Solta Medical Fraxel 1927, Fraxel DUAL 1927, Fraxel DUAL 1550, Fraxel 1550, Fraxel 1927 Operator's Manual
Page 1

Fraxel® 1550, Fraxel 1927 and Fraxel DUAL 1550/1927 Laser System
Fraxel® 1550, Fraxel 1927 and
Fraxel DUAL 1550/1927
Laser Systems Operator Manual
Fractional Resurfacing with IOTS
Intelligent Optical Tracking® System
Manufactured by
Solta Medical, Inc.
25881 Industrial Blvd
Hayward, CA 94545 U.S.A.
Phone: +1 510-782-2286
Customer Service +1 510-782-2286; or
Authorized Distributor
Part Number 10-05170 Rev. O Page 1 of 92
Page 2

Fraxel® 1550, Fraxel 1927 and Fraxel DUAL 1550/1927 Laser System
This manual may not be copied, translated, or reproduced in whole or in part without the
express written consent of Solta Medical, Inc. (henceforth “Solta”).
Solta Medical™, Fraxel, the Fraxel logo and Intelligent Optical Tracking are trademarks or
registered trademarks of Solta Medical, Inc. and its subsidiaries. Other names and brands
may be claimed as the property of others.
Various aspects of the Fraxel 1550, Fraxel 1927 and Fraxel DUAL 1550/1927 Laser
Systems are covered by U.S. Patents 5,897,549 and 6,083,217, 7,282,060, 7,372,606;
7,646,522, D604,843, D601,697 and foreign equivalents and by patents pending.
®
Cidex
and Enzol® are registered trademarks of Johnson and Johnson, Inc.
© Solta Medical, Inc.
June 2013
Published in the USA
Part Number 10-05170 Rev. O Page 2 of 92
Page 3

Fraxel® 1550, Fraxel 1927 and Fraxel DUAL 1550/1927 Laser System
Solta Medical, Inc.
Fraxel 1550, Fraxel 1927 and
Fraxel DUAL 1550/1927 Laser
Systems
THE SOLTA MEDICAL FRAXEL 1550 (previously known as the Fraxel re:store,
FRAXEL 1927 AND FRAXEL DUAL 1550/1927 (previously known as the Fraxel re:store
DUAL ) LASER SYSTEMS ARE ALL NON-ABLATIVE LASERS AND INTENDED FOR
USE ONLY BY PROPERLY TRAINED PHYSICIANS AND PROPERLY TRAINED
PERSONS UNDER THE SUPERVISION OF SUCH A TRAINED P HYSICIAN
(HENCEFORTH “THE PHYSICIAN”).
PLEASE READ THE SOLTA MEDICAL FRAXEL 1550, FRAXEL 1927 AND FRAXEL
DUAL 1550/1927 LASER SYSTEMS OPERATORS MANUAL IN ITS ENTIRETY PRIOR
TO USE OF THE LASER SYSTEM.
Part Number 10-05170 Rev. O Page 3 of 92
Page 4

Fraxel® 1550, Fraxel 1927 and Fraxel DUAL 1550/1927 Laser System
TableofContents
1.0 SYSTEMOVERVIEW.........................................................................................................................7
1.1 INDICATIONSFORUSE.................................................................................................................................8
1.2 DEVICEOVERVIEW.....................................................................................................................................9
1.3 INTRODUCTIONTOTHESYSTEM....................................................................................................................9
1.4 CONTROLFEATURES.................................................................................................................................13
1.5 SYSTEMSTATES.......................................................................................................................................15
2.0 SYSTEMOPERATION......................................................................................................................16
2.1 OVERVIEW..............................................................................................................................................16
2.2 ROUTINEPRECAUTIONSPRIORTOTREATMENT..............................................................................................16
2.3 SYSTEMSTATEDESCRIPTIONS.....................................................................................................................18
2.4 MEASUREMENT‐GUIDEDDOSIMETRY..........................................................................................................33
2.5 DEFINITIONS...........................................................................................................................................39
2.6 PARAMETERSELECTION.............................................................................................................................39
2.7 TOTALTREATMENTDENSITY(TOTALMTZS/CM2)........................................................................................40
2.8 SYSTEMAUDIBLEFEEDBACK.......................................................................................................................40
2.9 HANDPIECETRACKING(IOTS–INTELLIGENTOPTICALTRACKING®SYSTEM)........................................................41
2.10 TIPSELECTION,CLEANINGANDHIGHLEVELDISINFECTIONPROCEDURE..............................................................42
2.11 NORMALSHUT‐DOWNPROCEDURE.............................................................................................................45
3.0 SAFETY...........................................................................................................................................46
3.1 GENERALCONSIDERATIONSANDHAZARDPOTENTIAL.....................................................................................47
3.2 FIREPRECAUTIONS...................................................................................................................................47
3.3 OPTICALSAFETY......................................................................................................................................49
3.4 OPERATIONOFTHELASERSYSTEM..............................................................................................................53
3.5 ELECTRICALSAFETY..................................................................................................................................55
3.6 LASERTREATMENTCONTROLLEDAREA(LTCA)INFORMATION.........................................................................56
3.7 LASERSUITEACCESSANDREMOTEDOORINTERLOCK.....................................................................................56
3.8 PROPERLASERROUTINEMAINTENANCE,HANDLING,ANDSTORAGE..................................................................57
3.9 REGULATORYCOMPLIANCEANDDEVICESAFETYINFORMATION........................................................................59
3.10 LABELING...............................................................................................................................................61
4.0 PHYSICIANINFORMATION..............................................................................................................67
4.1 INDICATIONSFORUSE...............................................................................................................................67
4.2 CONSIDERATIONS.....................................................................................................................................67
4.3 CONTRAINDICATIONS................................................................................................................................68
4.4 TISSUEINTERACTION.................................................................................................................................68
4.5 PRE‐TREATMENTPATIENTRECOMMENDATIONS............................................................................................69
4.6 PRE‐OPERATIVEINSPECTION......................................................................................................................69
4.7 ANESTHESIA............................................................................................................................................69
4.8 PATIENTPREPARATION.............................................................................................................................71
4.9 SPOTTREATMENTS...................................................................................................................................72
4.10 IMMEDIATEPOST‐OPERATIVECARE.............................................................................................................72
4.11 POST‐OPERATIVEINFORMATION.................................................................................................................72
4.12 TREATMENTSREQUIREDANDTREATMENTINTERVALS.....................................................................................73
4.13 COMPLAINTSANDADVERSEEFFECTS...........................................................................................................73
4.14 COMPLICATIONS......................................................................................................................................74
5.0 MAINTENANCEANDWARRANTY....................................................................................................75
5.1 WARRANTYINFORMATION........................................................................................................................75
Part Number 10-05170 Rev. O Page 4 of 92
Page 5

Fraxel® 1550, Fraxel 1927 and Fraxel DUAL 1550/1927 Laser System
5.2 CLEANINGPROCEDURES............................................................................................................................75
5.3 DISPOSALANDRECYCLING.........................................................................................................................76
6.0 RE‐ORDERINFORMATION..............................................................................................................77
6.1 ACCESSORIES...........................................................................................................................................77
6.2 *EXTERNALSTEELEYEPROTECTION..............................................................................................................77
6.3 *CORNEALSHIELDS..................................................................................................................................77
6.4 *CORNEALSHIELDLUBRICATIONGEL...........................................................................................................77
6.5 *TOPICALCORNEALANESTHETIC................................................................................................................77
7.0 DECONTAMINATIONOFRETURNEDEQUIPMENT............................................................................78
8.0 SAFETYSTANDARDSANDREGULATORYCLASSIFICATIONS..............................................................79
8.1 GENERALSTANDARDS...............................................................................................................................79
9.0 FRAXELLASERSYSTEMSSPECIFICATIONS........................................................................................80
9.1 TREATMENTBEAM...................................................................................................................................80
9.2 CONSOLE................................................................................................................................................80
9.3 LASERSAFETYEYEWEAR............................................................................................................................81
9.4 CART.....................................................................................................................................................81
9.5 OPERATINGENVIRONMENT........................................................................................................................81
9.6 SHIPPINGANDSTORAGE(NON‐OPERATIONAL)..............................................................................................81
9.7 COMPATIBLEDELIVERYDEVICES.................................................................................................................81
9.8 ELECTROMAGNETICCOMPATIBILITYANDIMMUNITY........................................................................................81
10.0 EXTERNALFACTORYCALIBRATIONINSTRUCTIONS.........................................................................86
10.1 TOOLSANDPARTSREQUIRED.....................................................................................................................86
10.2 PROCEDURE............................................................................................................................................86
11.0 SHIPPING,INSTALLATIONANDSET‐UPREQUIREMENTS..................................................................87
11.1 SHIPPING................................................................................................................................................87
11.2 INSTALLATION.........................................................................................................................................87
11.3 SPACEREQUIREMENTS.............................................................................................................................. 87
11.4 ENVIRONMENTAL............................................................................................................................... ......88
11.5 ELECTRICAL.............................................................................................................................................88
12.0 LABELINGSYMBOLS.......................................................................................................................90
Part Number 10-05170 Rev. O Page 5 of 92
Page 6

Fraxel® 1550, Fraxel 1927 and Fraxel DUAL 1550/1927 Laser System
Warnings, Cautions, and Notes
Please note that the Fraxel 1550 was previously known as the Fraxel re:store and the
Fraxel DUAL 1550/1927 was previously known as the Fraxel re:store DUAL.
This operator’s manual contains certain warnings, cautions and notes designed to alert the
operator to the proper care and use of the Fraxel 1550, Fraxel 1927 and Fraxel DUAL
1550/1927 Laser Systems, (hereinafter referred to as “Fraxel Laser Systems”) and
accessories. This manual refers to the Fraxel 1550, the Fraxel 1927 and the Fraxel DUAL
1550/1927 laser systems unless specifically stated. The cool roller tip / focal cooling feature
described in this manual is not available on all models. The following illustrates each of
these messages and how to recognize them.
Warning: Message
The “Warning: Message” alerts the operator about safety and non-compliance issues that
are of the highest importance. Failure to observe the instructions in these alerts could result
in serious injury, fire, or damage to the laser system or surrounding equipment.
CAUTION:
The “CAUTION: Box” alerts the operator to proper operation and proper use of the Fraxel
Laser Systems and their accessories.
Note:
The “Note: Box” designates information of special interest.
Warning:
PLEASE READ THE FRAXEL LASER SYSTEMS OPERATOR’S MANUAL IN ITS
ENTIRETY PRIOR TO USE OF THE SOLTA FRAXEL 1550, FRAXEL 1927, OR FRAXEL
DUAL 1550/1927 LASER SYSTEMS. PLEASE CONTACT SOLTA CUSTOMER SERVICE
AT +1 510-782-2286 OR YOUR LOCAL AUTHORIZED DISTRIBUTOR OF SOLTA
MEDICAL LASERS IF YOU HAVE ANY QUESTIONS.
ALL OPERATORS ARE REQUIRED TO PARTICIPATE IN FRAXEL LASER SYSTEMS
TRAINING PROGRAM PRIOR TO STARTING INDEPENDENT TREATMENTS.
Part Number 10-05170 Rev. O Page 6 of 92
Page 7

Fraxel® 1550, Fraxel 1927 and Fraxel DUAL 1550/1927 Laser System
1.0 System Overview
This manual provides operating instructions for the Fraxel 1550, Fraxel 1927 and
Fraxel DUAL 1550/1927 Laser Systems for physicians and qualified operators under
physician supervision. Section 1 of this manual will provide a brief description of the
laser systems and their major components. The Fraxel Laser Systems should only
be used by physicians and staff who have been appropriately trained and who are
thoroughly familiar with the instructions and safety precautions provided in this
manual.
This manual covers the following part numbers:
Part #
MC-SYS-SR1500-D-US
MC-SYS-SR1500-D-UPG-US
MC-SYS-SR1500-P-US
MC-SYS-SR1500-P-UPG-US
MC-SYS-SR1500-CPO-US
MC-SYS-SR1500-D-INTL
MC-SYS-SR1500-D-UPG-INTL
MC-SYS-SR1500-P-INTL
MC-SYS-SR1500-P-UPG-INTL
MC-SYS-SR1500-CPO-INTL
MC-SYS-SR1900-US
MC-SYS-SR1900-INTL
Please note there are two differences between the International and Domestic systems:
1) An additional laser “Door Warning Sign” is shipped with International systems
2) There are two different power cords:
a. Part # 08-01615 for International
b. Part # 08-00475 for Domestic
PLEASE CONTACT SOLTA MEDICAL CUSTOMER SERVICE AT +1 510-782-2286 OR
CONTACT YOUR LOCAL AUTHORIZED DISTRIBUTOR OF SOLTA MEDICAL LASERS IF
YOU HAVE QUESTIONS.
Part Number 10-05170 Rev. O Page 7 of 92
Page 8

Fraxel® 1550, Fraxel 1927 and Fraxel DUAL 1550/1927 Laser System
1.1 Indications for use
The Fraxel 1550, Fraxel 1927, and Fraxel DUAL 1550/1927 Laser Systems are approved for
the following Indications for Use:
Geographic
Regions
For the
following
geographic
region:
U.S.A.
For the
following
geographic
regions:
Australia
Brazil
Canada
EU
Hong Kong
India
Japan
Korea
Mexico
Russia
Philippines
Singapore
Taiwan
Thailand
UK
Vietnam
1550 nm: The Fraxel 1550 nm laser is indicated for use in dermatological
procedures requiring the coagulation of soft tissue, as well as for skin
resurfacing procedures. It is also indicated for treatment of dyschromia
and cutaneous lesions, such as, but not limited to lentigos (age spots),
solar lentigos (sun spots), actinic keratosis, and melasma, and for
treatment of periorbital wrinkles, acne scars and surgical scars.
1927 nm: The Fraxel 1927 nm laser is indicated for use in dermatological
procedures requiring the coagulation of soft tissue, treatment of actinic
keratosis, and treatment of pigmented lesions such as, but not limited to
lentigos (age spots), solar lentigos (sun spots) and ephelides (freckles).
1550 nm: The Fraxel 1550 nm laser is indicated for use in dermatological
procedures requiring the coagulation of soft tissue, as well as for skin
resurfacing procedures. It is also indicated for treatment of dyschromia
and cutaneous lesions, such as, but not limited to lentigos (age spots),
solar lentigos (sun spots), actinic keratosis, and melasma, and for
treatment of periorbital wrinkles, acne scars and surgical scars.
1927 nm: The Fraxel 1927 laser is indicated for use in dermatological
procedures requiring the coagulation of soft tissue and the treatment of
actinic keratosis
Indications for Use
Part Number 10-05170 Rev. O Page 8 of 92
Page 9

Fraxel® 1550, Fraxel 1927 and Fraxel DUAL 1550/1927 Laser System
1.2 Device Overview
The Fraxel Laser Systems are designed for cutaneous laser treatment. These devices are
part of the family of Solta Medical products utilizing the Fractional Photothermolysis
principle.
The Fraxel 1550 consists of a single infra-red fiber laser with wavelength of 1550 nm.
The Fraxel 1927 consists of a single infra-red Thulium fiber laser with wavelength of 1927
nm.
The Fraxel DUAL 1550/1927 Laser System has two infra-red fiber lasers with wavelengths
of 1550 nm and 1927 nm. The two wavelength option allows for targeted treatment of
different tissue depths. The 1550 nm laser coagulates the epidermis and dermis with up to
1.6 mm depth of penetration. The 1927 nm laser coagulates epidermis and superficial
dermis with up to 0.3 mm depth of penetration. The laser sources are controlled by an
embedded processor. The solid state design of the Fraxel 1550, Fraxel 1927 and Fraxel
DUAL 1550/1927 Laser Systems typically result in limited maintenance and minimal utilities
requirements. The output of the fiber laser is focused into the skin using lenses contained
within the handpiece. Computer controlled motors within the handpiece and the software
control architecture direct the laser system to deliver focused spots in an evenly spaced
treatment pattern. These advanced features allow for precisely controlled delivery of the
fractional laser treatment.
Interchangeable tips allow for treatment of both small and large areas. The small tip design
is intended to allow for treatment of smaller areas (not available with all systems).
This manual is intended to help you get the most from your system as you make the Fraxel
1550, Fraxel 1927 and Fraxel DUAL 1550/1927 Laser Systems an integral part of your
practice. It is our intention that the information provided assists the operator in successful
use of this product. This manual is not intended to substitute for training by authorized Solta
Medical personnel prior to treatment of patients. If you have additional questions, please
contact your local Solta Customer Service representative directly.
Warning:
The Fraxel Laser Systems generate an intense beam of laser radiation which may cause
injury if not used properly. It is essential that this manual be read thoroughly and
understood in its entirety before operation.
1.3 Introduction to the System
The Fraxel 1550 and Fraxel 1927 systems both have a single fiber laser source, and the
Fraxel DUAL 1550/1927 Laser System has two fiber laser sources. The fiber laser controls
are contained in a single console. The console is electrically connected to the facility power
source. Laser energy produced by the unit is delivered to the tissue through the handpiece.
Contact with the tissue is maintained by removable disposable contact tips which attach to
the handpiece.
The features of the Fraxel Laser Systems are shown below:
Part Number 10-05170 Rev. O Page 9 of 92
Page 10

