Page 1

Application note
Control of whisker growth in Tin alloy coatings
1 Nature of whiskers and whisker mitigation
techniques
Some metals show an unusual metallurgical phenomenon: a single, microscopic crystal
filament of the metal grows “spon taneously” f rom its surface. The metals concerned include
Zinc, Cadmium, Silver , Tin and some of their allo ys. Because of the ir likeness to microscop ic
hair, these tiny filaments are commonly referred to as “whiskers”.
Scientists believe that whisker growth is mainly due to internal compressive stresses near
the metal surface. Under certain conditions the internal stress can reach a critical level,
leading to the formation of whiskers as a way of reducing the system’s internal energy.
Owing to their excellent electrical properties and solderability, and their low cost, pure Tinplated surfaces have been used for many decades by the electronics industry. Hundreds of
billions (trillions by some estimates taking passives and discretes into account) of
components have been supplied with pure Tin-plated surface finishes. On top of their low
cost, these components operate well and are highly reliable. Only does the occasional
occurrence of reliability problems caused by Tin whiskers tarnish their reputation. An easy
fix to whisker problems w as f ound, that consist ed in adding small amounts of Lead (Pb) – as
low as 3% – to the plating. In so doing, the growth of whiskers was effectively prevented.
AN2035
With the recent European Directive to eliminate Lead from electronic products, there is a
renewed interest in Tin and its alloys as a replacement for Lead-bearing alloys. A better
understanding of the factors which influence whisker formation and the application of new
techniques to control these f actors, alon g with the introduction of modern plating chemistries
and processes, allow the electronics industry to pursue this return to pure Tin-plating
surface finishes. Sin ce whisk er g ro wth is mainly caused by internal compressive stresses, a
number of strategies have been developed to prevent stress development within the Tinplated film. Internal stress in Tin-plated films may o riginate from a number of cause s, among
which are:
a) co-deposited impurities, e. g. organics
b) atomic defects, such as t hose caused by improper plating parameters
c) creation of new phases leading to local v olume changes . These ma y be caused by
either metallurgical or chemical reactions.
d) thermal stress caused by mismatches in the Coefficients of Thermal Expansion
(CTE) between the Tin film and the base metal (and/or additional films beneath
the Tin film).
April 2006 Rev. 2 1/11
www.st.com
Page 2

Contents AN2035 - Application note
Contents
1 Nature of whiskers and whisker mitigation techniques . . . . . . . . . . . . 1
2 Whisker assessment and process qualification . . . . . . . . . . . . . . . . . . 9
3 Whiskers and thermal cycles . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 11
4 Revision history . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 12
2/11
Page 3

