Page 1
acapella® choice Vibratory PEP System
Reference Guide
These instructions contain important information
for safe use of the product. Read the entire contents
of the Instructions for Use, including Warnings and
Cautions, before using this product. Failure to properly
follow warnings, cautions and instructions could
1. DESCRIPTION:
The acapella® choice vibratory PEP system is a
single patient use device that provides Positive
Expiratory Pressure (PEP) therapy for patients
who have Cystic Fibrosis, COPD, asthma, and lung
diseases with secretory problems, and patients
with atelectasis. All patients must be capable of
following instructions for PEP therapy. Review the
diagram and the product to become familiar with
all of the product features.
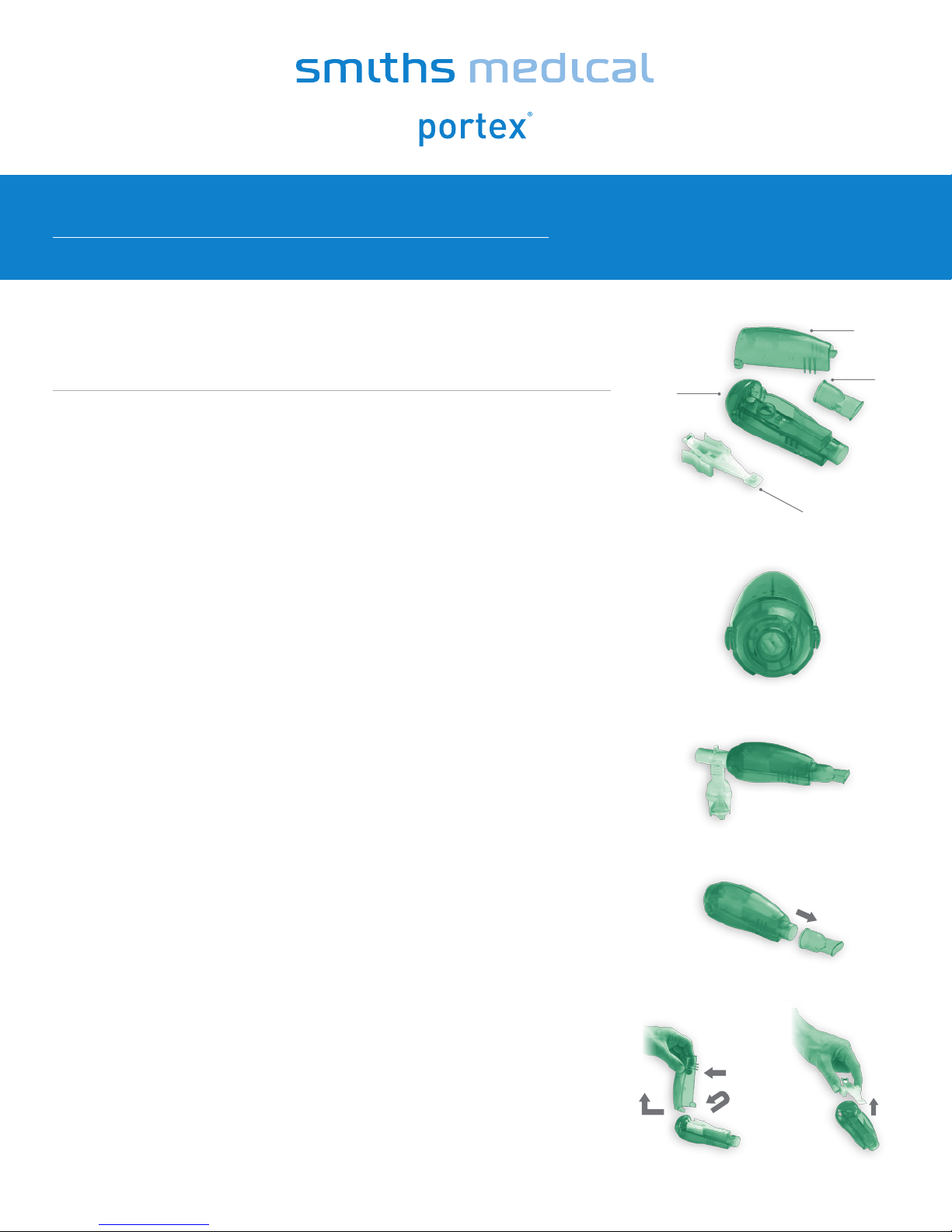
The acapella
one-way inspiratory valve and 22 mm male fittings
consists of: (See Figure 1)
A. Detachable mouthpiece
(device end adapts to mask)
B. Expiratory resistance/frequency adjustment dial
C. Detachable cover
D. Detachable rocker assembly – DO NOT
disassemble the rocker arm from its
platform base
®
choice Vibratory PEP system with
2. INDICATIONS:
The Smiths Medical acapella® choice system is
intended for use as a PEP device. It may also be
used simultaneously with nebulized aerosol
drug delivery.