Fraxel® 1550, Fraxel 1927 and Fraxel DUAL 1550/1927 Laser System
Umbilical
Cord and
Support Arm
Key Switch
Emergency
switch
Front Console with Zimmer Cart
Part Number 10-05170 Rev. O Page 10 of 92
Page 11

Fraxel® 1550, Fraxel 1927 and Fraxel DUAL 1550/1927 Laser System
Calibration Port
Power Connection
Footswitch Connection
Remote Door Interlock
Product Labeling
and Identification
Rear Console with Cart
Locking Casters
Part Number 10-05170 Rev. O Page 11 of 92
Page 12

Fraxel® 1550, Fraxel 1927 and Fraxel DUAL 1550/1927 Laser System
Treatment Handpiece with Cool Roller Tip installed
(Focal cooling not available on all models)
Part Number 10-05170 Rev. O Page 12 of 92
Page 13

Fraxel® 1550, Fraxel 1927 and Fraxel DUAL 1550/1927 Laser System
1.4 Control Features
1.4.1 On/Off Key Switch
The On/Off key switch turns the Laser System on and off. The key should always be
kept in a safe place (and not in the key switch), when not in use, to avoid
unauthorized use by untrained personnel and the possibility of injury or fire.
1.4.2 Touch Screen Display
The touch screen serves as the information center for the Fraxel Laser Systems.
The screen allows the operator to interact with the device. It displays the information
associated with the operating conditions, including: Laser ON / OFF, Machine Status,
Treatment Wavelength Selection (Fraxel 1550: 1550 nm; Fraxel 1927: 1927 nm and
DUAL 1550/1927: 1550 nm or 1927 nm), Laser Parameters for treatment,
Information Messages, Error Messages and System Prompts.
1.4.3 Emergency Stop Button
There is a red emergency stop button in the front center of the console. Press this
button during an emergency to stop the laser treatment beam. To restart, twist the
button and release it from the latched OFF position.
1.4.4 Calibration Port
The calibration procedure is enforced by the software.
CAUTION: The handpiece tip must be removed from the handpiece prior to
calibration. Take precautions to ensure that neither the handpiece internal sealing
window nor the tip window become contaminated before, during, or after the
calibration procedure as this may result in poor clinical outcome, damage to the
machine or injury to the physician or patient.
1.4.5 Umbilical Cord and Support Arm
The umbilical cord is managed by the adjustable support arm. When using focal
cooling with the Cool Roller Tip, the Zimmer cool air delivery hose may be clipped
onto the electrical umbilical cable and draped over the support arm as shown on
section 1.3 Ensure that there are no kinks in the hose.
CAUTION: Do not attempt to coil the umbilical beyond the resistance point of the
armoring. Do not use if the internal cables and wires are visible through holes or
gaps in the umbilical armoring as this may cause inadvertent exposure of laser
energy to persons in the room.
Part Number 10-05170 Rev. O Page 13 of 92
Page 14

Fraxel® 1550, Fraxel 1927 and Fraxel DUAL 1550/1927 Laser System
1.4.6 Treatment Tip
Removable disposable tips attach to the distal portion of the handpiece and MUST
be in place prior to any treatment. The tips are interchangeable and are available in
small and large sizes of approximately 7 and 15 mm, respectively. The different
sizes allow for treatment of both small and large areas. The system detects contact
between the tip and skin and translates handpiece motion into velocity information.
The tips have a finite treatment lifetime.
When using the tips, ensure that the cooling channel adaptor is clipped onto that side
of the handpiece as shown on section 1.3. Connect the distal end of the Zimmer air
cooler delivery hose to the white cylinder at the end of the clear tubing. Cool air will
flow down the cooling channel into the Cool Roller Tip. The air will flow across the
skin between the rollers and exhaust from the opposite side.
Inspect tip window for contamination before the tip is installed onto the handpiece.
The rollers used in the Fraxel Cool Roller Tip, are for single patient use only. Do not
reuse. Multiple patient use may create the potential risk of cross contamination of
biological agents from one patient to another.
Note: Handpiece tracking could be adversely affected by tip window fogging or
condensation. Remove tip from handpiece to observe both sides of the tip window.
CAUTION: The handpiece tip must be attached to the distal portion of the handpiece
prior to any treatment. If this is not done, the laser system will not function properly
which may result in injury to the physician or patient.
1.4.7 Power Cord
A hospital grade power cord connects the laser to the electrical outlet. The power
cord entry on the back of the console is a standard IEC60320 type C14 male
connector. The power cord needs to have a standard IEC60320 type C13 female
connector.
CAUTION: Do not substitute another power cord for the one supplied with the unit or
provided by your local representative. Do not use the power cord if it becomes frayed
or damaged in any way. Contact Solta Medical or your local authorized distributor of
Solta Medical lasers for a replacement, if necessary. Do not pull on the cord to pull
the plug out of the socket.
Part Number 10-05170 Rev. O Page 14 of 92
Page 15

Fraxel® 1550, Fraxel 1927 and Fraxel DUAL 1550/1927 Laser System
1.4.8 Cart
The cart serves as a platform for the console when counter space is limited. The
cart has wheels which allows for better mobility when moving the laser system, and
drawers available for storage of accessories.
CAUTION: Always lock the wheels prior to operation of the laser.
Warning:
If it is necessary to manually lift the Laser System Console and Cart, or to separate the
console from the cart, two people must perform the lifting operation. Failure to follow this
directive may result in serious injury or damage to the unit.
1.5 System States
The system has defined states of operation which include: utilities/home, system
test, simulate, and treat.
1.5.1 Utilities/Home
Allows access to various functional parameters of the system.
1.5.2 System Test Mode
Allows the system and handpiece to be tested. After successful power-on,
the system will move to this mode.
1.5.3 Simulate Mode
Allows the user to simulate treatment but without discharging laser energy.
1.5.4 Treat Mode
The system is ready and/or receiving input from the footswitch and
handpiece. This mode presents the highest potential risk of injury to the
physician and patient.
These functions are described in Section 2.0 in the context in which they
appear on the screen.
Part Number 10-05170 Rev. O Page 15 of 92
Page 16

Fraxel® 1550, Fraxel 1927 and Fraxel DUAL 1550/1927 Laser System
2.0 System Operation
2.1 Overview
This section provides a general guideline for the operation of the Fraxel 1550, Fraxel
1927 and Fraxel DUAL 1550/1927 Laser Systems. These laser systems have
specific operating requirements. It is the physician’s responsibility to fulfill these
requirements. Failure to do so can result in incorrect and/or intermittent operation or
damage to the laser.
2.2 Routine Precautions Prior to Treatment
2.2.1 Affix the laser warning sign, supplied with the system, to the door of
the room or the point denoting the perimeter of the Nominal Hazard
Zone (NHZ) (see Safety section of this manual).
2.2.2 Ensure that all persons in the NHZ are provided with the appropriate
eyewear (see Safety section of this manual).
2.2.3 Take precautions to mitigate the fire hazards associated with the
laser system, including the use of fire-retardant curtains, watersoaked towels, or gauze where necessary. See Section 3.0 for
more details.
2.2.4 Check the power cord for damage. Ensure that the appropriate
electrical utilities are available in the treatment room. Connect the
power cord to the laser and to the wall socket.
2.2.5 Make sure that the handpiece umbilical cable is not excessively
twisted from the unit. Do not tape the umbilical to any structure. Do
not attempt to coil the umbilical beyond the resistance point of the
armoring. Do not use if the internal cables and wires are visible
through holes or gaps in the umbilical armoring as this may cause
inadvertent exposure of laser energy to persons in the room. Do not
move the unit using the arm.
2.2.6 Check handpiece and tip cleanliness per sections. Do not use tips
with damaged windows.
2.2.7 Ensure that the red emergency switch is in disengaged position (not
pushed in) to enable the system for start up.
2.2.8 Insert the key into the key switch. Turn the unit on and verify that
the unit powers up normally with no error messages. Instructions
will appear on the screen to guide the operation of the system.
Part Number 10-05170 Rev. O Page 16 of 92
Page 17

Fraxel® 1550, Fraxel 1927 and Fraxel DUAL 1550/1927 Laser System
Warning:
All personnel within the Nominal Ocular Hazard Distance (NOHD) must be wearing
approved eye protection at this point. Failure to wear appropriate eyewear increases the
risk of serious eye injury.
Warning:
Laser equipment not in use should be protected against unauthorized operation by removing
the key from the key switch. Keep the key in a designated place accessible only to
authorized and trained personnel to avoid unauthorized use by untrained personnel and the
possibility of injury or fire.
Warning:
Do not attempt to coil the umbilical beyond the resistance point of the armoring. Do not use
if the internal cables and wires are visible through holes or gaps in the umbilical armoring as
this may cause inadvertent exposure of laser energy to persons in the room.
Warning:
If it is necessary to manually lift the Laser System console and cart, or to separate the
console from the cart, two people must perform the lifting operation. Failure to follow this
directive may result in serious injury or damage to the unit.
Warning:
SEVERE DAMAGE TO THE UNIT WILL RESULT FROM IMMEDIATE POWER UP
FOLLOWING MOVEMENT OF THE UNIT FROM A COLD ENVIRONMENT INTO A WARM
ENVIRONMENT. THIS CONDITION WILL OCCUR IF FOR EXAMPLE THE UNIT IS
MOVED FROM LOCATION TO LOCATION ON A DAILY BASIS AND IS STORED IN A
SHIPPING CONTAINER, AUTOMOBILE, OR TRUCK OVERNIGHT.
THE UNIT MUST BE ALLOWED TO EQUILIBRATE WITH ITS SURROUNDINGS FOR A
MINIMUM OF 90 MINUTES FOLLOWING A CHANGE OF ENVIRONMENT. FAILURE TO
OBSERVE THESE PRECAUTIONS WILL RESULT IN THE WARRANTY OF THE UNIT
BEING VOIDED.
NOTE: NEVER BLOCK THE COOLING SYSTEM VENTS. ENSURE ADEQUATE SPACE
AROUND THE UNIT AT ALL TIMES TO FACILITATE SYSTEM COOLING.
CAUTION: USE OF CONTROLS, ADJUSTMENTS, OR PROCEDURES OTHER THAN
THOSE SPECIFIED MAY RESULT IN HAZARDOUS ELECTROMAGNETIC RADIATION
EXPOSURE WHICH MAY RESULT IN INJURY, OR DAMAGE TO OTHER EQUIPMENT.
Part Number 10-05170 Rev. O Page 17 of 92
Page 18

Fraxel® 1550, Fraxel 1927 and Fraxel DUAL 1550/1927 Laser System
2.3 System State Descriptions
This section provides a general overview of the Fraxel Laser Systems operation.
These laser systems have specific operating requirements. It is the responsibility of
the physician to fulfill these requirements. Failure to do so can result in incorrect
and/or intermittent operation or damage to the laser.
While powering up, the system will briefly display an introductory splash screen.
2.3.1 System Test Mode
Upon completion of the system power-up, the system will enter the System Test mode.
Please follow the instructions as they appear on the touch screen display. The initial system
test screen will direct the placement of the handpiece into the calibration port.
In order to perform system testing, remove the tip from the handpiece. Place the tip in a
clean, dust-free container while the testing is in progress. Then, proceed by placing the
handpiece in the calibration port located on the top right hand side of the laser console. The
touch screen will indicate when testing has initiated.
Part Number 10-05170 Rev. O Page 18 of 92
Page 19

Fraxel® 1550, Fraxel 1927 and Fraxel DUAL 1550/1927 Laser System
If the testing is not successful, check the internal window and calibration port window for
damage, check the handpiece seating in the port to make sure it is correctly placed, and try
again, to a maximum of 3 times. After three attempts, contact Solta Medical Customer
Service.
If the system fails to calibrate to within 80% of the requested energy (more than 20% below
specification), it will not allow further treatment. Solta Medical then requires that the system
be serviced. Repeated power cycling will not clear this fault. Contact Solta Medical
Customer Service to schedule service.
Part Number 10-05170 Rev. O Page 19 of 92
Page 20

Fraxel® 1550, Fraxel 1927 and Fraxel DUAL 1550/1927 Laser System
2.3.2 Utilities/Home
Upon successful completion of the System Test, the touch screen display will immediately
transition to the Utilities/Home Mode. The Utilities/Home Mode allows various system
parameters to be selected, including Treatment Wavelength.
Fraxel DUAL 1550/1927
Home screen
Part Number 10-05170 Rev. O Page 20 of 92
Page 21

Fraxel® 1550, Fraxel 1927 and Fraxel DUAL 1550/1927 Laser System
Fraxel 1550
Home screen
Part Number 10-05170 Rev. O Page 21 of 92
Page 22

Fraxel® 1550, Fraxel 1927 and Fraxel DUAL 1550/1927 Laser System
Fraxel 1927
Home screen
The following parameters may be set in the Utilities/Home Mode:
Treatment Wavelength – Treatment Wavelength Controls allow either the 1550
nm or the 1927 nm laser to be selected on the Fraxel DUAL 1550/1927 Laser
System model.
From the Utilities/Home Mode, the user may access any of the other System modes,
including Simulate, Test System and Treat.
Part Number 10-05170 Rev. O Page 22 of 92
Page 23

Fraxel® 1550, Fraxel 1927 and Fraxel DUAL 1550/1927 Laser System
2.3.3 Simulate
Simulate Mode allows the user to simulate treatment but without discharging laser energy.
It is used during the training phase for new operators of the Fraxel Laser Systems and
should be utilized fully before the first patient treatment is performed. The Operator can also
select Simulate Mode in order to familiarize a patient with the feel of the treatment tip and
the tones of the laser before initiating treatment with a new patient.
Once the footswitch is depressed, the system will indicate that the simulate mode is
activated.
Part Number 10-05170 Rev. O Page 23 of 92
Page 24

Fraxel® 1550, Fraxel 1927 and Fraxel DUAL 1550/1927 Laser System
Part Number 10-05170 Rev. O Page 24 of 92
Page 25

Fraxel® 1550, Fraxel 1927 and Fraxel DUAL 1550/1927 Laser System
2.3.4 Treat
Warning:
Do not lift the handpiece from treatment area with the footswitch depressed during treatment
as this may result in injury to the patient due to inadvertent laser exposure.
When the final selection of utilities has been performed and treatment simulations have
been completed, the Operator may select to transition into Treat Mode. If “Treat” is
selected, the system will begin initializing. Initialization is an idle mode for the system.
If the tip is not yet attached to the handpiece, the touch screen display will notify the
Operator that no tip is installed. Attach tip to the handpiece as directed in this manual.
Part Number 10-05170 Rev. O Page 25 of 92
Page 26

Fraxel® 1550, Fraxel 1927 and Fraxel DUAL 1550/1927 Laser System
Once the tip is detected by the system, the touch screen display will indicate that
initialization is occurring, but will remain in standby mode. After initialization, the Operator
may adjust the treatment settings as desired, and then must press “Ready” in order to exit
Standby and enter Treat mode.
Part Number 10-05170 Rev. O Page 26 of 92
Page 27

Fraxel® 1550, Fraxel 1927 and Fraxel DUAL 1550/1927 Laser System
In order to initiate treatment, the Operator must depress the footswitch and initiate
handpiece motion.
Part Number 10-05170 Rev. O Page 27 of 92
Page 28

Fraxel® 1550, Fraxel 1927 and Fraxel DUAL 1550/1927 Laser System
Active treatment and laser emission will only occur when the footswitch is depressed and
the IOTS senses motion across the skin’s surface. When the system is actively treating, the
screen will reflect that laser energy is emitted and treatment is occurring.
Part Number 10-05170 Rev. O Page 28 of 92
Page 29