AN2035 - Application note List of figures
List of figures
Figure 1. Natural growth of InterMetallic Compound (IMC) at room temperature . . . . . . . . . . . . . . . . 4
Figure 2. Microscope View of Protection by Post-bake Treatment (1 hour at 150°C) . . . . . . . . . . . . . 5
Figure 3. Protection by post-bake treatment (1 hour at 150°C) . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 6
Figure 4. Protection by thickness. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 6
Figure 5. Temperature Cycling SnPb on FeNi42 (250 Cycles of –35 to 125°C) . . . . . . . . . . . . . . . . . 9
Figure 6. Temperature Cycling Sn 100% (500 Cycles of –35 to 125°C) . . . . . . . . . . . . . . . . . . . . . . . 9
3/11
Page 4
AN2035 - Application note
Experience has shown that proper plating practices and chemistries are important in
preventing whisker formation. Early Tin-plating chemistries were designed to produce a
cosmetically appealing shiny surface. This type of plating is known as “bright Tin plating”.
The shiny appearance of the tin-plate d surface is achieved by adding specialized chemicals
to the plating bath, that control the siz e of the gr ains (“gra in refiners”) and t he planarity of the
plated surface (“levelers”). Small grains and fl at surfaces help reflect the light, thus favoring
shiny surfaces. Due to the very nature of the chemistries and the high concentrations of
additives required to achieve bright finishes, these early bright Tin-plating chemistries were
prone to problems of organics co-deposition and atomic irregularities within the plated film,
leading to a higher susceptibility to whisker formation.
Modern chemistries and plating techniques have evolved with a view of preventing earlier
problems of contaminant co-deposition and atomic defect creation within the deposited film.
One major change in some Tin-plating chemistries is the use of much lower levels of grain
refining additives. The result is a duller (or matte) appearance of the Tin plating. For this
reason these chemistries are referred to as Matte Tin.
In a joint effort, Infineon, Philips, Freescale and ST Microelectronics (the so-called E4
Initiative) have tested a large number of modern plating chemistries for their resistance to
whisker growth. From this study a number of suitable commercial Matte Tin-plating
chemistries have been identified.
As mentioned previously, localized phase changes within the Tin film can also cause
localized compressive internal stresses. This happens when a volume increase is
associated with the phase change.
Since Tin and Copper normally form an intermetallic, Cu6Sn5, in a reaction which produces
a significant increase in volume, it is essential to take this into consideration for Tin-plated
copper leadframes.
When the Cu6Sn5 intermetallic forms at low temperatures (e.g. room temperature) the
reaction tends to take place more sp ecially along the grai n boundaries where the diffusion of
the combining elements is highest at lower temperatures due to solid-state diffusional
kinetics. The net result of the combined penetration and expansion of this growing
intermetallic may be envisioned as a “wedge” driven into the Tin layer at the grain boundary.
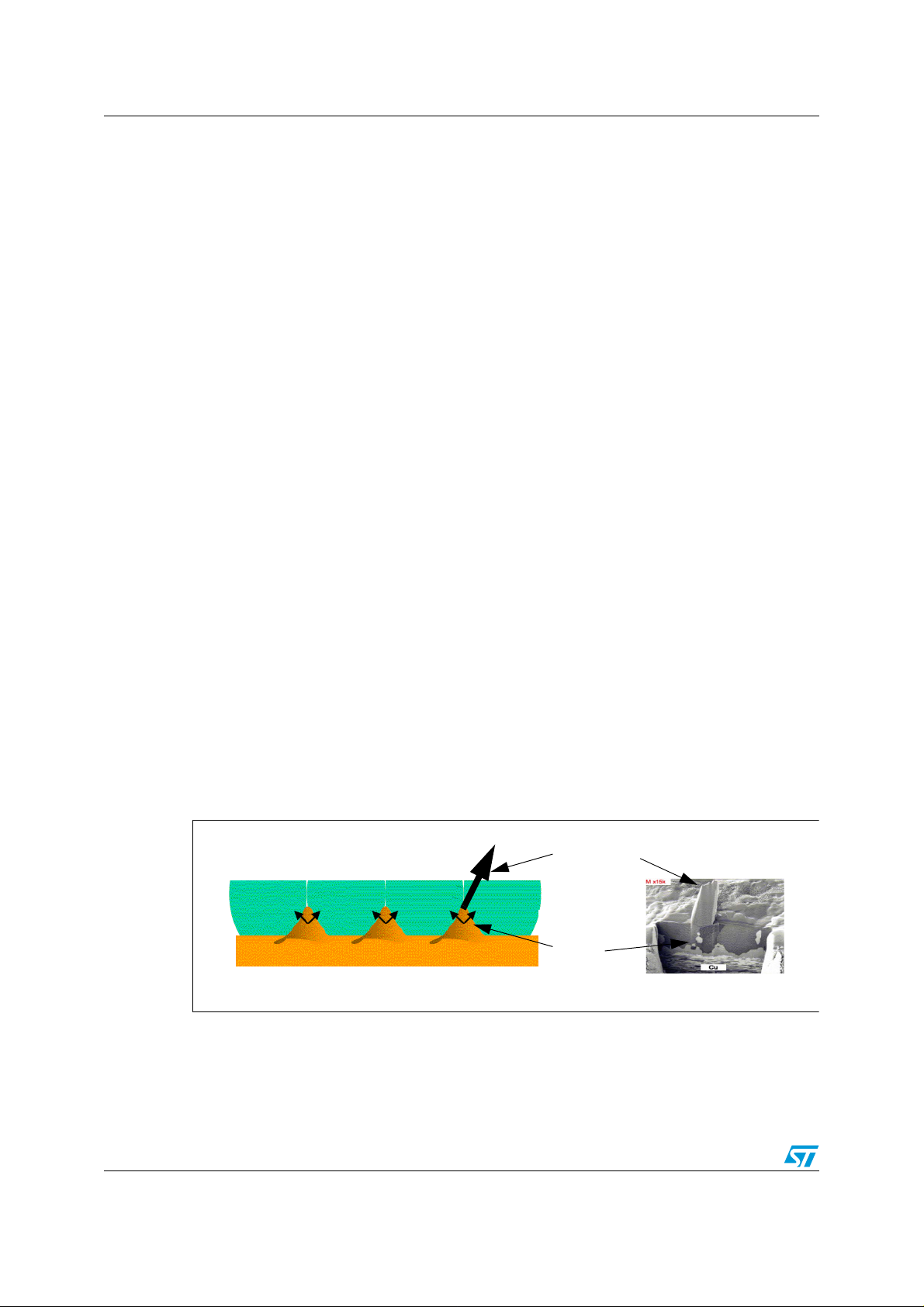
The penetration and growth of Cu6Sn5 intermetallics along grain boundaries is shown in
Figure 1 (schematic and photograph).
Figure 1. Natural growth of InterMetallic Compound (IMC) at room temperature
Tin Whisker
Cu6Sn
5
However, if the Cu
intermetallic is formed under higher temperature conditions (e.g.
6Sn5
around 150°C), a different and more desirable intermetallic structure forms. At higher
temperatures bulk diffusion is activated and the intermetallic reaction occurs more uniformly
across the entire Tin/Copper interface, not just at the grain boundaries. Since the reaction
4/11
Page 5