3. CONTRAINDICATIONS:
Although no absolute contraindications to the use
of PEP therapy have been reported, the following
should be carefully evaluated before a decision is
made to initiate therapy:
• Inability to tolerate increased work of breathing
• Hemodynamic instability
• Intracranial pressure (ICP) > 20 mm Hg
• Acute sinusitis
• Recent facial, oral or skull surgery or trauma
• Epistaxis
• Esophageal surgery
• Active hemoptysis
• Untreated pneumothorax
• Nausea
• Known or suspected tympanic membrane rupture
or other middle ear pathology
result in death or serious injury to the patient. It is the
responsibility of the healthcare practitioner to assure
that the instructions for use and maintenance are
understood by and provided to the caregiver.
4. WARNINGS:
4.1 Use of this device at excessive pressures may
have adverse effects. Expiratory pressures above
20 cm H2O in patients sensitive to increased
transpulmonary pressure may develop one or
more of the adverse side effects listed below.
4.2 Expert clinical judgment should be exercised in
the selection of the appropriate setting for each
individual patient. Failure to match the appropriate
resistance setting on the +/- dial indicator with
the patient’s expiratory flow may result in failure
to achieve therapeutic objectives of vibratory PEP
therapy or one or more adverse side effects below.
• Adverse reactions may include:
• Increased work of breathing that may lead to
hypoventilation and hypercarbia
• Increased cranial pressure
• Cardiovascular compromise
• Myocardial ischemia
• Decreased venous return
• Air swallowing with increased likelihood of
vomiting and aspiration
• Claustrophobia
• Skin break down and discomfort from mask
• Pulmonary barotraumas
4.3 The rocker assembly is not designed to be
disassembled - do not attempt to separate
the rocker arm from its platform base. Separating
these components may cause the acapella
choice system to function improperly, which may
deprive the patient of therapy and cause injury to
the patient.
®
5. PRECAUTIONS:
5.1 Bleach is not recommended for use on the
5.2 DO NOT MICROWAVE. The metal and magnet
5.3 It is the responsibility of the user to ensure all
5.4 Visually inspect the device to ensure that the
5.5 Verify all connections are secure.
®
acapella
nickel plated mechanism located in the interior
of the device.
might ignite.
sterility verification(s).
unit is free of contamination and foreign objects.
choice system. It may deteriorate the
90°
C.
Figure 1
Figure 2
Figure 3
Figure 4
Rib
B.
A.
D.
Figure 5
Figure 6
Page 2
6. SUGGESTED INSTRUCTIONS FOR USE:
6.1 Initial Settings:
6.1.1 If this is the first use of the acapella
system, ensure that the frequency adjustment
dial is turned counterclockwise to its lowest
frequency-resistance setting, indicated at “1”.
(See Figure 2)
6.1.2 Instruct the patient to relax while performing
diaphragmatic breathing and inspiring a
volume of air larger than normal tidal volume
(but not to total lung capacity).
6.1.3 Direct the patient to exhale to Functional
Residual Capacity (FRC) actively, but not
forcefully, through the device.
6.1.4 Adjust the dial to increase the resistance.
6.1.5 The patient should be able to exhale for 3-4
seconds while the device vibrates. Clockwise
adjustment increases the resistance of the
vibrating orifice; which will allow the patient to
exhale at a lower flowrate. Counter-clockwise
adjustment decreases the resistance.
6.1.6 Once the proper range has been identified, the
patient may be instructed to exhale harder or
softer, or dial adjustments may be made to
optimize the response the user “feels” from the
vibratory pressure.
6.2 Procedure for the User:
6.2.1 Ensure the adjustment dial is set to the correct
range as identified by your clinician.
6.2.2 Sit with your elbows resting comfortably on
the table.
6.2.3 Place the mouthpiece lightly in your mouth.
• Be sure to maintain a tight seal on the mouthpiece
during exhalation.
• Your clinician may recommend the use of a nose
clip, if necessary.
• If using a mask, apply the mask tightly but
comfortably over your nose and mouth.
6.2.4 Breathe from the diaphragm, as directed by your
clinician, taking in a larger than normal breath,
but not filling your lungs to capacity.
6.2.5 Hold your breath for 2-3 seconds.
6.2.6 Exhale actively, but not forcefully, through the
device. Exhalation should last approximately 3 to 4
times longer than inhalation.
6.2.7 Perform 10-20 PEP breaths as recommended by
your clinician.
6.2.8 Remove the mouthpiece (mask) and perform
2-3 “huff” coughs to raise secretions as
needed. Your clinician may direct you on proper
cough technique.