Fraxel® 1550, Fraxel 1927 and Fraxel DUAL 1550/1927 Laser System
In order to change treatment parameters or interrupt treatment, the Operator must select
“Standby”. The touch screen display will then indicate that the system is in Standby mode.
Part Number 10-05170 Rev. O Page 29 of 92
Page 30

Fraxel® 1550, Fraxel 1927 and Fraxel DUAL 1550/1927 Laser System
When in Standby, treatment parameters may be selected. Energy, treatment level and
number of passes are described in the table below. After selections are made, the Operator
must press “Ready” in order to exit Standby and re-enter Treat mode.
Symbol Interpretation
The Pulse Energy Level Indicator field should
be used to increase or decrease pulse energy.
The Treatment Level Indicator field should be
used to increase or decrease level of
treatment. This is a function of the treatment
density and coverage of the laser treatment.
The default display for treatment level is %
Coverage. The % Coverage is the percentage
of the skin that is treated after all the passes
are completed.
Display from % Coverage to Treatment Density
(MTZ / cm
2
) can be changed by the user by
simply pressing the area on the screen where
“% Coverage” is displayed.
Part Number 10-05170 Rev. O Page 30 of 92
Page 31

Fraxel® 1550, Fraxel 1927 and Fraxel DUAL 1550/1927 Laser System
The Handpiece Velocity Indicator displays
handpiece velocity in cm/s.
- White zone indicates normal operation
- Black zone indicates under-treatment
Tip information displays the amount of lifetime
that the currently installed tip has remaining
Part Number 10-05170 Rev. O Page 31 of 92
Page 32

Fraxel® 1550, Fraxel 1927 and Fraxel DUAL 1550/1927 Laser System
2.3.5 Pattern Preview Test
It is important to perform a Pattern Preview Test with each tip prior to use on a patient, every
time the Fraxel Laser Systems are used. Remember the following steps:
Use eye protection
Use settings listed on pattern prep paper
o 1550nm: 20mJ & 40mJ, TL7, 8 passes
o 1927nm: 10mJ & 20mJ, TL7, 8 passes
Perform only 1 pass for the test
If lines are missing do not perform treatment and contact Solta Medical Customer Product
Support immediately.
Performing Pattern Preview Test
Test paper with lines missing
Part Number 10-05170 Rev. O Page 32 of 92
Page 33

Fraxel® 1550, Fraxel 1927 and Fraxel DUAL 1550/1927 Laser System
2.4 Measurement-Guided Dosimetry
To ensure precise treatment dosimetry, actual and estimated kJ readings will be provided in
the kJ counter section on the bottom left corner of the touch screen. The “actual kJ” reading
will appear on the touch screen after treatment is completed. It is an indicator of the amount
of energy delivered though the tip during treatment.
To use the “Actual kJ” reading to determine whether the treatment was delivered as
intended, the Operator will need to measure the surface area of the treatment area. The
system handpiece will function as a measurement tool for assessment of surface area. After
surface area measurements are completed, the system will calculate the “Estimated kJ”
reading based on desired treatment level and measured surface area. This value should be
used as a point of reference in order to determine whether treatment dosimetry was
achieved as intended.
2.4.1 Surface Area Measurements
As described above, the handpiece may be used as a tool to measure the surface area of
the treatment region. Measurement selections may be performed while the system is in
Treat Standby or Simulate Standby mode. In order to begin, the Operator should select the
measurement icon.
2.4.2 Treatment Area Selection
After selection of the measurement icon, the touch screen display will prompt the Operator
to select a treatment area for measurement. Possible treatment areas include: the face,
neck, chest, forearm, hand and “other”. Vertical and horizontal measurements will be
required for measurement of each treatment region.
Part Number 10-05170 Rev. O Page 33 of 92
Page 34

Fraxel® 1550, Fraxel 1927 and Fraxel DUAL 1550/1927 Laser System
2.4.3 Measurement Process
The Operator must establish contact between the handpiece and the surface of the
treatment region. Vertical and horizontal measurements must be completed as directed by
the measurement touch screen display.
After completion of the measurements for a particular treatment area, the Operator may
select an additional treatment region for measurement. In order to do so, the Operator must
select “Change Treatment Region” and must select a new treatment location. After
completion of vertical and horizontal measurements for the selected region(s), the Operator
must press “Done”. Upon completion of the requested measurements, the system will
provide the value for surface area (cm
2
).
Part Number 10-05170 Rev. O Page 34 of 92
Page 35

Fraxel® 1550, Fraxel 1927 and Fraxel DUAL 1550/1927 Laser System
2.4.4 Measurement Example: Facial Surface Area
In order to measure facial surface area, the Operator will be prompted to complete
measurements along the vertical and horizontal axes. The length measurement should be
completed by administering a pass that begins at the lateral hairline and extends along the
vertical axis to the jawline. The vertical measurement should pass directly through the
lateral canthus (just past the lateral commissure). If the length was not measured properly
or needs to be repeated for any other reason, press “clear” and perform the vertical pass
again. After the length measurement is obtained, the Operator will be prompted to measure
the width of the treatment area.
Part Number 10-05170 Rev. O Page 35 of 92
Page 36

Fraxel® 1550, Fraxel 1927 and Fraxel DUAL 1550/1927 Laser System
The Operator shall measure the width of one half of the face. In order to begin, the
Operator must place the tip at the center of the philtrum (depression extending from the
base of the nose to the center of the lips) and complete a horizontal pass until the lateral
edge of the jawline (level with respect to the base of the earlobe). If the width measurement
was not completed properly, or needs to be repeated for any other reason, press “clear” and
perform the horizontal pass again. After the length and the width measurements are
obtained, the Operator must press “Done” in order to store the treatment area calculations.
Part Number 10-05170 Rev. O Page 36 of 92
Page 37

Fraxel® 1550, Fraxel 1927 and Fraxel DUAL 1550/1927 Laser System
After completion of all necessary measurements, the touch screen display will list the
“Estimated kJ” value and the treatment area measurement (cm
2
). If the surface area needs
to be recalculated, the Operator must select “reset all” and will need to perform the
measurements again.
Part Number 10-05170 Rev. O Page 37 of 92
Page 38

Fraxel® 1550, Fraxel 1927 and Fraxel DUAL 1550/1927 Laser System
2.4.5 Interpreting “Estimated kJ” Values
The “Estimated kJ” value provided by the system is calculation-based. The calculated
energy estimate incorporates the surface area of the treatment region, as measured by the
Operator, and the intended treatment level. After treatment is completed, the system will
display the “Actual kJ” reading. If the “Estimated kJ” reading is more than the “Actual kJ” of
energy delivered to the patient, the Operator may not have administered the treatment as
intended. Several technique-dependent factors may reduce the amount of energy delivered
to the patient, including: rapid hand speed, inappropriate delivery of treatment passes,
omission of sections within a treatment region, etc.
CAUTION: Measurement guidelines are intended to support the measurement
process. However, proper measurements must be performed by the Operator.
Inaccuracies in surface area measurements would compromise the interpretation of
estimated kJ calculations. Estimated kJ values are intended only to guide the
assessment of energy delivered during treatment, not to replace physician
assessment of treatment adequacy or assessment of clinical outcome.
2.4.6 Combination Wavelength Treatments
When combining both wavelengths in one treatment session, please note you must only tap
once on your chosen wavelength from the Home Screen.
After performing a treatment on a patient, using either wavelength, and then switching to the
second wavelength, it is important to tap the wavelength button only ONCE. If you tap the
screen twice (or any “even” number of screen taps), by mistake, the Total kJ count will
appear transposed from one wavelength to the other. This is a minor software bug that will
be rectified in future software updates, but can easily be avoided by ensuring your staff
conforms to the “one tap” protocol.
If you “double tap” the screen by mistake, tap the same button a third time to correct.
Please note, there is no safety issue and the transposition of Total kJ count does not impact
tip life in any way, nor does it actually change the total kJ count for either wavelength. It can
simply be confusing to the clinician if one desires to record the kilo Joule count prior to
switching wavelengths.
Part Number 10-05170 Rev. O Page 38 of 92
Page 39

Fraxel® 1550, Fraxel 1927 and Fraxel DUAL 1550/1927 Laser System
2.5 Definitions
This section of the manual should familiarize the physician with the terminology used in the
Solta Training Program and describe other features of the Fractional Photothermolysis
process. It is the responsibility of the physician to fully understand the indications for use,
safety considerations and laser function associated with the Fraxel 1550, Fraxel 1927, and
Fraxel DUAL 1550/1927 Laser Systems, and to make medical decisions regarding the
specific application of the Laser Systems to the individual patient.
MTZ
An MTZ is a Microscopic Treatment Zone, the region of coagulated tissue resulting from one
pulse from the handpiece.
Pass
A “pass” is a single, unidirectional hand motion laying down one segment of MTZs.
Magnified it will be similar to the pattern shown below although the spot pitch and stride may
not be identical.
MEND
A MEND is a Micro-Epidermal Necrotic Debris particle. This is a compact accretion of
necrotic epidermal debris which is being pushed up from the Dermal/Epidermal junction and
expelled as a part of the healing process. MENDS produced by the non-ablative Fraxel
Laser Systems typically flake off after 4 – 14 days. During the flaking process they give the
skin a slightly bronzed appearance.
2.6 Parameter Selection
The physician and all operators should be adequately trained in laser safety and should
make informed and appropriate selection of treatment parameters. The following parameter
set was used as part of the initial safety and efficacy IDE protocol for the first generation
non-ablative Fraxel SR Laser System. Refer to publications and clinical education materials
for additional guidelines.
“The directions for use provided in this section are based on the results of a clinical trial
conducted by Solta Medical, Inc. A total of 27 subjects received a total of 4 non-ablative
Part Number 10-05170 Rev. O Page 39 of 92
Page 40

Fraxel® 1550, Fraxel 1927 and Fraxel DUAL 1550/1927 Laser System
fractional laser treatments at 4-7 day intervals. One treatment consisted of ten passes with
the device to create a final treatment density of 2500 spots per cm
were made in one direction and five double passes were made in a perpendicular direction.
The average fluence per pass was 1.5 - 3 J/cm
2
, and (pulse energies) ranged from 6 to 12
2
. Five double passes
mJ/pulse. Each pulse had a duration of 1.5 - 5 ms. The results of the clinical trial indicated
that the Fraxel Laser System safely and effectively provided treatment for the cleared
indications for use listed in Sections 1.1 and 4.1 of this manual. It is the responsibility of the
physician to fully understand the indications for use and safety considerations associated
with the Fraxel Laser System.”
2.7 Total Treatment Density (Total MTZs / cm2)
Total treatment density is a function of the density per pass (MTZ / cm2 / pass) and the
number of passes delivered. For example, in order to achieve a desired final MTZ density of
1000 / cm
2
when the system is delivering 100 MTZ / cm2 / pass, 10 passes would be
required. System feedback displays density, energy and treatment level to confirm delivery
of desired treatment parameters.
2.8 System Audible Feedback
The laser system console will generate audible tones during normal operation which vary as
a function of handpiece velocity. This indicates that the tracking system is functioning. The
frequency of the tone will increase as hand velocity increases, and decrease as hand
velocity decreases. Physicians should practice with the system in Simulate Mode to gain an
understanding of the relationship between hand velocity and audible tones.
If the handpiece is moved at an unsustainable velocity, i.e. above that where the system will
produce the correct pulse energy and repetition rate, the sound will change to a higher
pitched tone designed to alert the operator. Slowing down will restore the audible tones to
normal.
There are two adjustments for volume control, “General” and “Alert” (see section 2.3.2). The
General volume control can be adjusted down, or turned off completely. If the General
volume is off, you will still hear a soft audible alarm if an alert presents itself.
The handpiece may get warm during treatment. This is normal and not typically a cause for
concern. If the handpiece gets abnormally hot, discontinue treatment and call Solta
Customer Service or your local authorized distributor of Solta Medical lasers.
Warning:
The tones generated by the machine indicate normal function. If the tone is unchanging but
hand velocity is changing, discontinue treatment and check the laser. If the fault persists,
call Solta Customer Service.
Part Number 10-05170 Rev. O Page 40 of 92
Page 41

Fraxel® 1550, Fraxel 1927 and Fraxel DUAL 1550/1927 Laser System
Warning:
Release the footswitch prior to lifting handpiece from the treatment area. Failure to do this
may result in burns or injury to the patient due to inadvertent laser exposure.
2.9 Handpiece Tracking (IOTS – Intelligent Optical
Tracking® System)
The handpiece is capable of sensing hand motion velocity during treatment, and maps
velocity feedback to ensure uniform delivery of the treatment beam. The IOTS is configured
to work in much the same way as a computer mouse does, in that it senses motion when
moved in a plane.
The IOTS senses velocity, i.e. not only the speed of the handpiece motion but also the
direction. This allows treatment to be bidirectional. The handpiece may be stopped after
one pass and the direction of motion reversed without loss of function. Simulate Mode
allows the operator to practice this hand motion and listen to the tones generated by the
machine without firing the laser.
NOTE: In order for the IOTS to stay locked during a treatment, it is important that the tip
stay in good physical contact and approximately perpendicular to the skin.
NOTE: There is a specific cadence preferred by physicians skilled in the use of the Fraxel
Laser systems. After selection of the desired treatment wavelength, the sequence is:
1. Place the laser in Treat mode.
2. Place the handpiece on the area to be treated.
3. Press the footswitch down.
4. Move the handpiece, performing as many passes as are required to complete treatment
of the selected area.
5. Release the footswitch. Lift the handpiece and move it to the next area to be treated.
6. Restart the sequence at step 3.
7. When finished, release the footswitch, remove the handpiece from the skin, clean and
store carefully.
Warning:
Do not lift the handpiece from treatment area with the footswitch depressed during
treatment. Release footswitch prior to lifting handpiece from treatment area. Failure to
follow these guidelines may increase the risk of injury to the patient.
Part Number 10-05170 Rev. O Page 41 of 92
Page 42

Fraxel® 1550, Fraxel 1927 and Fraxel DUAL 1550/1927 Laser System
Warning:
The tones generated by the machine indicate normal function. If the tone is unchanging but
hand velocity is changing, stop treatment to reduce the risk of injury to the patient. Test the
system in Simulate mode. Restart the laser if necessary. If the fault persists, call Solta
Customer Service.
Warning:
Do not move the handpiece in a direction parallel to the long axis of the treatment tip. Do
not twist the handpiece in a circular motion while treating. Failure to observe these
precautions may result in increased risk of injury to the patient.
2.10 Tip Selection, Cleaning and High Level Disinfection
Procedure
2.10.1 Tip Selection
The two interchangeable tips have treatment widths of approximately 7 mm (Small)
and 15mm (Large), respectively. The physician should select the appropriate tip
attachment based on the treatment area requirements. For example, for better
accuracy when targeting treatment of a small tissue area, selection of the smaller tip
may be more appropriate. As the treatment area increases, use of the larger tip may
be more efficient and may require fewer treatment passes. NOTE: The Fraxel 1927
and the Fraxel DUAL 1550/1927 only utilize the large treatment tip.
When selected, the tip should be attached to the distal portion of the handpiece. It
will latch magnetically when properly positioned, and will remain latched throughout
treatment with appropriate handling. The small and large tips attach in the same
manner and the dimensions of the tip bases are identical.
CAUTION: Make sure the tip is correctly latched prior to treatment. Check to make
sure that the surface of the handpiece where the tip attached is not contaminated.
Check in particular for obstructions or debris near the magnetic latch. The tip will not
seat properly if this surface is obstructed.
2.10.2 Tip Cleaning
Warning:
The tips cannot be sterilized or disinfected by EtO, or products containing glutaraldehyde.
Using these disinfection/sterilization techniques will severely reduce the tip lifetime and
possibly lead to immediate damage, such as cracking, and possible injury to the patient.
Part Number 10-05170 Rev. O Page 42 of 92
Page 43

