support.

62 DiagCORE Analyzer User Manual
Problem
Possible Cause
Solution
The touchscreen does not
respond.
The DiagCORE Analyzer is in
standby mode (status indicator
is blue).
Hardware failure.
Press the ON/OFF button on
the Operational Module.
Call Technical Support.
Barcode reader does not
scan.
Sample ID barcode feature is
not enabled.
Barcode reader has a
hardware or software problem.
Contact a laboratory
supervisor or instrument
administrator to configure
the barcode feature in the
DiagCORE Analyzer.
Call Technical Support.
The cartridge is stuck inside
the DiagCORE Analyzer.
Module mechanical failure.
Call Technical Support.
Lid of the cartridge
entrance port does not
open.
Module mechanical failure.
Call Technical Support.
The Run Test button is not
active.
A cartridge is still inside the
DiagCORE Analyzer and must
be ejected before the
DiagCORE Analyzer will allow a
new test execution.
The module is not available.
The status box of the module
in the Module status bar
should show the text “Eject
cartridge”. Press the status
box of the module and then
press the Eject.
Check that the bridge
between the Operational
Module and the Analytical
module is properly
connected.
Assay does not run.
The user has no rights to run the
test.
The assay is not installed on the
DiagCORE Analyzer.
Contact a laboratory
supervisor or instrument
administrator.
The assay needs to be
installed. Contact a
laboratory supervisor or
instrument administrator.

DiagCORE Analyzer User Manual 63
8.1 Errors and warning messages
Warning messages
Explanation
What to do
The AM in the slots has
changed.
The system detects that the
hardware configuration has
changed. At least one of the
Analytical Modules has been
changed to another
location.
Nothing needs to be done.
The system is self-configurable
after a module’s location
changes.
Making a backup is
recommended before
updating or restoring.
Data may be lost during the
update process if an error
occurs. A backup allows for
system and data recovery.
We highly recommend
making a backup of the
system before a system
restoration or update.
Shutdown not possible. Please
stop all tests and eject
cartridges.
When a test is running, the
DiagCORE Analyzer cannot
be shut down.
Wait until test completion, or
abort the test and then shut
down the system.
Calibration of nnn required in
ddd days.
Calibration action needed.
Contact Technical Support.
Free disc space ddd reached
warning or critical level.
System needs to be reviewed
by Technical Support to add
additional disk space.
Contact Technical Support.
The system was not shut down
properly last time.
The system was not shut
down following the
procedure. The last test data
could have been lost.
Test result with invalid data
found.
An unexpected error during
last test was found.
Try to re-run the test with a
new cartridge. If the problem
persists or occurs frequently,
contact Technical Support.
Unexpected behavior of AM
nnn.
General system failure.
Restart the system.
If the problem persists,
contact Technical Support.
Update data aborted, error
occurred.
Unexpected error occurred
while updating the
DiagCORE Analyzer.
Contact Technical Support.

64 DiagCORE Analyzer User Manual
Warning messages
Explanation
What to do
No backup file found!
A proper backup file .dbk
was not found in the USB
storage device.
Check if the file exists in the
USB storage device. If the
problem persists, contact
Technical Support.
Assay <assay_id> not
available.
Code: 0x400
The assay corresponding to
the cartridge has not been
imported to the DiagCORE
Analyzer.
Import the assay to the
DiagCORE Analyzer (see
section 6.7.2 Importing new
assays).
Assay <assay_name> not
active.
The assay is not active.
Activate the assay (see
section 6.7.1 Managing
available assays).
Assay <assay_name> already
imported. Code: 0x0304
There is an assay with the
same ID and version
available in the database.
The assay is already loaded in
the system. Nothing needs to
be done.
Import assay failed; the assay
file is invalid.
The assay file to be imported
is not correct.
Download the assay file
again from the STAT-Dx Life,
S.L. website. Contact
Technical Support if the issue
persists.
Importing ADF <adf_name>
failed.
Code: 0x0305
The assay file to be imported
is not correct.
Download the assay file
again from the STAT-Dx Life,
S.L. website. Contact
Technical Support if the issue
persists.
Login failed!
The login operation failed.
Contact the instrument
administrator.
Login failed!
The user is not activated.
User does not have
permission to use the
DiagCORE Analyzer.
Contact a laboratory
supervisor or instrument
administrator to activate the
user (see section 6.6.1
Accessing and managing the
list of users).