AN2035 - Application note
rate is virtually uniform across the Tin/Copper interface the resulting Cu6Sn5 structure has
practically no “wedges”.
The difference in the Cu6Sn5 structure is clearly demonstrated in the micrographs in
Figure 2. In these micrographs the Tin was selectively removed by chemical etching, thus
exposing the Cu
intermetallic as well as any copper not covered by the intermetallic.
6Sn5
In the micrographs on the left hand side of Figure 2, the intermetallic was allowed to form at
room temperature over a period of one month. The result is a large, blocky and irregular
Cu
structure located almost exclusively at the grain boundaries.
6Sn5
Note: In this image the large flat spaces between the blocky intermetallic are exposed copper.
By contrast the image on the right hand side of Figure 2 shows a Cu
formed by baking the part within 24 hours of plating at a temperature of 150°C. In this case
the image shows a very uniform layer of Cu
Note: All of the materials in this image are the Cu
after etching.
Figure 2. Microscope View o f Protection by Post-bake Treatment (1 hour at 150°C)
intermetallic
6Sn5
with virtually no “wedges”.
6Sn5
intermetallic. There is no exposed Copper
6Sn5
Large and Irregular IMC
(1 month at Room Temperature)
Thin and Uniform IMC
(1 hour at 150°C)
The ability of a 150°C bake to virtually eliminate the intermetallic “wedges” that lead to large
compressive stress at the g rain boundaries serves as the basis f or the second component of
the whisker mitigation strategy adopted by STMicroelectronics.
The second component of the str ateg y to mit igate whisk er gro wth is to apply a 1-hour 1 50°C
bake within 24hrs to freshly plated Mat te-Tin finishes . This has sev er al beneficial eff ects: the
film is annealed and stress due to atomic-level plating defects is reduced. In addition, and
more importantly, a stable and uniform Cu
layer is created that protects against further
6Sn5
localized penetration of the intermetallic at grain boundaries, thus avoiding the “wedge”
effect that creates compressive stress. Furthermore, the bake creates an additional
beneficial layer of Cu
Sn, underneath the Cu6Sn5 layer.
3
5/11
Page 6

AN2035 - Application note
)
The beneficial effects of the 150°C bake can be seen in the whisker test results shown in
Figure 3. Regardless of the plating thickness none of the samples had grown whiskers after
400 days of storage at room temperature.
Figure 3. Protection by post-bake treatment (1 hour at 150°C)
Time (in days
Length of Longest Whisker (in µm)
Figure 4. Protection by thickness
1.82µm
3.5µm
5.35µm
7.10µm
10.10µm
Length of Longest Whisker (in µm)
Time (in days)
The above whisker mitigation techniques have been agreed with all subcontractors and are
therefore applied to all ST products.
A “Whisker Risk Free” coating does not develop whiskers that can impact electrical
performance, or component reliability. The E4 have chosen 50µm as the maximum whisker
length at the end of the device life (at the end of whisker assessment tests).
6/11
Page 7