6.2.9 Repeat steps 6.2.2 to 6.2.7 as prescribed.
Note: See nebulizer set-up section.
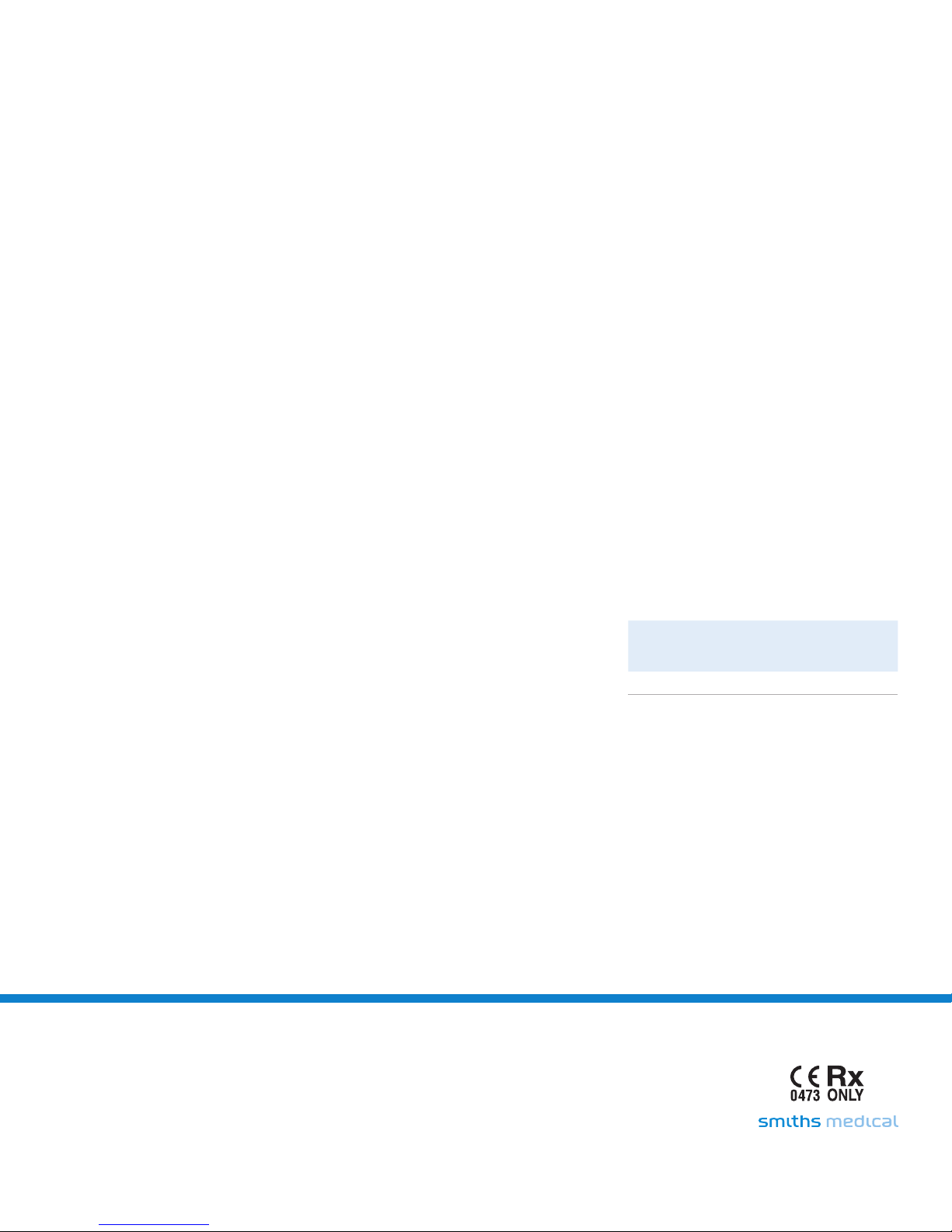
6.3 Set-up Nebulizer
6.3.1 Review the diagrams contained with the devices.
6.3.2 A possible nebulizer and acapella
set up is reflected below. (See Figure 3)
®
choice
®
choice system
6.3.3 Follow set-up instructions for each device.
6.3.4 Follow cleaning instructions contained with
each device.
6.3.5 Inspect device(s) on a routine basis to ensure
proper use and function.
6.3.6 If damaged, do not use.
6.3.7 Verify all connections are secure.
7. CLEANING AND DISINFECTING
INSTRUCTIONS:
Precaution: Bleach is not recommended for use on the
®
acapella
plated mechanism located in the interior of the device.