DiagCORE Analyzer User Manual 65
Warning messages
Explanation
What to do
Login failed!
Wrong Password!
The entered password is
incorrect.
After three failed attempts to
enter the password, the user
must wait one minute before
trying to log in again. In case
the password has been
forgotten, contact the
instrument administrator to set
a new one.
Login failed!
User identification does not
exist.
User has not been added to
the system.
Contact the administrator or
laboratory supervisor to add
the new user.
Passwords are not identical!
To set up a new password, it
must be entered identically
two times.
Invalid Password! Min. length
6 characters. Max. length 15
characters. Allowed
characters: 0-9, a-z A-Z, _,
space.
Password does not comply
with security policies.
Set a password with a
minimum length of 6
characters and maximum
length of 15 characters,
containing only the allowed
characters: 0-9, a-z A-Z, _,
space.
Export failed!
An unexpected error
occurred during the results
export operation.
Retry the operation. If the
problem persists, contact
Technical Support.
USB Device not found.
USB storage device is not
detected in the DiagCORE
Analyzer.
Insert a USB storage device in
the USB port.
Barcode reading failed.
Barcode reader malfunction.
Contact Technical Support.
Failed to scan barcode.
There is no assay loaded in
the system for this barcode.
The barcode may be
damaged. Use a different
cartridge.
Contact Technical Support if
the problem persists.

66 DiagCORE Analyzer User Manual
Warning messages
Explanation
What to do
Test failed, Error:
<error_code>.
Test failed with an error.
Retry executing the test with
a new cartridge. If the
problem persists, contact
Technical Support and
provide the error code
message.
User has no right to execute
assay <assay_name>.
Code: 0x0402
Permission to execute the
assay has not been given to
the user.
Permission can be granted
from the User Management
screen (refer to section 6.6
User management).
Cartridge already used.
A previously used cartridge
cannot be re-used.
Dispose of the used cartridge
according to relevant safety
and disposal regulations. Run
the test using a new
cartridge.
Cartridge expired.
The cartridge cannot be
used because its expiration
date has elapsed.
The cartridge can no longer
be used. Dispose of the
cartridge according to
relevant safety and disposal
regulations.
Different cartridge inserted.
The cartridge inserted does
not match the cartridge
detected by the barcode
reader.
Insert the same cartridge
scanned by the barcode
reader.
Failed to create file.
The backup file could not be
created.
The USB storage device is not
working. Retry using a
different USB storage device.

DiagCORE Analyzer User Manual 67
9 Appendices
9.1 Technical specifications
Description
Details
Power Requirements
90–264 VAC
50–60 Hz
IEC 60320-1 C14 socket
Fuse
1x8A time-lag
Operating Conditions
Temperature: 15–30 °C (59–86 °F)
Humidity conditions: 20–80% relative, non-condensing
Altitude 0–2200 m
Light up to 4000 lux
Shipping Conditions
Temperature: 0 to +38 °C (+32 to +100 °F), maximum 85% relative
humidity, non-condensing
Dimensions and Weight
Operational Module: Width/Height/Depth [mm]: 234/326/517; 5
kg
Analytical Module: Width/Height/Depth [mm]: 153/307/428; 16 kg
EMC Requirements
Compliant with IEC 61326 Class A
The equipment has been designed and tested to CISPR 11 Class
A. In a domestic environment it may cause radio interference, in
which case, you may need to take measures to mitigate the
interference.
Ethernet Interface
1x 10/100 –Base-T Ethernet
USB Ports
1 front and 3 rear
9.2 Disposal information
Recycling of the DiagCORE Analyzer can be provided by STAT-Dx Life upon request at additional
cost. In the European Union, in accordance with the specific WEEE recycling requirements and
where a replacement product is being supplied, free recycling of its WEEE-marked electronic
equipment is provided.