Fraxel® 1550, Fraxel 1927 and Fraxel DUAL 1550/1927 Laser System
Treatment tips are provided clean and non-sterile by the manufacturer; however, it is
the responsibility of the user to clean the tip before treatment use. The tip should be
wiped with 70% alcohol (preps) and inspected for debris and other contamination.
After treatment AND WHILE STILL ATTACHED TO THE HANDPIECE, the tip should
be wiped with 70% alcohol (preps) and inspected for debris and other contamination.
The tip should then be removed from the handpiece and inspected again. Any
remaining debris should be removed. Care should be taken not to allow debris,
cleaning materials, or other contaminants to fall into the mouth of the tip. If the tip
was used with off-white plastic rollers, remove used rollers and replace with new set.
The rollers used in the Fraxel Cool Roller Tip, are for single patient use only. Do not
reuse. Multiple patient use may create the potential risk of cross contamination of
biological agents from one patient to another.
CAUTION: Tip cleaning and care protocols are critical to optimizing tip lifetime.
Treating through a contaminated tip may accelerate the degradation process and may
result in poor clinical outcomes, including possible injury to the patient, and suboptimal tip lifetimes.
Warning:
Do not allow the metal part of the handpiece to come into prolonged contact with corrosive
chemical solutions such as acetone, bleaches or disinfectants.
2.10.3 Handpiece Window Cleaning
With the tip removed, wipe the handpiece window with 70% isopropyl alcohol and inspect for
debris and other contamination.
2.10.4 High Level Disinfection Procedure
Solta Medical has tested and qualified a disinfection method using Cidex OPA, which
disinfects the tips to the reusable medical device classification of high level disinfection for
semi-critical devices.
Warning:
Wear gloves and other appropriate body protection as directed by the materials
safety data sheets (MSDS) and instructions for use accompanying Cidex® OPA
solution.
Part Number 10-05170 Rev. O Page 43 of 92
Page 44

Fraxel® 1550, Fraxel 1927 and Fraxel DUAL 1550/1927 Laser System
Warning:
Do not allow the chip inside the tip to come into contact with any liquid such as the
®
Cidex
OPA solution, or water.
Do not allow the metal part of the handpiece to come into prolonged contact with any
liquid such as the Cidex
®
OPA solution.
Pre-cleaning Procedure
After tip use, remove and discard the tip rollers. Wipe off the outside of the tip with 70%
isopropyl alcohol (IPA) wipes until there is no visual sign of soil. Tips can then be placed, but
not submerged, in the Cidex solution (per the Cidex OPA solution instructions). DO NOT
submerge the tip completely. DO NOT allow the cleaning solution to come in contact with
the chip inside the tip.
If desired, pre-cleaning can be done with a solution of an enzymatic detergent, e.g. Enzol
®
enzymatic detergent, (per the manufacturer’s recommendations). Pre-clean devices by
immersing the soiled portion of the tips in the prepared detergent.
During cleaning or disinfection, DO NOT allow any liquid to come into contact with the chip
inside the tip.
Rinse the part of the tip exposed to either IPA or enzymatic detergent with water for a
minimum of 30 seconds, being careful not to expose the chip to any liquid.
High Level Disinfection Procedure
Pour the Cidex® OPA solution into a container and immerse the base of the tip (where the
white rollers attach). Ensure that the Cidex
®
OPA solution completely covers the base of the
tip. Allow the tip to soak in the disinfectant for 12 minutes (or per Cidex OPA instructions for
use).
Following disinfection with Cidex
®
OPA solution, rinse the tip with water and then dry the tip
thoroughly and attach new unused rollers before use.
The rollers used in the Fraxel Cool Roller Tip, are for single patient use only. Do not
reuse. Multiple patient use may create the potential risk of cross contamination of
biological agents from one patient to another.
More details on the Enzol
®
and Cidex® OPA products can be found at:
http://www.aspjj.com/us/products/enzol/features-and-benefits
and
http://www.aspjj.com/us/products/cidex-opa/features-and-benefits
Part Number 10-05170 Rev. O Page 44 of 92
Page 45

Fraxel® 1550, Fraxel 1927 and Fraxel DUAL 1550/1927 Laser System
More details on preventing infection from reusable medical equipment can be found at:
http://www.biomedcentral.com/1471-2334/2/4
2.11 Normal Shut-down Procedure
2.11.1 Secure the handpiece safely in the recess in the support arm.
2.11.2 Turn off the unit at the key switch.
2.11.3 Remove the key from the key switch and store safely.
Part Number 10-05170 Rev. O Page 45 of 92
Page 46

Fraxel® 1550, Fraxel 1927 and Fraxel DUAL 1550/1927 Laser System
3.0 Safety
The following Fraxel systems are each classified as Class 4 lasers according to Center for
Devices and Radiological Health (CDRH) and IEC / EN regulations: Fraxel 1550, Fraxel
1927 and the Fraxel DUAL 1550/1927 Lasers. This classification represents the highest
power laser. Users and staff must take precautions to prevent exposure of laser energy to
the eye and/or skin from either direct or diffusely reflected laser beams. In addition,
precautions must be taken in the surgical environment to prevent hazards of fire and
electrical injury.
This section discusses the primary safety concerns when operating the both Fraxel 1550,
Fraxel 1927 and Fraxel DUAL 1550/1927 Laser Systems. The instructions are intended to
raise awareness of the safety issues and provide insight so that hazardous optical,
mechanical, electrical, and chemical risks may be minimized.
CAUTION: Prior to use of the Fraxel Laser Systems, this manual must be thoroughly
read and understood by the personnel involved with operating the System. Improper
use may cause personal injury and damage to the System and may invalidate any
warranty agreement.
CAUTION: Improper service, repair or modifications performed by unauthorized
personnel may pose a hazard and will invalidate any warranty agreement.
CAUTION: United States Federal law restricts this device to sale by or on the order of
a physician.
CAUTION: The availability of electronic alarms does not relieve operators of their
obligation to carefully monitor the entire system during operation. CAUTION: Laser
plumes may contain viable tissue particles.
Although the Solta system is a contact non-ablative system which normally cannot
produce any smoke or plume, any laser plume (debris) generated by the laser is
potentially hazardous. It may obscure the laser operating field and may contain
viable airborne pathogens. Any plume generated as part of a laser procedure must
be evacuated promptly and effectively. If such plumes are observed during normal
operation of the laser as instructed by this manual and as directed in the Solta
training, immediately stop treating, shut off the laser system and contact Solta
Customer Service
Part Number 10-05170 Rev. O Page 46 of 92
Page 47

Fraxel® 1550, Fraxel 1927 and Fraxel DUAL 1550/1927 Laser System
3.1 General Considerations and Hazard Potential
Laser light presents a severe eye and burn hazard to physician, patient and bystanders, and
has a potential for starting a fire. In order to avoid injury, avoid unintentional exposure to the
laser beam. Take all necessary protective measures in areas where the laser is being used.
To reduce the potential for injury, follow the Standard Operating Procedures set forth by the
physician and Facility Laser Safety Officer, and heed the warnings, cautions, notes and
guidelines in this manual. Failure to do so could result in increased risk of injury or fire or
damage to equipment.
3.2 Fire Precautions
Warning:
Do not use this device in the presence of critical concentrations of flammables or explosives,
such as volatile anesthetics, alcohol, certain surgical preparation solutions, and other
substances known to have these fire hazards. An explosion and/or fire could occur and
severe injury could result.
Warning:
Take extra precautions when using this laser around an airway (endotracheal) oxygen tube.
Firing the laser into the airway oxygen tube could start a fire that will spread rapidly because
of the presence of oxygen which could in turn cause severe injury.
Warning:
The use of flammable anesthetics or oxidizing gases such as nitrous oxide (N2O) and
oxygen should be avoided. Some materials, for example cotton wool, when saturated with
oxygen, may be ignited by the high temperatures produced during NORMAL OPERATION
of the LASER EQUIPMENT.
Warning:
Flammable solutions used for cleaning/disinfecting and the solvents of adhesives should be
allowed to evaporate before the LASER EQUIPMENT is used. An explosion and/or fire
could occur and severe injury could result if these precautions are not observed.
Warning:
Do not attempt to coil the umbilical beyond the resistance point of the armoring. Do not use
if the internal cables and wires are visible through holes or gaps in the umbilical armoring as
this may cause inadvertent exposure of laser energy to persons in the room. An explosion
and/or fire could occur and severe injury could result.
Part Number 10-05170 Rev. O Page 47 of 92
Page 48

Fraxel® 1550, Fraxel 1927 and Fraxel DUAL 1550/1927 Laser System
3.2.1 A designated Facility Laser Safety Officer (F-LSO) and the
physician shall control and enforce the safety aspects of the laser in
the health care facility.
3.2.2 A fire extinguisher shall always be available to extinguish any
flames.
3.2.3 Know the location of the nearest fire extinguisher in case of fire.
3.2.4 Never test-fire the laser over a patient who is receiving oxygen
through an endotracheal (airway) tube as inadvertent exposure may
start an airway fire which could result in serious injury.
3.2.5 Exercise great care with the use of oxygen, especially as an airway
gas. In the event of fire, oxygen will accelerate the progress of a fire
which could cause serious injury.
3.2.6 Do not attempt to operate the laser without a non-flammable
backstop downrange from the handpiece. Without the nonflammable
backstop, inadvertent exposure of the laser beam may cause a fire
and serious injury could result.
3.2.7 Always moisten gauze with water before treating to minimize the fire
risk.
3.2.8 Hair in the treatment area should be shaved prior to the procedure
to reduce the potential for interference with the operation of the
laser system, including the IOTS, which may increase the risk of
burns or other injury.
3.2.9 If alcohol is used for cleaning and disinfection of the skin, allow the
area to dry completely before using the laser in order to reduce the
possibility of alcohol fuelling a fire. Replace the cover on the alcohol
container and remove the alcohol from the immediate area prior to
initiating treatment to reduce the possibility of alcohol fuelling a fire
which could cause serious injury.
Part Number 10-05170 Rev. O Page 48 of 92
Page 49
Fraxel® 1550, Fraxel 1927 and Fraxel DUAL 1550/1927 Laser System
3.3 Optical Safety
3.3.1 The designated Facility Laser Safety Officer (F-LSO) and physician
shall control and enforce the safety aspects of the laser in the health
care facility.
3.3.2 The F-LSO and physician are responsible for determining the
appropriate Nominal Ocular Hazard Distance (NOHD), Nominal
Hazard Zone (NHZ) and Maximum Permissible Exposure (MPE) per
ANSI Standard Z136.1-2000, Z136.3-2005 and/or IEC 60825-1.
3.3.3 The F-LSO and physician are responsible for providing appropriate
laser safety items including, but not limited to, the correct laser
safety eyewear, and enforcing the use of these items through laser
safety protocols and Standard Operating Procedures.
3.3.4 A laser-trained physician, nurse, or technician shall be responsible
for enforcing safety guidelines during operation.
3.3.5 All operators shall attend the appropriate laser training course and
shall receive certification and/or credentialing prior to patient
treatment.
3.3.6 Laser safety eyewear is routinely required with most lasers. Failure
to use appropriate eyewear with the Fraxel Laser Systems
increases the risk of injury to the cornea, sclera and soft tissue of
the eye.
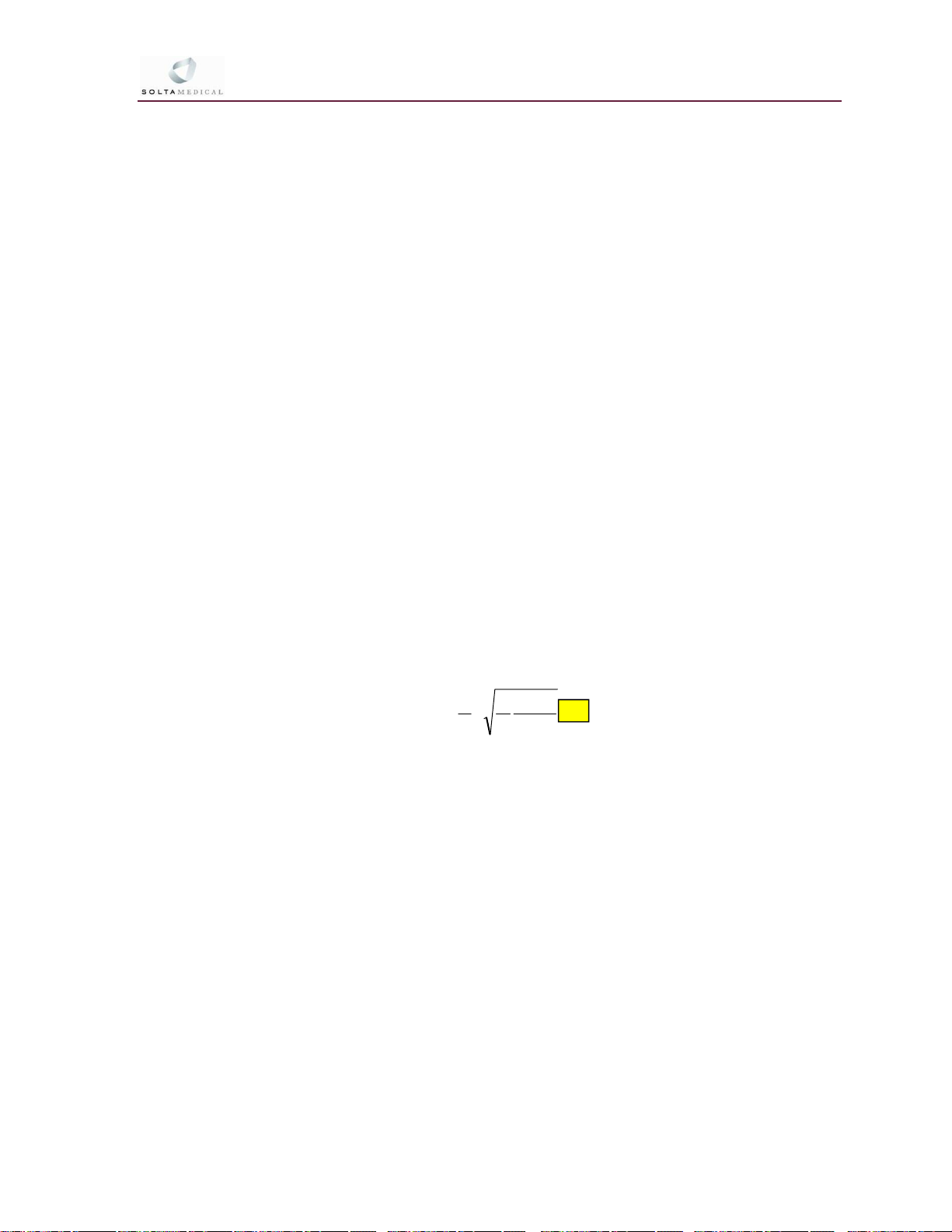
3.3.7 The following formula, taken from ANSI Standard Z136.1, was used
to calculate the Worst Case NOHD for the Fraxel Laser Systems.
NOHD
Where:
NOHD = the Nominal Ocular Hazard Distance, defined as the distance from the
source required to reduce the energy density incident on a 7 mm aperture
to less than the Maximum Permissible Exposure,
θ (theta) = the full angle beam divergence, is measured in radians,
Φ (phi), the energy or power of the laser is measured over one aversion response
time (Watts or Joules),
MPE, the Maximum Permissible Exposure, as defined per ANSI 136.1 and ANSI
136.3, is in Watts per unit area.
a = the beam waist diameter at the e point in units of length, and the beam waist is
at the exit window.
Note: Length units for “a” and MPE must be the same.
41
MPE
a
Part Number 10-05170 Rev. O Page 49 of 92
Page 50

Fraxel® 1550, Fraxel 1927 and Fraxel DUAL 1550/1927 Laser System
Fraxel 1927 or Fraxel DUAL
Parameter
Fraxel 1550 1550 nm
Laser Output
1550/1927
1927 nm
Laser Output
Power (Φ) 30 W 12 W
Beam Waist Diameter (a) 63 (microns 1/e) 537 (microns 1/e)
Divergence (°) 14.1 (mrad 1/e) 2.5 (mrad 1/e)
Wavelength 1550 (min:1520nm,
max:1560nm)
1927 (min:1905nm,
max:1945nm)
MPE 0.10 (W cm-2) 0.10 (W cm-2)
3.3.8 Using this formula, and the values for the parameters above:
Worst Case NOHD for the Fraxel Laser Systems when operating at 1550 nm
is 45 feet / 13.8 meters. When operating at 1927 nm the worst case NOHD is
159 feet / 49 meters.
All personnel within the NOHD are required to wear eye protection with a
minimum Optical Density of 3.49 at 1550 nm and 3.10 at 1927 nm. Appropriate
eye wear should be used that supports the minimum optical density for both
given wavelengths.
For operators complying with EN 207, all persons shall wear eyewear with
protection class of DL4.
CAUTION: It is recommended that protective eyewear will be worn by the operator
and any individuals in the treatment room when the Fraxel Laser Systems are in use.
3.3.9 Patients must have appropriate eye protection. The F-LSO and
physician are responsible for ensuring that the patient is adequately
protected during treatment. Failure to use appropriate eyewear with
the Fraxel Laser Systems increases the risk of injury to the cornea,
sclera and soft tissue of the eye.
3.3.10 Extra precautions must be taken if treating inside the bony orbital
rim of the eye, including but not limited to treatment on the upper
and lower eyelid. Failure to use appropriate eyewear with the
Fraxel Laser Systems increases the risk of injury to the cornea,
sclera and soft tissue of the eye.
Part Number 10-05170 Rev. O Page 50 of 92
Page 51