7.1 There are four parts to clean:
A. The mouthpiece
B. The base unit (with frequency adjustment dial)
C. The cover
D. The rocker assembly – DO NOT disassemble the
7.2 Disassembly
7.2.1 Detach the mouthpiece or mask. (See Figure 4)
7.2.2 Detach the cover by placing your forefinger and
7.2.3 Gently press on the ribbed surface and lift
7.2.4 Detach the rocker assembly by placing your
7.3 Cleaning/Disinfecting Instructions
7.3.1 Cleaning: This should be done prior to
choice system. It may deteriorate the nickel
rocker arm from its platform base
thumb on the (3) ribs located on the unit toward
the mouthpiece.
the cover up.
Note: The cover should only be lifted to a 90°
angle. (See Figure 5)
Warning: The rocker assembly is not designed to
be disassembled - do not attempt to separate the
rocker arm from its platform base. Separating
these components may cause the acapella
®
choice system to function improperly, which may
deprive the patient of therapy and cause injury to
the patient.
forefinger and thumb.
As per the Cystic Fibrosis Foundation’s cleaning
and disinfecting guidelines entitled, “Respiratory,
Stopping the Spread of Germs” 2003, below are
the guidelines for the acapella
®
Note: The acapella
choice system is for single
®
choice system.
patient use
Disinfecting (7.3.2)
Cleaning with Liquid Dish Detergent:
As needed, detach the mouthpiece (mask) then
soak the device and mouthpiece in warm, soapy
water as required to remove visible contaminants.
Use a liquid dish detergent (Dawn
®
or equivalent),
mixing two (2) tablespoons of detergent per one (1)
gallon water. Rinse thoroughly with sterile water,
and allow parts to air dry.
Drain the device by placing it in a normal resting
position. (See Figure 2)
7.3.2 Disinfecting:
• Boiling – Boil the device in water (100°C/212°F)
up to twice daily for five (5) minutes. In addition,
Smiths Medical suggests the use of distilled
or sterile water to lessen the potential of local
community, mineral-rich tap waters from
calcifying the acapella
®
choice systems metallic
components.
®
• Autoclaving – The acapella
choice system
will functionally withstand autoclaving at
temperatures not to exceed 136°C/277°F
for a maximum of 30 cycles.
®
• Automatic Dishwasher – The acapella
choice
system is dishwasher safe. It is recommended to
place the parts on the top shelf.
• Alcohol – Soak five (5) minutes, twice daily.
The acapella
®
choice system is compatible with
70% isopropyl alcohol. Rinse with sterile water.
You can make water sterile by boiling for five
(5) minutes.
®
• Glutaraldehydes (Cidex
The acapella
®
choice system will maintain its
or equivalent) –
integrity using cold sterilizing solutions such
as glutaraldehydes.
8. DISPOSAL:
Dispose of the acapella® choice system in a safe
manner according to Federal/State/Local
regulations and guidelines for disposal of
contaminated medical waste.
9. ESTIMATED DEVICE LIFETIME:
Assuming the manufacturer recommended
cleaning protocol is followed, the acapella
system devise should have a useful life of six (6)
months under normal and customary usage.
The six (6) month usage duration is measured
from the date of initial use.
®
acapella
Ref
choice
system Catalog
Number
Mouthpiece 27-7000 10
®
choice
Quantity Per
Carton
f ~ : ; ‚
< = J 2 6
Caution • Latex Free • Do not use if package is
damaged • Non-sterile • Contains or Presence of
Phthalate: bis(2-ethylhexyl) phthalate (DEHP)
[www.smiths-medical.com/phthalates] • Catalogue
Number • Batch Code • Date of Manufacture
Caution: Federal (U.S.A.) law restricts this device to sale
by or on the order of a physician. SINGLE PATIENT USE
For more information visit our website at www.smiths-medical.com
PRODUCT(S) DESCRIBED MAY NOT BE LICENSED OR AVAILABLE FOR SALE IN CANADA AND OTHER COUNTRIES
Smiths Medical ASD, Inc.
Keene, NH 03431, USA
Phone: +1-603-352-3812
Toll-Free USA 1-800-258-5361
www.smiths-medical.com
Find your local contact information at: www.smiths-medical.com/customer-support
Smiths Medical is part of the global technology business Smiths Group plc. acapella and the Smiths Medical design mark are trademarks of Smiths Medical. The symbol ® indicates the trademark is
registered in the U.S. Patent and Trademark Office and certain other countries. All other names and marks mentioned are the trademarks or service marks of their respective owners. ©2016 Smiths
Medical. All rights reserved. RE194318EN-082016
Smiths Medical ASD, Inc.
6000 Nathan Lane North
Minneapolis, MN 55442, USA
Tel: 1-614-210-7300
Toll-Free USA: 1-800-258-5361
www.smiths-medical.com
MKEECA-0059
 Loading...
Loading...