68 DiagCORE Analyzer User Manual
To recycle electronic equipment, contact STAT-Dx Life for the required return form. Once the form
is submitted, we will request follow-up information to schedule collection of the electronic waste or
provide an individual quote.
9.3 Declaration of Conformity
Name and address of the legal manufacturer:
STAT-Dx Life, S.L.
Baldiri Reixac 4-8
08028 Barcelona, Spain
An up-to-date Declaration of Conformity can be requested from STAT-Dx Life, S.L.
9.4 Glossary
Analytical Mode (AM): The main DiagCORE Analyzer hardware module, in charge of executing
tests on DiagCORE cartridges. It is controlled by the Operational Module.
Assay Definition File: An Assay Definition File is a file need in order to execute an assay on a
DiagCORE analyzer. The content of the file describes what can be measured, how to measure it
and how to evaluate the raw measurement results. The file should be imported in the DiagCORE
Analyze before executing an assay the first time
GUI: Graphical user interface.
IFU: Instructions for use.
Operational Module (OM): The dedicated DiagCORE Analyzer hardware that provides the user
interface for one to four Analytical Modules (AM).
User: A person who operates the DiagCORE Analyzer in the intended way.
9.5 Trademarks and copyright
DiagCORE® is a registered trademark of STAT-Dx Life, S.L. Windows® is a registered trademark of
Microsoft Corporation.
All other trademarks are the property of their respective owners.
This DiagCORE Analyzer User Manual is protected by copyright. Neither this manual, nor any part
thereof, may be reproduced, modified, or distributed by any means now known or later
developed.
© 2017 STAT-Dx Life, S.L. — ALL RIGHTS RESERVED
9.6 Copyright, disclaimer and warranty
INFORMATION IN THIS DiagCORE Analyzer USER MANUAL IS PROVIDED IN CONNECTION WITH STATDx Life DiagCORE Analyzer PRODUCT. NO LICENSE, EXPRESS OR IMPLIED, BY ESTOPPEL OR
OTHERWISE, TO ANY INTELLECTUAL PROPERTY RIGHTS IS GRANTED BY THIS DOCUMENT. EXCEPT AS
PROVIDED IN STAT-Dx Life TERMS AND CONDITIONS OF SALE FOR THE DiagCORE Analyzer, STAT-Dx
Life ASSUMES NO LIABILITY WHATSOEVER AND DISCLAIMS ANY EXPRESS OR IMPLIED WARRANTY
RELATING TO THE USE OF THE DiagCORE Analyzer INCLUDING LIABILITY OR WARRANTIES RELATING