Fraxel® 1550, Fraxel 1927 and Fraxel DUAL 1550/1927 Laser System
3.3.11 Protective eyewear of the correct optical density for the laser
wavelength must be worn at all times during the laser procedure
and during laser verification and calibration. Failure to use
appropriate eyewear with the Fraxel Laser Systems increases the
risk of injury to the cornea, sclera and soft tissue of the eye.
3.3.12 All protective glasses or goggles must bear a label from the
manufacturer identifying the wavelengths for which their use is
intended and the optical density at those wavelengths.
3.3.13 All protective eyewear must have side shields for protection against
inadvertent exposure from the side. Failure to use appropriate
eyewear with the Fraxel Laser Systems increases the risk of injury
to the cornea, sclera and soft tissue of the eye.
3.3.14 Laser safety eyewear shall be properly stored away from direct
sunlight to prevent degradation of the absorbing media, such as
photobleaching.
3.3.15 When cleaning eyewear, do not use solvents or abrasives that might
remove protective coatings. Follow the manufacturers’
recommendations for cleaning.
3.3.16 Two sets of appropriate protective eyewear are provided with each
Fraxel Laser System. Additional sets may be purchased from Solta
(See Section 6.0 – Re-Order Information). One set of the eyewear
is designed to be used over optical spectacles.
3.3.17 Examples of patient-specific eye protection, not tested and not
endorsed by Solta, but used by practitioners skilled with the use of
this and similar lasers, are given in the “Re-Order Information –
Section 6.0” of this manual. The presence of these products in this
manual does not constitute an all-inclusive list of products, nor does
it constitute an endorsement of any product or a testimonial of
safety or efficacy.
3.3.18 Discard eyewear if it becomes scratched, chipped or if the
protection is in any other way compromised as serious injury may
result.
3.3.19 Contact lenses and regular glasses do not provide adequate
protection. Laser protective eyewear must be worn. Failure to use
appropriate eyewear with the Fraxel Laser Systems increases the
risk of injury to the cornea, sclera and soft tissue of the eye.
3.3.20 The treatment room door shall be kept closed during operation of
the laser. Door interlocks can optionally be installed to
automatically disable the laser when the treatment room door is
open.
3.3.21 Keep reflective objects out of the treatment field to avoid accidental
reflection. Remember that stainless steel eye shields may cause
some reflection of the laser beam.
Part Number 10-05170 Rev. O Page 51 of 92
Page 52

Fraxel® 1550, Fraxel 1927 and Fraxel DUAL 1550/1927 Laser System
Warning:
All persons within the NOHD shall wear appropriate eye protection with a minimum optical
density of 4.58. For operators complying with EN 207, all persons shall wear eyewear with a
protection class of DL4. Do not use eyewear that is visibly damaged in any way. Failure to
use appropriate eyewear with the Fraxel Laser Systems increases the risk of injury to the
cornea, sclera and soft tissue of the eye.
Warning:
Use extra caution when treating close to the eyes. The laser wavelengths used in the
Fraxel Laser Systems are extremely hazardous to the cornea and soft tissue around the
eye. All operators must have a comprehensive understanding of the risks involved and
knowledge of the safety precautions required for these lasers to ensure the safety of the
patients and others within the NOHD.
Warning:
Never look directly into the path of the laser as this may cause permanent eye damage. In
addition, direct exposure to the laser beam can cause permanent damage to the safety
eyewear lens.
Warning:
A warning sign shall be placed on the outside of the treatment room door or controlled area.
Warning:
Do not attempt to coil the umbilical beyond the resistance point of the armoring. Do not use
if the internal cables and wires are visible through holes or gaps in the umbilical armoring as
this may cause inadvertent exposure of laser energy to persons in the room.
Warning:
Never look into the handpiece exit window if the laser is turned on. Severe eye damage
could occur. Turn off the power to the laser before inspecting any of the optical
components.
Part Number 10-05170 Rev. O Page 52 of 92
Page 53

Fraxel® 1550, Fraxel 1927 and Fraxel DUAL 1550/1927 Laser System
Warning:
Contact lenses and spectacles do not provide adequate ocular protection. Do not substitute
for the specified eye protection. Failure to use appropriate eyewear with the Fraxel Laser
Systems increases the risk of injury to the cornea, sclera and soft tissue of the eye.
3.4 Operation of the Laser System
Warning:
This manual is not intended to be a complete guide to the use of the Fraxel 1550, Fraxel
1927 and Fraxel DUAL 1550/1927 Laser Systems. Solta recommends that all qualified
personnel who operate the laser system first seek training that includes, but is not limited to,
the following aspects of laser operation:
Laser Safety
Laser/Tissue Interaction
Hands-On training by a qualified Solta representative
CAUTION: Solta does not and cannot recommend standard operating clinical
practice. The following guidelines are suggested as a starting point from which
operators may become familiar with good clinical laser technique through study of
the medical literature. The guidelines in ANSI Standards Z136.1, Z136.3, IEC60825-1
and EN207 are also recommended as reference material.
3.4.1 Check the laser prior to every procedure before admitting the patient
into the room.
3.4.2 Prepare the patient as directed with appropriate pain management,
and recommended contact lubricant (Refer to Section 4.8).
3.4.3 The footswitch should be controlled only by the operator to prevent
accidental firing of the laser.
3.4.4 The laser is designed to fire only when:
a) Either the 1550 nm or the 1927 nm laser is selected, and
b) The system is in “Treat” mode, and
c) The footswitch is depressed, and
d) The Intelligent Optical Tracking System (IOTS) senses
handpiece motion when the tip is in contact with the skin. See
Section 2.9 of this manual for a more detailed explanation of the
IOTS.
3.4.5 Press the footswitch only after positioning the laser handpiece over
the treatment area.
Part Number 10-05170 Rev. O Page 53 of 92
Page 54

Fraxel® 1550, Fraxel 1927 and Fraxel DUAL 1550/1927 Laser System
3.4.6 Do not allow the tip to lose contact with the skin during treatment.
3.4.7 The audible tones emitted by the unit during treatment are an
indication of hand velocity. If you are moving your hand at different
velocities, but you hear an unchanging monotonic sound, stop
treatment immediately. This indicates an IOTS fault. Stop treatment
and go to Simulate mode to test the IOTS. If the fault persists,
restart the system. If this fault condition persists, call Solta
Customer Service or your local authorized distributor of Solta
Medical lasers.
3.4.8 Never place the system in Treat mode and depress the footswitch
without intending to initiate a treatment.
3.4.9 Refer to the list of “Indications for Use” and “Contraindications”
before treatment.
3.4.10 Do not use visibly damaged (cracked, crazed, cloudy) tips.
Warning:
Do not move the footswitch while the laser is in Treat mode other than to initiate a treatment.
Do not move the footswitch by placing a foot in the housing and lifting it up. Do not pull the
footswitch by its cord. Do not move the laser by the footswitch cord. Do not place the
footswitch or cord in an exposed area where it may be damaged by for example, being
tripped over, or where a sharp object such as a scalpel may fall on it.
Warning:
Laser equipment not in use should be protected against unauthorized operation by removing
the key from the key switch. Keep the laser key in a designated place and allow only trained
personnel access to the key.
Warning:
Do not allow the tip to lose physical contact with the skin during treatment. Serious injury or
undertreatment may result if the IOTS enters a cycle of rapid starts and stops due to loss of
signal.
Warning:
Do not attempt to coil the umbilical beyond the resistance point of the armoring. Do not use
if the internal cables and wires are visible through holes or gaps in the umbilical armoring as
this may cause inadvertent exposure of laser energy to persons in the room.
Part Number 10-05170 Rev. O Page 54 of 92
Page 55

Fraxel® 1550, Fraxel 1927 and Fraxel DUAL 1550/1927 Laser System
Warning:
Laser treatment can only be initiated when the screen shows Treat mode, when the
footswitch is pressed down, and when the IOTS registers handpiece motion. Do not leave
the system in Treat mode and the footswitch pressed down when no treatment is desired,
otherwise inadvertent exposure could occur and serious injury could result.
CAUTION: Solta systems are intended for use solely by trained physicians and
authorized staff trained in the operation of these devices.
CAUTION: Unauthorized calibration of this device, or the use of controls or
adjustments to performance not specified or approved by Solta Medical could cause
injury or harm to the patient, or may result in unintentional laser exposure, or may
void any warranty.
3.5 Electrical Safety
3.5.1 Electrical hazards with the laser are similar to any high-powered
electrical device. Care should be taken when plugging the unit into
the wall outlet. The area must be free of water and your hands must
be dry to avoid electric shock. Always disconnect the laser by
grasping and pulling the plug and not the power cord.
3.5.2 Place the power cord and footswitch cord where they cannot
become hazards.
CAUTION: Never operate the footswitch in water. Serious injury could result. The
footswitch must remain dry.
3.5.3 The high voltages of the Fraxel Laser Systems are not accessible
unless the protective housing is opened. Do not open the protective
panels unless trained and authorized to do so.
3.5.4 Do not attempt to coil the umbilical or power cable beyond the
resistance point of the armoring. Do not use if internal cables and
wires are visible through holes or gaps in the umbilical armoring as
this may cause inadvertent exposure of electrical energy to persons
in the room.
3.5.5 Servicing high voltage and other components is to be done only by
Solta authorized personnel. If service is required call Solta
Customer Service or your local authorized distributor of Solta
Medical lasers.
3.5.6 The laser should never be left turned on while opened.
Part Number 10-05170 Rev. O Page 55 of 92
Page 56

Fraxel® 1550, Fraxel 1927 and Fraxel DUAL 1550/1927 Laser System
CAUTION: The Fraxel Laser Systems are susceptible to Electrostatic Discharge
(ESD) and proper ESD procedures should be followed.
3.6 Laser Treatment Controlled Area (LTCA) Information
3.6.1 Warning signs for laser exposure must be placed on all doors
allowing entrance into the LTCA.
3.6.2 The signs must display "Danger" or "Warning", and the type and
wavelength of laser in use in accordance with ANSI Z35.1, the
specification for accident prevention signs, or per your local or
regional regulations. Examples of suitable signs are also shown in
ANSI Z136.1, Z136.3 and IEC60825-1.
3.6.3 Appropriate protective eyewear should be placed outside of the
laser treatment room to facilitate safe entry. Failure to use
appropriate eyewear with the Solta Medical Fraxel Laser Systems
increases the risk of injury to the cornea, sclera and soft tissue of
the eye.
3.6.4 All LTCA windows should be covered with opaque non-flammable
material or a non-flammable window filter specific for the
wavelength being used in order to prevent inadvertent escape of
laser light. Note that a material that appears opaque to the eye may
not be opaque at the treatment wavelength. Most common ordinary
window glass will allow the laser wavelength used in the Solta
Medical Fraxel 1550, Fraxel 1927 and Fraxel DUAL 1550/1927
Laser Systems to escape.
Warning:
Do not attempt to coil the umbilical beyond the resistance point of the armoring. Do not use
if the internal cables and wires are visible through holes or gaps in the umbilical armoring as
this may cause inadvertent exposure of laser energy to persons in the room.
3.7 Laser Suite Access and Remote Door Interlock
3.7.1 Always limit personnel in the surgical procedure room to those
essential to the procedure. To protect personnel from possible
injury due to entry into the procedure room during treatment, an
optional remote door interlock can be connected from the laser
system to the procedure room entrance door.
3.7.2 When connected, the interlock will automatically disable the laser if
the door is opened during the procedure. When the door is closed,
the interlock is reconnected and treatment can resume once the
message is acknowledged.
Part Number 10-05170 Rev. O Page 56 of 92
Page 57

Fraxel® 1550, Fraxel 1927 and Fraxel DUAL 1550/1927 Laser System
3.7.3 Access to the remote door interlock is through a plug and socket
located on the back panel of the laser. The appropriate pin-outs
and wiring are shown in the figure below. The laser system is
delivered with this plug shorted internally. Cut the internal wire and
join to your external circuit when this is required. The circuit used
should be low impedance and use twisted-pair wire connections.
Consult an electrical professional if unsure about this.
3.7.4 Do not connect other power supplies to this interlock. Connecting
devices to the unit other than instructed may cause severe damage
to the unit and will result in voiding of the device warranty.
3.8 Proper Laser Routine Maintenance, Handling, and
Storage
3.8.1 Only Solta personnel or personnel approved and trained by Solta
shall install or adjust the Laser Systems.
3.8.2 After each patient treatment, the portion of the handpiece tip that
came in contact with the patients’ skin should be wiped down with a
70% alcohol prep pad and visually inspected for damage, debris,
dirt, contamination, crazing or cracks. Allow the alcohol to dry
before initiating another treatment in order to mitigate the risk of fire,
or serious injury could result.
3.8.3 Make sure that the handpiece umbilical cable is not excessively
twisted from the unit. Do not tape the umbilical to any structure. Do
not attempt to coil the umbilical beyond the resistance point of the
armoring. Do not use if the internal cables and wires are visible
through holes or gaps in the umbilical armoring as this may cause
inadvertent exposure of laser energy to persons in the room.
3.8.4 Do not use acetone on the tip as this may cause damage preventing
the tip from functioning properly.
3.8.5 To clean the internal handpiece sealing window, carefully use an
alcohol prep pad or gauze moistened with 70% alcohol. Do not hold
the prep pad or gauze with bare hands while performing this
operation. Allow the alcohol to dry before initiating treatment in
order to mitigate the risk of fire from which serious injury could
result.
3.8.6 Be careful not to contaminate the internal handpiece optics with any
substance used for cleaning and disinfecting the tip or with the
topical anesthesia gel.
3.8.7 Do not allow the inside of the tip window to become contaminated
while cleaning the handpiece sealing window.
3.8.8 Do not set liquids of any kind on top of the laser.
3.8.9 The accessories should be stored in an area accessible only to
qualified and trained personnel.
Part Number 10-05170 Rev. O Page 57 of 92
Page 58

Fraxel® 1550, Fraxel 1927 and Fraxel DUAL 1550/1927 Laser System
3.8.10 Minimize the presence of dust particles and corrosive substances
such as salts and acids in the storage environment in order to
extend the lifetime of the laser system.
3.8.11 Do not store the laser outside.
3.8.12 Examine the handpiece frequently to check for debris on the
window.
3.8.13 Do not allow the plastic or metal parts of the handpiece to come into
prolonged contact with enzymatic detergents, Cidex
®
OPA or other
chemically aggressive cleaning solutions of chemicals.
3.8.14 Solta Medical suggests that the operators clean and disinfect the
exterior chassis of the laser system with a soft cloth dampened with
mild soap and water.
Warning:
Inspect the handpiece umbilical armoring for damage on a routine basis. Do not use the
device if the umbilical is damaged as inadvertent exposure to the laser may occur which
may result in serious injury.
Warning:
Never move the unit by pulling on the handpiece support arm.
Warning:
Never move the unit by pulling on the footswitch cable.
Warning:
Do not immerse or soak the handpiece or the umbilical cable containing the optical fiber.
Warning:
If the handpiece tip or internal sealing window is cleaned with alcohol prior to use, allow time
for the alcohol to evaporate before restarting the laser to mitigate the risk of fire and injury.
Warning:
Do not allow the metal part of the handpiece to come into prolonged contact with enzymatic
detergent, Cidex® OPA solution, or other chemically aggressive cleaning solutions.
Part Number 10-05170 Rev. O Page 58 of 92
Page 59