AN2035 - Application note Whisker assessment and process qualification
2 Whisker assessment and process qualification
On 1st March 2006, the JEDEC standard organization released the International
Standardized Methodology for the assessment of whisker risk: JESD 201 (Environmental
Acceptance Requirements for Tin Whisker Susceptibility of Tin and Tin Alloy Surface
Finishes) to be implemented together with JESD 22A121 (Test Method for Measuring
Whisker Growth on Tin and Tin Alloy Surface Finishes).
STMicroelectronics will abide by this standard and re-qualify the Tin plating line in
accordance with it.
Since no international standards were available 4 years ago when STM beg an preparing for
the RoHS initiative, customer specifications guided our testing strategy to qualify both our
internal and subcontractor plating lines. This qualification plan satisfies more than eighty
percent of our customers.
The qualification plan used the following stresses and criteria:
All customer specifications imposed a maximum permissible whisker length of 50µm.
● Ambient storage (15°C to 30°C, at 60 % RH) for 6 months
● Dry air: 50°C to 55°C for 6 months
● Thermal cycles: (–35°C to +125°C) for 500 cycles
● Temperature & Humidity: 85°C at 85%RH for 500 hrs.
A total of about 1500 units from 40 different package types have been tested in this phase,
with no evidence of whiskers.
The electronics industry’s search for an appropriate set of stresses for testing the
susceptibility of Tin-surface finishes to whisker formation has continued over the last 4
years. This search has been strongly influenced by several commercial consortia.
Most attention has been focused on the High Humidity stress since some studies have
indicated that this stress may in fact fa v or whisker gro wth. How ev er, there is concern that the
conclusions of some of these studies were flawed, the results being confounded by the
presence of corrosion.
Unfortunately, now as more industry data is being generated, it is apparent that the highhumidity environmental stress state has the ability to introduce corrosion in the test sample
and the corrosion may in turn produce spurious secondary whisker f ormation that is not truly
representative of the integrity of the surface finish under study . As a result, the various
commercial organizations providin g input to the JEDEC task group have changed their
recommendations for the humidity st r ess state used for whisker evaluations at least 4 times
(60°C/95%RH, 60°C/93%RH, 60°C/87%RH and 55°C/85% RH) over the last year.
It is still not clear whether applying high-humidity stress conditions for a long period of time
either relates to a whisker gro wth mechanism or to act ual field usage. For e x ample, a re vie w
of the literature shows no e xamples of whisk ers associa ted with corrosion and it is cle ar that
whiskers have been observed even in vacuum.
7/11
Page 8

Whisker assessment and process qualification AN2035 - Application note
STMicroelectronics along with their E4 partners have continued to study the Matte Tinplating process and the various stresses used to evaluate these processes.
● Tes t Set #1 (started Jan 2004) completed with final readout at 9360 hrs (13
months)
– Package : QFP 176L Cu leadframes
– Plated in Muar plant
– Solder thickness > 7µm
– Maximum whisker length after 2000 TC (thermal cycles) single whiskers on 1 unit
(27 micron length)
– Maximum whisker length after 9360 hrs (13 months) at 60°C/93%RH, 1 single
whiskers on 1 unit (25 micron length) without associated corrosion.
– No whiskers observed at ambient storage after 9360 hrs (13 months).
● Test Set # 2 (started April 2004) completed with final readout at 3600 hr s (5
months)
– Pac kage: QFP10 x10 Cu Leadframe
– Plated in Malta
– Solder thickess > 7µm
– Mounting on board with SAC solde r paste at 250° C max solder reflo w temperatu re
– Maximum whisker length after 3096 hrs (5 months) at 60°C/93% RH, 1 single
whisker on 1 unit (10 micron length ).
● Test Set # 3 (started April 2004), completed with final readout at 3600 hrs (5
months)
– Pac kage: QFP14 x14 Cu Leadframe
– Plated in Muar
– Solder thickess > 7µm
– Mounting on board simulated at 215°C max solder reflow temperature
– Maximum whisker leng th after 3689 hrs (6 months) at 60°C/93% RH, no whiskers
in all tests.
● Test Set # 4 (started Nov. 2004), actual read out 6700 hrs ( 9 months)
– Package: Power SO36L (#2 pcs), Power So20L (#1 pc), DPak (#2 pcs)
– Plated in Muar and Shenzhen plant (2 different chemicals)
– Solder thickness > 7µm
– Mounted on Board with SAC solder paste (NO clean flux)
– NO whiskers found after 6700 hrs exposure at 60°C/93%RH.
8/11
Page 9