DiagCORE Analyzer User Manual 69
TO MERCHANTABILITY, FITNESS FOR A PARTICULAR PURPOSE, OR INFRINGEMENT OF ANY PATENT,
COPYRIGHT, OR OTHER INTELLECTUAL PROPERTY RIGHT ANYWHERE IN THE WORLD.
The DiagCORE Analyzer is equipped with an Ethernet port. The Purchaser of the DiagCORE Analyzer
is solely responsible for preventing any and all computer viruses, worms, trojans, malware, hacks, or
any other type of cybersecurity breaches. Stat Diagnostica assumes no liability for computer
viruses, worms, trojans, malware, hacks, or any other type of cybersecurity breaches.
9.7 Patent statement
TO THE EXTENT PERMITTED BY LAW, NO RIGHTS FOR RESALE ARE CONFERRED WITH THE PURCHASE OF
THIS PRODUCT.
Purchase of the DiagCORE Analyzer, and/or associated DiagCORE cartridges, does not grant any
rights under patents owned or controlled, now or later, by STAT-Dx Life except to the extent
necessary to operate the DiagCORE Analyzer as specified in this DiagCORE Analyzer User Manual.
9.8 Software License Agreement
STAT-Dx Life, S.L. is the owner, or licensee with right to sublicense, of all rights necessary to provide
to the Purchaser of the DiagCORE Analyzer and associated DiagCORE cartridges, a license to the
software embedded within the DiagCORE Analyzer or associated DiagCORE cartridges, software
supplied on physical media by STAT-Dx Life, or software downloaded to the DiagCORE Analyzer or
associated standard PC (see Glossary, section 9.5 above), collectively the “Software.” Physical
media and copies thereof in any form are, and remain, the property of STAT-Dx Life. The Software
is on loan to the Purchaser of the DiagCORE Analyzer and associated DiagCORE cartridges for the
term of this Software License Agreement. Purchaser must not cause or permit the decompilation,
disassembly, or reverse engineering of the Software; nor transfer the Software in whole or in part to
any third party without the prior written consent of STAT-Dx Life, which will not be unreasonably
withheld.
STAT-Dx Life grants to Purchaser of the DiagCORE Analyzer and associated DiagCORE cartridges a
non-exclusive, non-transferable license to use one copy of the Software in connection with one
DiagCORE Analyzer, and to make one back-up copy. This Software License Agreement is effective
until terminated, and may be terminated by STAT-Dx Life if Purchaser of the DiagCORE Analyzer
and associated DiagCORE cartridges fails to comply with the terms and conditions of this Software
License Agreement, and is given written notice by STAT-Dx Life. Upon termination of this Software
License Agreement the Purchaser of the DiagCORE Analyzer and associated DiagCORE cartridges
must destroy all copies of the Software.
PURCHASER OF THE DIAGCORE ANALYZER EXPRESSLY ACKNOWLEDGES AND AGREES THAT USE OF THE
SOFTWARE IS AT YOUR SOLE RISK. TO THE MAXIMUM EXTENT PERMITTED BY APPLICABLE LAW, THE
SOFTWARE IS PROVIDED "AS IS" AND “AS AVAILABLE,” WITH ALL FAULTS AND WITHOUT WARRANTY OF ANY
KIND, AND STAT-Dx Life HEREBY DISCLAIMS ALL WARRANTIES AND CONDITIONS WITH RESPECT TO THE
SOFTWARE, EITHER EXPRESS, IMPLIED, OR STATUTORY, INCLUDING, BUT NOT LIMITED TO, THE IMPLIED
WARRANTIES AND/OR CONDITIONS OF MERCHANTABILITY, OF SATISFACTORY QUALITY, OF FITNESS FOR A
PARTICULAR PURPOSE, OF ACCURACY, OF QUIET ENJOYMENT, AND OF NONINFRINGEMENT OF THIRDPARTY RIGHTS. NO ORAL OR WRITTEN INFORMATION OR ADVICE GIVEN BY STAT-Dx Life OR ITS AUTHORIZED
REPRESENTATIVE(S) SHALL CREATE A WARRANTY. SHOULD THE SOFTWARE PROVE DEFECTIVE, YOU ASSUME
THE ENTIRE COST OF ALL NECESSARY SERVICING, REPAIR, OR CORRECTION. SOME JURISDICTIONS DO