Fraxel® 1550, Fraxel 1927 and Fraxel DUAL 1550/1927 Laser System
3.9 Regulatory Compliance and Device Safety Information
3.9.1 Features
The Fraxel Laser Systems provide a means for setting and measuring the
laser output of each laser source, and also contains the required interlock
connectors, key control, and labeling.
3.9.2 Emergency Stop Button
The laser has an emergency stop button that, when pushed, turns off (deenergizes) the laser in emergency situations.
3.9.3 Key Control
The key switch must be turned to the “ON” position to allow the laser to be
energized. Laser light can be produced by the system only when the key
switch is on. The key may not be removed while the key switch is in the “ON”
position.
3.9.4 Touch Screen Display
Allows the operator of the laser to calibrate the laser prior to use, set the
system and treatment parameters prior to treatment, simulate treatment,
manage tip usage, and perform treatments.
3.9.5 Laser Emission Indicator
After a self-test, treatment can only begin when the laser is in the “Treat”
mode. The laser also emits an audible sound when the laser is pulsing.
3.9.6 The laser can only fire when the following conditions are met:
a) The tip is installed correctly, and
b) The footswitch is depressed, and
c) The handpiece senses motion when the handpiece tip is placed
in contact with the skin.
3.9.7 Handpiece Connection
The electrical connections providing power to the handpiece are completely
enclosed by the console and umbilical. There is no access to electrical
sources form the console to the handpiece. The optical path comprising the
laser energy conduit from the console to the handpiece is completely and
redundantly enclosed from the source to the handpiece window.
3.9.8 Foot Pedal Connector
The Foot Pedal connector allows the Foot Pedal supplied with the system to
be connected to the main unit.
Part Number 10-05170 Rev. O Page 59 of 92
Page 60

Fraxel® 1550, Fraxel 1927 and Fraxel DUAL 1550/1927 Laser System
3.9.9 Remote Interlock Connector
The remote interlock connector permits the operator to connect a remote
barrier interlock, remote emergency stop switch, or similar device. The
interlock is wired internally such that, when the terminals of the connector are
open, the laser is disabled to prevent laser emission.
3.9.10 Protective Housing
The laser has a protective housing which prevents unintended access to
laser radiation. No sections of the housing can be removed without
specialized tooling.
The laser optical path is enclosed by four separate layers of protection,
comprising the fiber buffer, the fiber jacketing, the metal armoring on the fiber
jacket and the metal armoring on the handpiece umbilical cable. There is no
exposed optical path between the console (laser enclosure) and the delivery
device. The only exposed laser portal is the handpiece exit window.
CAUTION: DO NOT ATTEMPT TO REMOVE ANY PANEL FROM THE LASER
CONSOLE. ANY ATTEMPT TO DO SO COULD RESULT IN SERIOUS INJURY. THE
LASER WARRANTY WILL BE VOIDED.
Part Number 10-05170 Rev. O Page 60 of 92
Page 61

Fraxel® 1550, Fraxel 1927 and Fraxel DUAL 1550/1927 Laser System
3.10 Labeling
The Fraxel Laser Systems contain the required manufacturing warning labels.
The location, positioning and formatting of labels are subject to change.
Emergency Stop Button
Part Number 10-05170 Rev. O Page 61 of 92
Page 62

Fraxel® 1550, Fraxel 1927 and Fraxel DUAL 1550/1927 Laser System
Regulatory Compliance Labels
The location, positioning and formatting of labels are subject to change.
1550 nm and 1927 nm
Part Number 10-05170 Rev. O Page 62 of 92
1550 nm
Page 63

Fraxel® 1550, Fraxel 1927 and Fraxel DUAL 1550/1927 Laser System
Regulatory Compliance Labels
The location, positioning and formatting of labels are subject to change.
Part Number 10-05170 Rev. O Page 63 of 92
Page 64

Fraxel® 1550, Fraxel 1927 and Fraxel DUAL 1550/1927 Laser System
Part Number 10-05170 Rev. O Page 64 of 92
Page 65

Fraxel® 1550, Fraxel 1927 and Fraxel DUAL 1550/1927 Laser System
Regulatory Compliance Labels
The location, positioning and formatting of labels are subject to change.
Part Number 10-05170 Rev. O Page 65 of 92
Page 66

Fraxel® 1550, Fraxel 1927 and Fraxel DUAL 1550/1927 Laser System
Part Number 10-05170 Rev. O Page 66 of 92
Page 67

Fraxel® 1550, Fraxel 1927 and Fraxel DUAL 1550/1927 Laser System
4.0 Physician Information
Warning:
The information which follows below is provided by Solta only as an information service to
physicians and is made available with the understanding that it does not constitute the
rendering of medical advice. Physicians should make their own assessment of and decision
on the use of any of the information.
4.1 Indications for Use
Refer to Section 1.1.
4.2 Considerations
MEDICAL JUDGEMENT SHOULD BE USED WHEN TREATING PATIENTS
WITH ANY OF THE FOLLOWING CONDITIONS:
4.2.1 Predisposition to post inflammatory hyperpigmentation (PIH)
4.2.1.1 Prophylactic bleaching regimen for skin types IV to VI for
4-6 weeks prior to first treatment, throughout treatment
regimen, and 4-6 weeks post final treatment
recommended
4.2.2 Lesion suspicious for malignancy
4.2.3 Active infection: bacterial, viral, fungal
4.2.4 Active tanning, UV exposure
4.2.5 Medication that can affect coagulation and predispose patient to
petechiae
4.2.6 Compromised ability to heal
4.2.7 Topical retinoids or retinols
4.2.8 History of cold sores
4.2.8.1 Prophylactic acyclovir regimen recommended
4.2.9 No studies on cosmetic tattoos, pregnant or nursing women, or
silicone implants
Part Number 10-05170 Rev. O Page 67 of 92
Page 68

Fraxel® 1550, Fraxel 1927 and Fraxel DUAL 1550/1927 Laser System
4.3 Contraindications
4.3.1 Not all patients will necessarily react the same way to the laser
treatment. The laser system should not be used on any patient who
is ineligible for general surgery. Appropriate medical judgment
should be exercised at all times.
4.3.2 The following contraindications are routine for many laser
treatments and may also be associated with non-ablative Fraxel
1550, Fraxel 1927 and Fraxel DUAL 1550/1927 Laser Systems
treatments. Pre-screening and informed consent should include,
(but not be limited to):
a) predisposition to keloid formation or excessive scarring,
b) pigmentation changes following surgery,
c) skin indentations and textural changes following surgery,
d) systemic steroids (e.g. prednisone, dexamethasone), which
should be rigorously avoided prior to and throughout the course
of treatment,
e) patients undergoing Accutane™ treatment or with drugs in a
similar class.
4.3.3 Solta has no clinical information or experience concerning the
use of the Fraxel 1550, Fraxel 1927 and Fraxel 1550/1927 DUAL
Laser Systems on pregnant women or nursing mothers.
4.4 Tissue Interaction
In skin, water is the primary absorber (chromophore) of the 1550 nm and 1927 nm laser
wavelengths used in the Fraxel 1550, Fraxel 1927 and Fraxel DUAL 1550/1927 Laser
Systems.
Tissue damage can occur with the application of incorrect laser energy settings, incorrect
use of the handpiece or inappropriate pretreatment regimens. Extreme caution should be
exercised until you are familiar with the system.
Part Number 10-05170 Rev. O Page 68 of 92
Page 69

Fraxel® 1550, Fraxel 1927 and Fraxel DUAL 1550/1927 Laser System
4.5 Pre-Treatment Patient Recommendations
4.5.1 Stop use of isotretinoin 6-12 months prior to treatment
4.5.2 Stop use of the following 2 weeks prior to treatment:
®
4.5.2.1 Retinols: Retin A, Tazorac
4.5.2.2 Glycolic acid products
4.5.2.3 Salicylic acid products
4.5.2.4 Tanning or sun exposure
4.5.2.5 Complementary/supplementary non-ablative laser
treatments (i.e., IPL, Hair Removal, etc.)
4.5.3 Stop use of the following 7-10 days prior to treatment
4.5.3.1 Waxing
4.5.3.2 Abrasive scrubs
4.5.3.3 Microdermabrasion treatments
, ‘anti-aging’ products
4.6 Pre-Operative Inspection
Do not use the Fraxel Laser Systems on patients meeting the contraindication criteria.
Warning:
The information which follows below is provided by Solta only as an information service to
physicians and is made available with the understanding that it does not constitute the
rendering of medical advice. It also does not constitute a statement that Solta has tested
these items for safety or efficacy, nor is it an endorsement or warranty of the safety or
efficacy of these products. Physicians should make their own assessment of and decision
on the use of any of the information.
4.7 Anesthesia
NOTE: The physician must be fully versed in the nature, presentation and treatment of
anesthesia toxicity. Physicians should inquire before starting this procedure as to whether
the patient has a history of potential sensitivity to amide-type anesthetics if, for example,
lidocaine is used. Do not use topical lidocaine or similar systems if contact allergies or
systemic allergic conditions have occurred in the past.
An example of a test of an anesthesia protocol as performed by a physician skilled in the
use of a Fraxel 1550, Fraxel 1927 and Fraxel DUAL 1550/1927 Laser Systems might be:
“After the anesthetic has been administered to the treatment area for an appropriate period
of time, perform a sensitivity test to gauge readiness for treatment. For example, perform an
“ice cube test”. This consists of placing a plastic-wrapped ice cube on the area coated with
topical ointment for 7 seconds and then placing a plastic-wrapped ice cube on the nontreatment area for 7 seconds. The patient should score the sensation felt within the
Part Number 10-05170 Rev. O Page 69 of 92
Page 70

Fraxel® 1550, Fraxel 1927 and Fraxel DUAL 1550/1927 Laser System
treatment on a scale from 0 to 5, with “0” equal to no sensation of cold and “5” equaling the
sensation of cold felt on the non-treated region. If the patient scores the sensation within the
treatment region as being greater than 1, reapply the topical anesthetic and wait 15 more
minutes. Repeat until the patient has scored the cold sensation within the treatment region
as a “0” or a “1”. Another test may be used to gauge patient sensitivity and readiness for
treatment, however in the interest of maintaining an appropriate pain management protocol,
aim to begin treatment only when the anesthetic appears to have reached optimal
effectiveness.”
Part Number 10-05170 Rev. O Page 70 of 92
Page 71

Fraxel® 1550, Fraxel 1927 and Fraxel DUAL 1550/1927 Laser System
4.8 Patient Preparation
4.8.1 Ensure patient does not have any contraindications
4.8.2 Have patient sign consent forms
4.8.3 Provide verbal and written post-procedure care instructions to
patient
4.8.4 Prepare patient record forms
4.8.4.1 Treatment record forms are located in the Treatment
Form section of the Fraxel Treatment Reference Guide
and are available for download on Solta Space,
www.soltaspace.com
4.8.5 Remove all topical make-up and/or lotions from treatment area
4.8.6 Remove all jewelry
4.8.7 Cleanse treatment area
4.8.8 Take “Before” photos
4.8.8.1 Photo set-up instructions are available in the Fraxel
Treatment Reference Guide
4.8.9 Apply topical anesthetic:
4.8.9.1 Wipe treatment area with alcohol
4.8.9.2 Apply moderate amount of topical anesthetic to
treatment area, massaging into skin
4.8.9.3 Exceed the anticipated treatment area/border (i.e.,
hairline)
4.8.9.4 High concentration topical (i.e., 23/7, 7/7) – two
treatment areas maximum (1550 nm)
4.8.9.5 Lower concentration topical (i.e., EMLA/LMX) – multiple
treatment areas (1927 nm)
4.8.9.6 Leave topical on the skin for 60-90 minutes
4.8.9.7 Remove topical with dry gauze
4.8.9.8 Do not wash or apply water to treatment area after
removing topical anesthetic
4.8.10 Provide protective eyewear for the patient
4.8.11 Apply Tracking Gel if desired:
4.8.11.1 1550: Optional
4.8.11.2 1927: Do not use
Part Number 10-05170 Rev. O Page 71 of 92
Page 72

Fraxel® 1550, Fraxel 1927 and Fraxel DUAL 1550/1927 Laser System
4.9 Spot Treatments
Targeting certain areas at deeper settings than the rest of the treatment area:
Spot treat first, then continue treating entire area
Use erythema from spot treatment as a boundary to avoid re-treating the same area
4.10 Immediate Post-Operative Care
Remove any topical anesthetic residue from the skin using a gentle facial cleanser
(Neutrogena™, Kinerase™, Hydraphase™ etc), plenty of water and a wash cloth. Another
method of removing the residue consists of gently wiping the skin with commerciallyavailable, unscented “baby wipes”. Used towelettes or washcloths may be washed using
ordinary laundry detergents.
Warning:
The information which follows below is provided by Solta only as an information service to
physicians and is made available with the understanding that it does not constitute the
rendering of medical advice. Physicians should make their own assessment of and decision
on the use of any of the information.
4.11 Post-Operative Information
4.11.1 Protecting New Skin
For the Fraxel 1550, Fraxel 1927 and Fraxel DUAL 1550/1927 Laser System’s non-ablative
wavelengths, histology indicates that new epithelium (new skin) will start to form within about
24 hours, but that it will remain covered by the old stratum corneum and MENDS from 4 -14
days. Desquamation of MENDS may progress more slowly on non-facial skin and may vary
depending on individual patient response to the treatment. During that time normal /
accelerated exfoliation will reveal the repaired epidermal tissue and new stratum corneum.
Patients should avoid injury and sun exposure.
Skin Protection: Patients should plan to use a high SPF sunscreen on a regular basis for at
least 6 months whenever they are outside. Patients should apply a dual UVA/UVB
sunscreen containing both a physical sun block (either or both zinc oxide or titanium dioxide)
with a sun protection factor of at least 30 SPF. In addition, patients should be instructed to
avoid direct sunlight and wear sun-protective clothing (i.e. a wide-brimmed hat).
4.11.2 Routine Skin Care
Most skin care products can be used 3 weeks after treatment. Avoid the use of retinoids
and topical corticosteroids for 1 – 2 weeks following treatment. Avoid systemic steroids e.g.
prednisone, dexamethasone throughout the course of the Fraxel treatment.
Part Number 10-05170 Rev. O Page 72 of 92
Page 73

Fraxel® 1550, Fraxel 1927 and Fraxel DUAL 1550/1927 Laser System
4.11.3 Expected Responses to Treatment
Edema: Mild to moderate edema (swelling) typically develops immediately after treatment
and diminishes, or resolves within the first several days post-treatment. A small degree of
swelling may last longer in some cases.
Erythema: Mild to moderate erythema (redness) typically develops immediately after
treatment and diminishes or resolves, within the first several days post-treatment. A small
degree of redness may last longer in some cases.
Itching / Dryness of the Treated Area: These are common symptoms once the skin has
healed initially. Flakiness and dry crusting will gradually clear. Use of bland moisturizers
and/or moisturizing sunscreens that have previously been shown to not cause irritation
should help this condition.
Pain or Discomfort: Post-treatment, most patients report a feeling similar to sun
overexposure, which usually dissipates within 1 to 3 hours. Post-treatment ice packs may
be applied to alleviate discomfort. Over-the-counter itch and pain relievers may be used if
necessary (e.g. Benadryl, Claritin, Tylenol etc). Solta has no knowledge of the effects of
NSAIDS on the outcome of treatment.
Petechiae/ Pinpoint Bleeding: Although rare, may occur and typically self-resolve without
sequelae
Laser Cuts: Although rare, may occur and typically self-resolve without sequelae
4.12 Treatments Required and Treatment Intervals
Everyone’s skin is different, but clinical studies suggest that for most patients, maximum
results can be achieved in 3-5 sessions with the 1550 nm wavelength or 1-3 sessions with
the 1927 nm wavelength. Treatments are usually spaced 2-6 weeks apart, at physician’s
discretion.
4.13 Complaints and Adverse Effects
Complaints and Adverse Events are reportable under FDA Guidelines. Solta Medical
follows MDR (Medical Device Reporting) rules for complaint and adverse event handling.
Solta Customer Service should be contacted if the Fraxel Laser Systems are involved in a
complaint or adverse event.
Part Number 10-05170 Rev. O Page 73 of 92
Page 74