AN2035 - Application note Whiskers and thermal cycles
)
3 Whiskers and thermal cycles
In thermal cycles, a different cause of whisker growth exists, which is linked to the mismatch
in thermal coefficient of expansion (TCE) between base material and coating layer, rather
than to localized internal stress. The TCE of Tin, Copper and A42 are 23ppm /°C , 17 ppm/°C
and 4 ppm/°C, respectively. Therefore, whisker growth will be more significant on A42 than
on Copper. Whisker length depends also on the extreme lower limit of the thermal cycles:
the lower this limit, the longer the whisker.
It must be noted that PbSn finishings, too, have a TCE that is close to the one of Matte Tin.
They are exposed to the same whisker growth, and give the same whisker length.
Therefore, Matte Tin behaves in a very similar way to the traditional SnPb coating.
Figure 5. T emperature Cycling SnPb on FeNi42 (250 Cycles of –35 to 125°C)
Whisker on
SnPb15 (10µm
(over FeNi42)
Figure 6. Temperature Cycling Sn 100% (500 Cycles of –35 to 125°C)
Whisker on
SnPb3 (18µm)
(over FeNi42)
9/11
Page 10

Revision history AN2035 - Application note
4 Revision history
Table 1. Document revision history
Date Revision Changes
04-Nov-2004 1 First Issue
Title added to Section 1: Nature of whiskers and whisker
mitigation techniques, section detailed and clarified.
10-Apr-2006 2
Section 2: Whisker assessment and process qualification
updated to latest results, Additional Tests (NEMI evaluation
methodology) title removed and section moved under Section 2.
10/11
Page 11

AN2035 - Application note
Please Read Carefully:
Information in this document is provided solely in connection with ST products. STMicroelectronics NV and its subsidiaries (“ST”) reserve the
right to make changes, corrections, modifications or improvements, to this document, and the products and services described herein at any
time, without notice.
All ST products are sold pursuant to ST’s terms and conditions of sale.
Purchasers are solely res ponsibl e fo r the c hoic e, se lecti on an d use o f the S T prod ucts and s ervi ces d escr ibed he rein , and ST as sumes no
liability whatsoever relati ng to the choice, selection or use of the ST products and services described herein.
No license, express or implied, by estoppel or otherwise, to any intellectual property rights is granted under this document. If any part of this
document refers to any third pa rty p ro duc ts or se rv ices it sh all n ot be deem ed a lice ns e gr ant by ST fo r t he use of su ch thi r d party products
or services, or any intellectua l property c ontained the rein or consi dered as a warr anty coverin g the use in any manner whats oever of suc h
third party products or servi ces or any intellectual property contained therein.
UNLESS OTHERWISE SET FORTH IN ST’S TERMS AND CONDITIONS OF SALE ST DISCLAIMS ANY EXPRESS OR IMPLIED
WARRANTY WITH RESPECT TO THE USE AND/OR SALE OF ST PRODUCTS INCLUDING WITHOUT LIMITATION IMPLIED
WARRANTIES OF MERCHANTABILITY, FITNESS FOR A PARTICUL AR PURPOS E (AND THEIR EQUIVALE NTS UNDER THE LAWS
OF ANY JURISDICTION), OR INFRINGEMENT OF ANY PATENT, COPYRIGHT OR OTHER INTELLECTUAL PROPERTY RIGHT.
UNLESS EXPRESSLY APPROVED IN WRITING BY AN AUTHORIZE REPRESENTATIVE OF ST, ST PRODUCTS ARE NOT DESIGNED,
AUTHORIZED OR WARRANTED FOR USE IN MILITARY, AIR CRAFT, SPACE, LIFE SAVING, OR LIFE SUSTAINING APPLICATIONS,
NOR IN PRODUCTS OR SYSTEMS, WHERE FAILURE OR MALFUNCTION MAY RESULT IN PERSONAL INJURY, DEATH, OR
SEVERE PROPERTY OR ENVIRONMENTAL DAMAGE.
Resale of ST products with provisions different from the statements and/or technical features set forth in this document shall immediately void
any warranty granted by ST fo r the ST pro duct or serv ice describe d herein and shall not cr eate or exten d in any manne r whatsoever , any
liability of ST.
ST and the ST logo are trademarks or registered trademarks of ST in various countries.
Information in this document su persedes and replaces all information previously supplied.
The ST logo is a registered trademark of STMicroelectronics. All other names are the property of their respective owners.
© 2006 STMicroelectronics - All rights reserved
STMicroelectronics group of compan ie s
Australia - Belgium - Brazil - Canada - China - Czech Republic - Finland - France - Germany - Hong Kong - India - Israel - Italy - Japan -
Malaysia - Malta - Morocco - Singapore - Spain - Sweden - Switzerland - United Kingdom - United States of America
www.st.com
11/11
 Loading...
Loading...