70 DiagCORE Analyzer User Manual
NOT ALLOW THE EXCLUSION OF IMPLIED WARRANTIES OR LIMITATIONS ON APPLICABLE STATUTORY RIGHTS
OF A CONSUMER, SO THE ABOVE EXCLUSION AND LIMITATIONS MAY NOT APPLY TO YOU.
TO THE EXTENT NOT PROHIBITED BY LAW, IN NO EVENT SHALL STAT-Dx Life BE LIABLE FOR PERSONAL INJURY
OR ANY INCIDENTAL, SPECIAL, INDIRECT, OR CONSEQUENTIAL DAMAGES WHATSOEVER, INCLUDING,
WITHOUT LIMITATION, DAMAGES FOR LOSS OF PROFITS, LOSS OF DATA, BUSINESS INTERRUPTION, OR ANY
OTHER COMMERCIAL DAMAGES OR LOSSES, ARISING OUT OF OR RELATED TO YOUR USE OF OR INABILITY
TO USE THE SOFTWARE, HOWEVER CAUSED, REGARDLESS OF THE THEORY OF LIABILITY (CONTRACT, TORT,
OR OTHERWISE) AND EVEN IF LICENSOR HAS BEEN ADVISED OF THE POSSIBILITY OF SUCH DAMAGES. SOME
JURISDICTIONS DO NOT ALLOW THE LIMITATION OF LIABILITY FOR PERSONAL INJURY, OR OF INCIDENTAL
OR CONSEQUENTIAL DAMAGES, SO THIS LIMITATION MAY NOT APPLY TO YOU. IN NO EVENT SHALL STATDx Life S.L.’s TOTAL LIABILITY TO YOU FOR ALL DAMAGES (OTHER THAN AS MAY BE REQUIRED BY
APPLICABLE LAW IN CASES INVOLVING PERSONAL INJURY) EXCEED THE PURCHASE PRICE OF THE
DIAGCORE ANALYZER. THE FOREGOING LIMITATIONS WILL APPLY EVEN IF THE ABOVE STATED REMEDY
FAILS OF ITS ESSENTIAL PURPOSE.
To the extent that any terms and conditions in this DiagCORE Analyzer User Manual differ from the terms
and conditions in the Purchase or Sale Agreement for the DiagCORE Analyzer, the terms and conditions
in the Purchase or Sale Agreement will control.

DiagCORE Analyzer User Manual 71
10 Index
A
Aborting a test ................................................. 28
Adding users ..................................................... 44
Amplification curves ....................................... 30
Appendices ...................................................... 68
Assay management ....................................... 45
Available assays ......................................... 45
Importing new assays ................................ 46
Automatic log out .................................... 40, 50
B
Backing up the system ................................... 55
Barcode ............................................................ 24
Biological safety............................................... 11
C
Cartridge
Disposal ........................................................ 27
Ejecting the cartridge ................................ 27
Inserting a cartridge ................................... 26
Preparing the cartridge ............................. 23
Removal ....................................................... 27
Changing passwords ...................................... 56
Chemical safety .............................................. 10
Contact information .................................. 2, 61
Content area ................................................... 38
Controls
Controls tab ................................................. 30
Failed controls ............................................. 31
Passed controls ........................................... 31
Copyright, disclaimer and warranty ............ 69
Customer and technical support ................... 2
D
Declaration of conformity ............................. 69
DiagCORE Analyzer status ............................. 56
Disposal information ........................................ 68
E
Electrical safety ................................................ 10
Ending a test ..................................................... 28
Errors and warning messages ........................ 64
Exporting results ............................................... 34
F
Filtering results................................................... 34
G
General settings ............................................... 49
Automatic log off ....................................... 50
Exclude modules ........................................ 50
Patient ID mandatory ................................ 50
Prefer patient ID barcode ......................... 50
Prefer sample ID barcode ........................ 50
Require password ....................................... 50
Restore factory default.............................. 51
Sample ID mandatory ............................... 50
Use Patient ID .............................................. 50
User access control .................................... 49
General status bar ........................................... 35
Glossary ............................................................. 69
H
HIS/LIS settings .................................................. 53
I
Installation ......................................................... 16
Repackaging for shipment ....................... 21
Site ................................................................. 16
System components .................................. 16
Unpacking and installing........................... 18
Intended use ...................................................... 7
L
Limitations of use ............................................... 7
Logging out ...................................................... 40
Login screen ..................................................... 38
M
Main menu bar ................................................ 37
Main screen ...................................................... 35
Content area .............................................. 38
General status bar ...................................... 35
Main menu bar ........................................... 37
Module status bar ....................................... 36
Maintenance ................................................... 58
Analyzer repair ............................................ 60
Cleaning the Analyzer ............................... 58
Decontaminating the Analyzer ............... 59
Replacing the air filter ............................... 60
Module status bar............................................ 36
N
Network settings............................................... 52
O
Options menu .................................................. 40