Fraxel® 1550, Fraxel 1927 and Fraxel DUAL 1550/1927 Laser System
4.14 Complications
The following complications may be associated with the non-ablative Fraxel 1550, Fraxel
1927 and Fraxel DUAL 1550/1927 Laser Systems. This is not intended to be an allinclusive list, nor a substitute for an informed consent form, which should be provided to
every patient.
Blistering and burns: Blistering or burns may develop over the treated areas.
Discoloration: The possibility of temporary and permanent skin color change is
known to exist with any laser treatment. Post-Inflammatory hypo-pigmentation and
hyper-pigmentation are known complications of many laser treatments and may
occur with Fraxel laser treatment.
Eye injury: Protective eyewear or goggles are provided for the operator and
assistant. As discussed in the Safety Section (Section 3.0), it is important to keep
the eye protection on at all times during treatment in order to protect your eyes from
accidental laser exposure and serious injury.
Infection: A risk of infection exists whenever the skin is wounded. The possibility for
infection exists even with non-ablative fractional laser devices such the Fraxel 1550,
Fraxel 1927 or Fraxel DUAL 1550/1927 Laser Systems. If observed, infection should
be treated appropriately with topical and/or systemic medications.
Keloid formation: A thickened scar can result from excessive growth of fibrous tissue.
Please refer to the contraindications section.
Prolonged redness: Mild-moderate transient erythema is an expected response.
However, if erythema is severe or persists significantly longer than expected, retreatment should be avoided until the condition resolves. Reaction may vary on a
patient-by-patient basis.
Scarring: The possibility for scarring exists even with non-ablative fractional laser
devices such the Fraxel 1550, Fraxel 1927 and Fraxel DUAL 1550/1927 Laser
Systems. Local scarring may occur directly from laser exposure if treatment
procedures are not followed properly, or from infection or physical irritation such as
picking and rubbing.
Delayed wound healing / skin textural changes: Following laser treatment,
reepitheliazation may not occur as expected due to patient physiology, i.e. poor
wound healing ability, or post-treatment care. This may result in undesirable textural
changes.
Temporary bruising: Temporary bruising may develop over the treated areas.
Part Number 10-05170 Rev. O Page 74 of 92
Page 75

Fraxel® 1550, Fraxel 1927 and Fraxel DUAL 1550/1927 Laser System
5.0 Maintenance and Warranty
The Fraxel 1550, Fraxel 1927 and Fraxel DUAL 1550/1927 Laser Systems should not
require any specific maintenance by the operator. Routine regular preventative
maintenance should be performed on the system as described in this manual. The device is
covered by the warranty described in the Solta Master Purchase Agreement and the
included attachments thereto, such as the Terms and Conditions of Sale.
5.1 Warranty Information
Please refer to the Terms and Conditions of Sale document attached to the Master
Purchase Agreement. Specific warranty terms and procedures are discussed in this
document.
Warning and Disclaimer:
Factory Calibration of the Fraxel 1550, Fraxel 1927 and Fraxel DUAL 1550/1927 Laser
Systems as described in Section 10 are to be carried out only by factory-qualified engineers
or technicians, or by individuals who have taken the Solta Medical Training Program on
service of lasers and who have passed the examination. Factory Calibration or other
adjustment of this device by persons other than those identified above will result in the
warranty of the unit being voided.
5.2 Cleaning Procedures
5.2.1 Exterior Surfaces
The exterior surfaces of the laser console may be cleaned with a soft cloth damped
with a solution of soap and water. Do not use harsh detergents or acetone to clean
the exterior surfaces, including the console. A hospital grade disinfectant may be
used when the system is returned to Solta Medical. The display screen should be
cleaned with antistatic glass or plastic cleaner. Do not pour cleaning products
directly on the display screen, as this could potentially cause damage to the system.
Part Number 10-05170 Rev. O Page 75 of 92
Page 76

Fraxel® 1550, Fraxel 1927 and Fraxel DUAL 1550/1927 Laser System
5.2.2 Handpiece
WITH A TREATMENT TIP IN PLACE, wipe the exterior surface with a gauze pad
moistened with a small amount of 70% alcohol solution. The handpiece must be
thoroughly dried prior to initiation of laser treatment. Do not use harsh detergents or
acetone to clean the handpiece.
5.2.3 Treatment Tip
ALWAYS CLEAN THE TIP BEFORE REMOVING IT FROM THE HANDPIECE.
Wipe the exterior surface of the tip attachment with 70% alcohol. Remove the tip
from the handpiece, turn the tip with the treatment surface up and carefully clean
any remaining debris around. Ensure that no debris, cleaning materials or other
contamination enters the tip. Be sure to completely dry the tip prior to attachment to
the handpiece. Visually inspect the tip for scratches, pits, or damage. Do not use if
these conditions exist.
5.2.4 Component Replacement/Service
Solta Medical, Inc. offers full service warranty to cover all necessary modifications,
replacements, and repairs for the Fraxel Laser Systems. Contact Solta Medical
Customer Service to confirm return of the device for these purposes.
CAUTION: All equipment must be properly decontaminated before return to Solta.
Failure to do this will result in delays and charges to the operator.
5.3 Disposal and Recycling
Do not dispose of laser console as unsorted municipal waste. End of life electrical /
electronic equipment must be collected separately to prevent ill effects to the environment
and human health.
Contact your local recycling facility for separate collection or Solta Medical, Inc. for
appropriate disposal information.
Part Number 10-05170 Rev. O Page 76 of 92
Page 77

Fraxel® 1550, Fraxel 1927 and Fraxel DUAL 1550/1927 Laser System
6.0 Re-order Information
To reorder accessory items contact Solta Customer Service or your local authorized
distributor of Solta Medical™ lasers.
6.1 Accessories
Accessory items include:
Large (15mm) Cool Roller treatment & replacement tips
MC-RS-CRTL-US or MC-RS-CRTL-INTL
(for use with systems with Focal Cooling Feature)
Large (15mm) and small (7mm) treatment tips
MC-F2-RT1L or MC-F2-INTL-RT1L
*Laser Protective Eyeglasses and Shields
MC-LG-GLASS
*Additional information on these items is available from their respective suppliers and/or
their regional distributors.
6.2 *External steel eye protection
The P2 Design is made by Trinity Technologies, 4110 Central Avenue, Minneapolis,
MN 55421. USA Phone (763) 788-8278, Fax (763) 788-8283. The Durette II Regular is
made by Oculoplastik, Inc., 200 Sauve W., Montreal, Quebec, Canada H3L 1Y9, Phone
(888) 381-3292, Fax (800) 879-1849.
6.3 *Corneal Shields
The Cox II Ocular Shield is made by Oculoplastik, Inc., 200 Sauve W., Montreal, Quebec,
Canada H3L 1Y9, Phone (888) 381-3292, Fax (800) 879-1849
Goldman / Stefanovsky Laser Eye Shields are distributed by Rockwell Laser Industries, Inc.,
PO Box 43010, Cincinnati, OH 45243 USA, Phone (513) 271-1568, (800) 945-2737, Fax
(513) 271-1598
6.4 *Corneal Shield Lubrication Gel
Celluvisc is made by Allergan, P.O. Box 19534, Irvine, CA 92623 USA, Phone: (714) 2464500, Fax: (714) 246-4971.
www.allergan.com/site/products/consumers/home.asp?id=celluvisc&largeText,
6.5 *Topical Corneal Anesthetic
Proparacaine USP 0.5% Hydrochloride Ophthalmic Solution is made by Bausch and Lomb
Vision Care, 1400 N. Goodman St. P.O. Box 450 Rochester, NY 14603-0450 USA Phone:
585-338-6000, 1-800-553-5340, Fax: 585-338-6896
Part Number 10-05170 Rev. O Page 77 of 92
Page 78

Fraxel® 1550, Fraxel 1927 and Fraxel DUAL 1550/1927 Laser System
7.0 Decontamination of Returned Equipment
US Federal Postal and Transportation Law (Postal Law Title 18, US Code Section 1716,
Department of Transportation Rules CFR 49, Section 173.386 and 173.387) mandates that
equipment returned to Solta for repair or return must be properly decontaminated with a
chemical cleared for use as a “Hospital Disinfectant” and which is commercially available.
To ensure that transportation workers and Solta personnel are not exposed to pathogens, a
signed statement of decontamination must be enclosed with the return shipment.
If this is not done, Solta will not accept the shipment and the sender will be liable for all
costs and delays.
All decontamination inquiries should be addressed to Solta Customer Service.
The text of the Decontamination Certificate must include the following statements or
information:
The undersigned individual, at the (institution) in
(City and State) certifies that the Solta equipment being
returned has undergone decontamination with a commercially available Hospital
Grade disinfectant / germicide, and is clear and free from biohazards including but
not limited to blood, dermal matter, other body tissues and fluids or components and
constituents thereof.
The undersigned also agrees to reimburse Solta for any costs incurred in shipping,
handling, cleaning of the enclosed equipment in the event that the items received by
Solta are found to be in a contaminated condition.
Model
Serial Number
Date of Manufacture
Solta RMA Number
Print Name
Signature
Date
Part Number 10-05170 Rev. O Page 78 of 92
Page 79

Fraxel® 1550, Fraxel 1927 and Fraxel DUAL 1550/1927 Laser System
8.0 Safety Standards and Regulatory
Classifications
8.1 General Standards
The Fraxel 1550, Fraxel 1927 and Fraxel DUAL 1550/1927 Laser Systems are compliant to
and have been certified as meeting the following FDA, CDRH standards. Formal
compliance certifications to the IEC 60601-1 based standards will be obtained through TUV
Rheinland of N.A. and TUV Rheinland GmbH.
FDA – CDRH 21 CFR 1040
FDA – Laser Notice 50
Warning:
The Fraxel 1550, Fraxel 1927 and Fraxel DUAL 1550/1927 Laser Systems are classified to
contain Class 4 lasers by the Center for Devices and Radiological Health and by IEC / EN.
This classification represents the highest power laser. The output power is in excess of 300
times the maximum permissible exposure per ANSI Z136 and IEC60825-1. The physician
and associated staff must take precautions to prevent exposure of laser energy to the eye
and unintended skin exposure from either direct or diffusely reflected laser beams. In
addition, precautions must be taken in the surgical environment to prevent hazards of fire
and electrical injury.
Warning:
In compliance with CAN/CSA-Z386-08, it is the responsibility of the user to be aware of the
requirements for safe use of the Fraxel 1550, Fraxel 1927 and Fraxel DUAL 1550/1927
Laser Systems. This includes training in laser safety and responsibility for the physical
facility and its signs, proper use of protective eyewear and other safety measures, both for
protection of the patient, or others who may be potentially exposed to hazards associated
with use of the device. This also includes maintenance and other practices required for safe
operation of the device. If needed, the user should consult a safety expert, a consultant, or
other sources regarding safety related issues such as site assessment, hazard evaluation,
problem solving, or compliance enforcement.
European Community Authorized Representative
MDSS GmbH
Schiffgraben 41
D-30175 Hannover
Germany
Part Number 10-05170 Rev. O Page 79 of 92
Page 80

Fraxel® 1550, Fraxel 1927 and Fraxel DUAL 1550/1927 Laser System
9.0 Fraxel Laser Systems Specifications
9.1 Treatment Beam
Invisible Laser Radiation Invisible Laser Radiation
Product Fraxel 1550 Fraxel 1927 and Fraxel
Equipment
classification
IEC60601-1
CDRH Laser
Classification
IEC 60825
Classification
1550 min:1520
Wavelength
Maximum Power 30 W 30 W / 12 W
Maximum Pulse
Energy
Maximum Pulsewidth 10 ms 10 ms
Pulse Repetition Rate 0 – 3kHz 0 – 3kHz
Class I Class I
Class 4 Class 4
Class 4 Class 4
max:1560
70 mJ 70 mJ / 20 mJ
DUAL 1550/1927
1550 min:1520
max:1560
1927 min:1905
max:1945
9.2 Console
Dimensions / in 19.25w x 17.75h x 17.5d
(/cm) 48.9w x 45.1h x 44.45d
Weight ~ 55 lbs
Warning:
SEVERE DAMAGE TO THE UNIT MAY RESULT FROM IMMEDIATE POWER UP
FOLLOWING MOVEMENT OF THE UNIT FROM A COLD ENVIRONMENT INTO A WARM
ENVIRONMENT. THIS CONDITION WILL OCCUR IF FOR EXAMPLE THE UNIT IS
MOVED FROM LOCATION TO LOCATION ON A DAILY BASIS AND IS STORED IN A
SHIPPING CONTAINER, AUTOMOBILE, OR TRUCK OVERNIGHT.
THE UNIT SHOULD BE ALLOWED TO EQUILIBRATE WITH ITS SURROUNDINGS FOR A
MINIMUM OF 90 MINUTES FOLLOWING A CHANGE OF ENVIRONMENT. F AILURE TO
OBSERVE THESE PRECAUTIONS WILL RESULT IN THE WARRANTY OF THE UNIT
BEING VOIDED.
Part Number 10-05170 Rev. O Page 80 of 92
Page 81

Fraxel® 1550, Fraxel 1927 and Fraxel DUAL 1550/1927 Laser System
9.3 Laser Safety Eyewear
Refer to “Optical Safety” – Section 3.0 of this document
9.4 Cart
Dimensions / in 20w x 30h x 22d
(/cm) 50.8w x 76.2h x 55.9d
Weight <100 lbs
9.5 Operating Environment
Maximum Recommended Ambient Air
Temperature for Treatment
Operating Temperature Range 15 to 40°C, 59 to 104°F
Humidity 30 – 75% Non-condensing
Altitude < 10000 feet / 3075 meters
35°C / 95°F
9.6 Shipping and Storage (Non-Operational)
Temperature Range -15 to 45°C, 5 to 113°F
Humidity 30 – 75% Non-condensing
Altitude < 45000 feet / 13846 meters
9.7 Compatible Delivery Devices
Only use original Fraxel 1550, Fraxel 1927 and Fraxel DUAL 1550/1927 Disposable Tips.
Contact your local authorized distributor of Solta Medical lasers for more information.
9.8 Electromagnetic compatibility and immunity
This guidance and manufacturer's declaration information pertains to the following Solta
Medical products: Fraxel DUAL 1550/1927 Laser System
Special precautions concerning electromagnetic compatibility (EMC) must be taken for all
medical electrical equipment.
All medical electrical equipment must be installed and put into service in accordance with
the EMC information provided in this document.
Portable and mobile RF communications equipment can affect the behavior of medical
electrical equipment.
The Fraxel DUAL 1550/1927 Laser System comply with all applicable and required
standards for electromagnetic interference:
It does not normally affect nearby equipment and devices.
Part Number 10-05170 Rev. O Page 81 of 92
Page 82

Fraxel® 1550, Fraxel 1927 and Fraxel DUAL 1550/1927 Laser System
It is not normally affected by nearby equipment and devices.
It is not safe to operate the Fraxel DUAL 1550/1927 Laser System in the presence of
high-frequency medical equipment.
It is good practice to avoid using the Fraxel DUAL 1550/1927 Laser System in extremely
close proximity to other equipment.
Guidance and manufacturer’s declaration – electromagnetic emissions
The Fraxel DUAL 1550/1927 Laser System is intended for use in the electromagnetic
environment specified below. The customer or user of the Fraxel DUAL 1550/1927 Laser System
should assure that it is used in such an environment.
Emissions test Compliance Electromagnetic environment - guidance
RF Emissions
CISPR 11
RF Emissions
CISPR 11
Harmonic emissions
IEC 61000-3-2
Voltage fluctuations /
flicker emissions
IEC 61000-3-3
WARNING: The Fraxel DUAL 1550/1927 Laser System should not be used adjacent to or stacked with other equipment and
that if adjacent or stacked use is necessary, the Fraxel DUAL 1550/1927 Laser System should be observed to verify normal
operation in the configuration in which it will be used.
Group 1
Class A
Class A
Complies
The Fraxel DUAL 1550/1927 Laser System uses RF energy
only for its internal function. Therefore, its RF emissions are
very low and are not likely to cause any interference in nearby
electronic equipment.
The Fraxel DUAL 1550/1927 Laser System is suitable for use
in all establishments other than domestic and those directly
connected to the public low-voltage power supply network
which supplies buildings used for domestic purposes.
Part Number 10-05170 Rev. O Page 82 of 92
Page 83

Fraxel® 1550, Fraxel 1927 and Fraxel DUAL 1550/1927 Laser System
Guidance and manufacturer’s declaration – electromagnetic immunity
The Fraxel DUAL 1550/1927 Laser System is intended for use in the electromagnetic
environment specified below. The customer or user of the Fraxel DUAL 1550/1927 Laser System
should assure that it is used in such an environment.
Immunity test IEC 60601 test
level
Electrostatic
discharge (ESD)
IEC 61000-4-2
Electrical fast
transient / burst
IEC 61000-4-4
Surge
IEC 61000-4-5
Voltage dips, short
interruptions and
voltage variations on
power supply input
lines
IEC 61000-4-11
Power frequency
(50/ 60Hz) magnetic
field
IEC 61000-4-8
NOTE: U
is the a.c. mains voltage prior to application of the test level.
T
±2 kV, ±4 kV, ±6 kV
contact
±2 kV, ±4 kV, ±8 kV air
±2 kV on AC mains lines
±1 kV for signal input /
output lines
±0.5 kV, ±1 kV lines to
lines
±0.5 kV, ±1 kV, ±2 kV
lines to ground
<5 % UT
(>95 % dip in U
) for 0.5
T
cycle
40 % U
T
(60 % dip in U
) for 5
T
cycles
70 % U
T
(30 % dip in U
) for 25
T
cycles
(>95 % dip in
<5 % U
T
) for 5 sec
U
T
3 A/m 3 A/m
Compliance level Electromagnetic environment
- guidance
±2 kV, ±4 kV, ± 6kV
contact
±2 kV, ±4 kV, ± 8kV air
±2 kV on AC mains lines
signal input / output lines
not applicable – no signal
cables longer than 3 m.
±0.5 kV, ±1 kV lines to
lines
±0.5 kV, ±1 kV, ±2 kV
lines to ground
<5 % U
(>95 % dip in U
T
) for 0.5
T
cycle
40 % U
T
(60 % dip in U
) for 5
T
cycles
70 % U
T
(30 % dip in U
) for 25
T
cycles
<5 % U
T
(>95 % dip in U
) for 5
T
sec
Floors should be wood, concrete or
ceramic tile. If floors are covered with
synthetic material, the relative humidity
should be at least 30%.
Mains power quality should be that of a
typical commercial or hospital
environment.
Mains power quality should be that of a
typical commercial or hospital
environment.
Mains power quality should be that of a
typical commercial or hospital
environment. If the user of the Fraxel
DUAL 1550/1927 Laser System
requires continued operation during
power mains interruptions, it is
recommended that the Fraxel DUAL
1550/1927 Laser System be powered
from an uninterruptible power supply or
a battery.
Part Number 10-05170 Rev. O Page 83 of 92
Page 84

Fraxel® 1550, Fraxel 1927 and Fraxel DUAL 1550/1927 Laser System
Guidance and manufacturer’s declaration – electromagnetic immunity (continued)
The Fraxel DUAL 1550/1927 Laser System is intended for use in the electromagnetic
environment specified below. The customer or user of the Fraxel DUAL 1550/1927 Laser System
should assure that it is used in such an environment.
Immunity test IEC 60601 test
level
Conducted RF
IEC 61000-4-6
Radiated RF
IEC 61000-4-3
3 V
rms
150 kHz to 80 MHz
3 V / m
80 MHz to 2,5 GHz
Compliance
level
3 V
rms
3 V / m
Electromagnetic environment -
guidance
Portable and mobile RF communications
equipment should be used no closer to any part of
the Fraxel DUAL 1550/1927 Laser System,
including cables, than the recommended separation
distance calculated from the following equations
applicable to the frequency of the transmitter.
Recommended separation distance
,√
,√
,√
P is the maximum output power rating of
where
the transmitter in watts (W) according to the
transmitter manufacturer and
recommended separation distance in metres (m).
Field strengths from fixed RF transmitters, as
determined by an electromagnetic site survey
should be less than the compliance level in each
frequency range
Interference may occur in the vicinity of equipment
marked with the following symbol:
80 MHz to 800 MHz
800 MHz to 2,5 GHz
b
.
d is the
a
,
NOTE 1: At 80 MHz and 800 MHz, the higher frequency range applies.
NOTE 2: These guidelines may not apply in all situations. Electromagnetic propagation is affected by absorption and
reflection from structures, objects, and people.
a
Field strengths from fixed transmitters, such as base stations for radio (cellular / cordless) telephones and land mobile
radios, amateur radio, AM and FM radio broadcast and TV broadcast cannot be predicted theoretically with accuracy. To
assess the electromagnetic environment due to fixed RF transmitters, an electromagnetic site survey should be
considered. If the measured field strength in the location in which the Fraxel DUAL 1550/1927 Laser System is used
exceeds the applicable RF compliance level above, the Fraxel DUAL 1550/1927 Laser System should be observed to
verify normal operation. If abnormal performance is observed, additional measures may be necessary, such as reorienting or relocating the Fraxel DUAL 1550/1927 Laser System.
b
Over the frequency range 150 kHz to 80 MHz, field strengths should be less than 3 V/m.
Part Number 10-05170 Rev. O Page 84 of 92
Page 85

Fraxel® 1550, Fraxel 1927 and Fraxel DUAL 1550/1927 Laser System
Recommended separation distances between portable and mobile RF
communications equipment and the Fraxel DUAL 1550/1927 Laser System
The Fraxel DUAL 1550/1927 Laser System is intended for use in the electromagnetic
environment in which radiated RF disturbances are controlled. The customer or the user of the
Fraxel DUAL 1550/1927 Laser System can help prevent electromagnetic interference by
maintaining a minimum distance between portable and mobile RF communications equipment
(transmitters) and the Fraxel DUAL 1550/1927 Laser System as recommended below, according
to the maximum output power of the communications equipment.
Rated
maximum
output power
(watts) of
transmitter
0,01 0.12 0.12 0.23
0,1 0.38 0.38 0.73
1 1.2 1.2 2.3
10 3.8 3.8 7.3
100 12 12 23
For transmitters rated at a maximum output power not listed above, the recommended separation distance d in metres
(m) can be estimated using the equation applicable to the frequency of the transmitter, where P is the maximum output
power rating of the transmitter in watts (W) according to the transmitter manufacturer.
NOTE 1: At 80 MHz and 800 MHz, the separation distance for the higher frequency range applies.
NOTE 2: These guidelines may not apply in all situations. Electromagnetic propagation is affected by absorption and
reflection from structures, objects, and people.
Separation distance (meters) according to frequency of transmitter
150 kHz to 80 MHz
,√
80 MHz to 800 MHz
,√
800 MHz to 2,5 GHz
,√
Part Number 10-05170 Rev. O Page 85 of 92
Page 86

Fraxel® 1550, Fraxel 1927 and Fraxel DUAL 1550/1927 Laser System
10.0 External Factory Calibration Instructions
Regulations state that laser-based devices that are classified as CDRH Class 4 shall have
instructions for external Factory Calibration of the power output.
External Factory Calibration of the Fraxel 1550, Fraxel 1927 and Fraxel DUAL 1550/1927
Laser Systems must be performed by a factory-qualified engineer or technician. Please
contact Solta Customer Service with questions relating to the external power calibration of
the Fraxel laser.
10.1 Tools and Parts Required
Serial Port Cable
Personal Computer (PC) running Windows 95 or more recent version
Must have a 9 or 25 pin Serial Port Connector (COMM1 or COMM2)
Laser Power Meter
16 mm Sensor Aperture and > 30W power handling at 1550 & 1927 nm
Fraxel Utility Update CD-ROM
Laser Safety Glasses: P/N 09-00827 and 09-00828
10.2 Procedure
10.2.1 Connect the PC and the Fraxel 1550, Fraxel 1927 or Fraxel DUAL
1550/1927 Laser System using the Serial Connector. Both systems
must be “OFF” at time of connection.
10.2.2 Turn on the computer. Once this has started, turn on the laser
system. Wait for Self-Test to complete.
10.2.3 Calibrate the system.
10.2.4 Select External Calibration Option.
10.2.5 Follow the instructions on the screen.
10.2.6 When finished, power down the computer and the Laser System,
and disconnect the units by removing the serial connector.
Warning and Disclaimer:
Factory Calibration of the Fraxel 1550, Fraxel 1927 and Fraxel DUAL 1550/1927 Laser
Systems are to be carried out only by factory-qualified Solta employees, or by individuals
who have taken a Solta taught course on service of lasers who have passed the
examination and are currently listed by Solta as factory-qualified technicians. Factory
Calibration or other adjustment of this device by persons other than those identified above
will result in the warranty of the unit being voided.
Part Number 10-05170 Rev. O Page 86 of 92
Page 87

Fraxel® 1550, Fraxel 1927 and Fraxel DUAL 1550/1927 Laser System
11.0 Shipping, Installation and Set-Up Requirements
11.1 Shipping
The Fraxel 1550, Fraxel 1927 and Fraxel DUAL 1550/1927 Laser Systems will be shipped in
a specially designed container. When the shipment is received, inspect the exterior
container for damage. Inspect the shock indicators for indications of shipping damage. If
there is exterior damage, DO NOT ACCEPT OR UNPACK THE SYSTEM. Contact Solta
Customer Service at +1 510-782-2286 or your local authorized distributor of Solta Medical
lasers and the shipping company immediately.
DO NOT ATTEMPT TO UNPACK THE SYSTEM even if the system is undamaged and the
shock indicators appear normal. Unpacking will be done as a part of installation by Solta
authorized representatives.
Store the system indoors and at a temperature similar to the temperature of the facility in
which it is to be installed. When the system has been successfully installed, return the
shipping containers to Solta with the enclosed shipping documents.
Warning and Disclaimer:
Storage at excessively low temperatures, such as outdoors in the winter, may result in a
delay during installation and subsequent shortening of the time available for training by the
Solta Customer Service representative.
11.2 Installation
The Fraxel Laser Systems will be installed by Solta Customer Service. At this time the
system, its use, indications and safety features will be explained to facility personnel as part
of the Solta Training Program. It is the customers’ responsibility to prepare the site. See
Site Preparation Guide for installation utilities, space, and other prerequisites.
11.3 Space Requirements
The dimensions of the Fraxel 1550, Fraxel 1927 and Fraxel DUAL 1550/1927 Laser
Systems are listed in the specifications above. Allow adequate space for ventilation (at least
a 12 inch perimeter all around the laser system) to prevent overheating.
Allow extra space for placement of the footswitch. The footswitch must remain accessible
during all treatments. The unit must not be moved by pulling on the footswitch cable, the
handpiece support arm, or the handpiece umbilical.
Part Number 10-05170 Rev. O Page 87 of 92
Page 88

Fraxel® 1550, Fraxel 1927 and Fraxel DUAL 1550/1927 Laser System
11.4 Environmental
The temperature in the laser room should ideally remain between 65º to 80º F, (18 – 27°C)
both for patient comfort and for optimum operation of the laser. Patients may feel more
discomfort if the temperature of the room is excessively high. A maximum ambient
operating temperature of 104°F / 40ºC is allowed. The cooling system for the laser is forced
air, and no other external utilities are required.
The Fraxel 1550 and Fraxel DUAL 1550/1927 Laser Systems will emit a maximum of 600W
/ 2050BTUh into the environment around the console.
11.5 Electrical
The Fraxel 1550 and Fraxel DUAL 1550/1927 Laser Systems contain a universal, medical
grade, power-factor corrected power supply. The line voltage and power switch amperage
requirements are given in the specifications section of the manual. Solta recommends a
dedicated circuit for the laser system. Place the power cord where it cannot become a
hazard, for example by being tripped over or having sharp objects dropped on it.
The worst-case current draw is anticipated to be:
100-240V 50/60Hz 5 2.5A
Warning:
The operator is responsible for ensuring that the proper electrical specifications are met at
the installation site.
Warning:
Laser equipment not in use should be protected against unauthorized use by removing the
key from the key switch. Keep the key in a designated place accessible only to designated
and trained personnel. Moving the Laser
11.5.1 Shut down the laser and then remove the power cord from the
wall and from the power switch housing in the laser.
11.5.2 Remove the footswitch cable plug from the rear of the system
and carefully wrap the footswitch cable around the footswitch
housing. Store the footswitch in the basket in the rear of the
cart. If the external interlock is connected, remove this plug.
11.5.3 Lock the handpiece umbilical management arm prior to
movement. Unlock the cart wheels.
Part Number 10-05170 Rev. O Page 88 of 92
Page 89

Fraxel® 1550, Fraxel 1927 and Fraxel DUAL 1550/1927 Laser System
11.5.4 Using the console handle, move the system to the new
location. Be careful when pushing the unit over elevator
thresholds, carpet-linoleum interfaces, and other step-like
features in the path of motion. Do not allow the unit to tip over
when in motion. The safest way to cross a high threshold is to
back the unit into the threshold and pull it across, so that the
larger wheels cross the threshold first.
11.5.5 When the Fraxel Laser Systems are parked in their working
location, the brakes located on the base of the console must be
locked thus avoiding accidental movement and reducing the
possibility of tilting the laser system.
11.5.6 The Fraxel Laser Systems have been fully tested and is
compliant to the Class A limits of the EN60601-1-2 medical
device standards. The Class A limits in the EN60601-1-2
standard are used to provide reasonable protection against
harmful electromagnetic interference caused by medical
equipment. To avoid interference with other devices, use the
electrical cord provided with the Fraxel Laser systems and
always plug the cord into a grounded outlet.
.
Warning:
If it is necessary to manually lift the Laser System Console and Cart, or to separate the
console from the cart, two people must perform the lifting operation. Failure to follow this
directive may result in serious injury, or damage to the unit.
Warning:
Never move the unit by pulling on the handpiece support arm or by pulling on the footswitch
cable. Do not utilize an undersized substitute cart to move the system.
Warning:
SEVERE DAMAGE TO THE UNIT WILL RESULT FROM IMMEDIATE POWER UP
FOLLOWING MOVEMENT OF THE UNIT FROM A COLD ENVIRONMENT INTO A WARM
ENVIRONMENT. THIS CONDITION WILL OCCUR IF FOR EXAMPLE THE UNIT IS
MOVED FROM LOCATION TO LOCATION ON A DAILY BASIS AND IS STORED IN A
SHIPPING CONTAINER, AUTOMOBILE, OR TRUCK OVERNIGHT.
THE UNIT MUST BE ALLOWED TO EQUILIBRATE WITH ITS SURROUNDINGS FOR A
MINIMUM OF 90 MINUTES FOLLOWING A CHANGE OF ENVIRONMENT. FAILURE TO
OBSERVE THESE PRECAUTIONS WILL RESULT IN THE WARRANTY OF THE UNIT
BEING VOIDED.
Part Number 10-05170 Rev. O Page 89 of 92
Page 90

Fraxel® 1550, Fraxel 1927 and Fraxel DUAL 1550/1927 Laser System
12.0 Labeling Symbols
Description Symbol
Caution / Warning: See Instructions for Use
Authorized Representative in the EU
Date of Manufacture
Do Not Discard in Trash
European Conformity
Footswitch
Fuse
Manufacturer
Type “B” Symbol
Invisible Laser Radiation Hazard
Emergency Laser Stop
Part Number 10-05170 Rev. O Page 90 of 92
Page 91

Fraxel® 1550, Fraxel 1927 and Fraxel DUAL 1550/1927 Laser System
Description Symbol
ESD Sensitive Device
Laser Aperture, Avoid exposure. Invisible laser radiation
Key Switch Power On and Off
Heavy, Lift with two persons
Handpiece Connector
Service Key (For Service Technicians Only)
USB port (This port is used for system updates/upgrades. This
port is utilized by qualified Service Technicians Only)
Do not insert any cables or wires into any of the service ports, as
this may potentially cause damage to the equipment
Remote Interlock Connector
Part Number 10-05170 Rev. O Page 91 of 92
Page 92

Fraxel® 1550, Fraxel 1927 and Fraxel DUAL 1550/1927 Laser System
Description Symbol
Communication Connectors (These ports are used in case a
system error has occurred. This port is utilized by qualified
Service Technicians Only)
Do not insert any cable or wires to any of the service ports as
this may potentially cause damage to the equipment
No special protection against moisture
TUV Rheinland logo
MC-SYS-SR1500-D-US
MC-SYS-SR1500-D-UPG-US
MC-SYS-SR1500-P-US
MC-SYS-SR1500-P-UPG-US
MC-SYS-SR1500-CPO-US
MC-SYS-SR1500-D-INTL
MC-SYS-SR1500-D-UPG-INTL
MC-SYS-SR1500-P-INTL
MC-SYS-SR1500-P-UPG-INTL
MC-SYS-SR1500-CPO-INTL
REF
Part Number 10-05170 Rev. O Page 92 of 92
 Loading...
Loading...