72 DiagCORE Analyzer User Manual
P
Passwords .......................................................... 56
Patent statement ............................................ 70
Printer settings .................................................. 51
Printing reports .......................................... 29, 34
R
Regional settings.............................................. 47
Date .............................................................. 47
Language .................................................... 48
Time ............................................................... 47
Repackaging for shipment ............................ 21
Results
Amplification curves .................................. 30
Controls ........................................................ 31
Controls tab ................................................. 30
Exporting results........................................... 34
Filtering results .............................................. 34
Linear scale .................................................. 31
Logarithmic scale ....................................... 31
Melting curves ............................................. 31
Pathogens tab ............................................ 30
Possible outcomes ...................................... 34
Results summary .......................................... 28
Searching results ......................................... 34
Test details.................................................... 32
View results list ............................................. 33
Results summary ............................................... 27
Running a test .................................................. 23
Aborting a test ............................................ 28
Ejecting the cartridge ................................ 27
Inserting the cartridge ............................... 26
Preparing the cartridge ............................. 23
Scanning the cartridge barcode ............ 24
Scanning the sample barcode ................ 24
Selecting the sample type ........................ 25
Starting the Analyzer .................................. 23
Viewing results ............................................. 28
S
Safety information ............................................. 9
Biological safety .......................................... 11
Chemical safety ......................................... 10
Electrical safety ........................................... 10
General safety precautions ........................ 9
Transport precautions ................................ 10
Waste disposal ............................................ 12
Saving reports............................................ 29, 34
Screensaver ...................................................... 40
Searching results .............................................. 34
Shipping the Analyzer ..................................... 21
Shutting down the instrument ....................... 57
Software license agreement ......................... 70
Starting the Analyzer ....................................... 23
Status LEDs ........................................................ 56
Symbols ............................................................... 6
System components ....................................... 16
System configuration ...................................... 47
General settings .......................................... 49
HIS/LIS settings ............................................. 53
Network settings .......................................... 52
Printer settings ............................................. 51
Regional settings ......................................... 47
System backup ........................................... 55
System log .................................................... 53
System update ............................................ 55
Technical log ............................................... 54
Version information .................................... 54
System description .......................................... 13
DiagCORE Analyzer ................................... 13
DiagCORE cartridge .................................. 14
System features ................................................ 35
Logging out ................................................. 40
Login screen ................................................ 38
Main screen ................................................. 35
Options menu.............................................. 40
Screensaver ................................................. 40
User management ..................................... 41
System log ......................................................... 53
System update ................................................. 55
T
Technical log .................................................... 54
Technical specifications ................................. 68
Technical support ............................................ 61
Test details ........................................................ 32
Trademarks and copyright ............................ 69
Transport precautions ..................................... 10
Troubleshooting ............................................... 61
Errors and warning messages ................... 64
U
Unpacking and installation ............................ 18
User access control ......................................... 49
User management .......................................... 41
Adding users ................................................ 44
Assay statistics ............................................. 44
Assign assays ............................................... 43
Assign user profiles ...................................... 43
Managing users .......................................... 42
Multi user mode .......................................... 41
Single user mode ........................................ 41
User profiles .................................................. 41
User profiles ....................................................... 41
V
Viewing results .................................................. 28
W
Waste disposal ................................................. 12

DiagCORE System, Revision 1.0 © 2017 STAT-Dx Life S.L. All rights reserved.
STAT-Dx Life S.L. · Baldiri Reixac 4, Barcelona, Spain +34 93 448 51 24 · http.support.qiagen.com
DiagCORE®
Analyzer
 Loading...
Loading...