Smart Medical Device DM VME03S User Manual
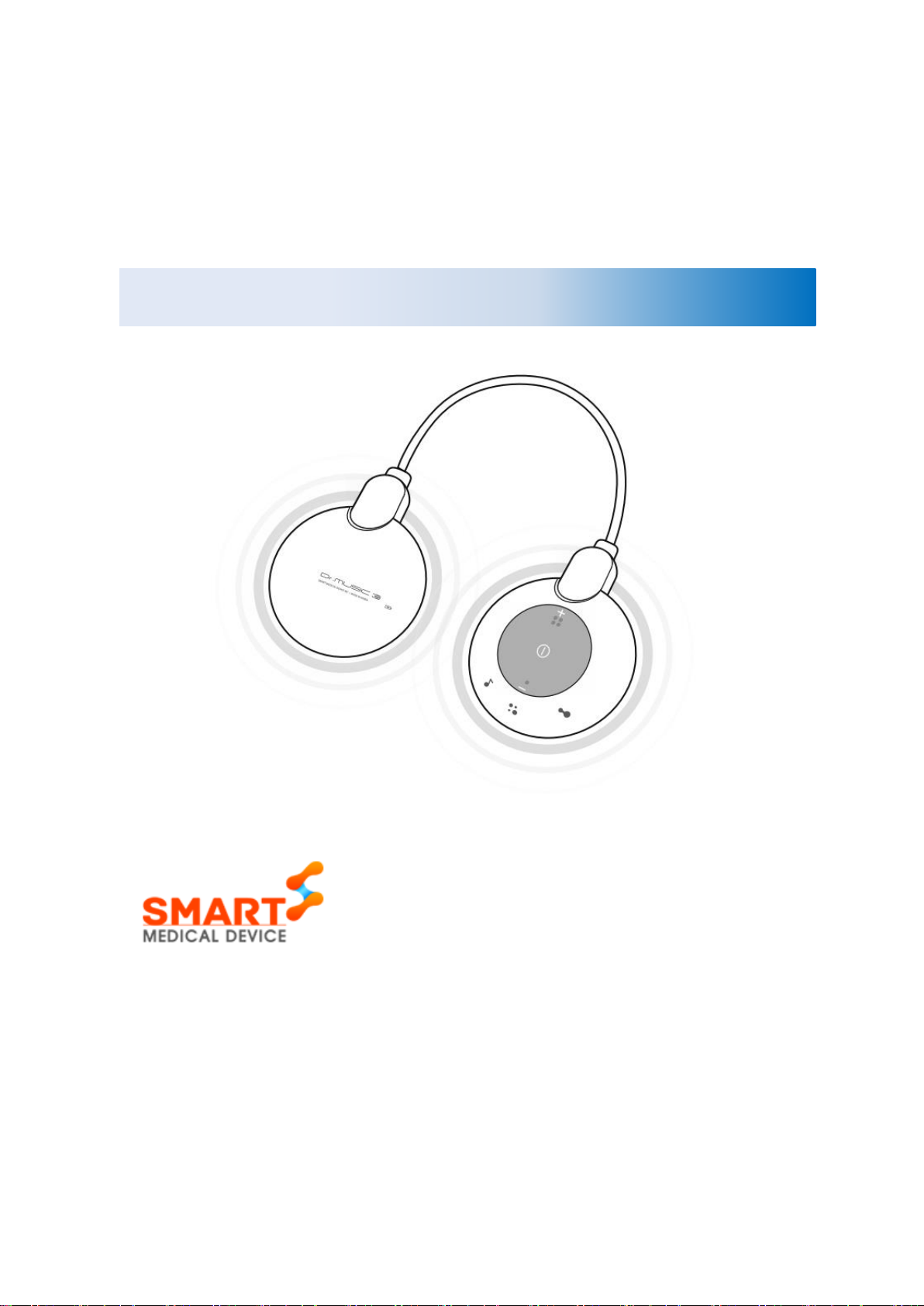
Dr.MUSIC 3s
(Model: DM-VME03S)
SmartMedicalDevice Co.,Ltd.
#803, 32-19, Gobong-ro, Ilsandong-gu, Goyang-si
Gyeonggi-do, Korea
Tel: +82-70-7525-2104, Fax: 82-504-983-1173
Website: Http://www.smd21.com
E-mail: sales@smd21.com
© 2016.SmartMedicalDevice Co.,Ltd.
All rights reserved. Reproduction in whole or in part in any form or by any means, electrical,
mechanical or otherwise is prohibited without the written consent of the copyright holder.
Manual status: SMD-IFUE-DMV-001 (ver. 0), Date of preparation: 16/03/15
User ’s Manual

Dr.MUSIC 3s (Model: DM-VME03S)
SMD-IFUE-DMV-001 Rev 0 2
About this manual
• Read and understand all instruction in this manual before attempting to use the D ev ice .
• SmartMedicalDevice Co., Ltd. shall not be liable for any incidental, or consequential damages
that occurred by not complying with the content of the User’s Manual.
• Assist user in the safe and effective operation of the product.
• Explains procedures for the product setup, control and function.
• Keep this manual with the product.
• Content of this manual may be changed or improved.
• All references to standards / regulations and their revisions are valid for the time of
publication of this instruction for use.
• The screen graphics and illustrations in this manual are for illustrative purposes only and may
be different from what is displayed on the screen or device.
Conventions
Throughout the text in these instructions for use, warnings and other information essential when
using this unit, such as cautionary or prohibited items, appear classified as per the following.
Mark
Description
WARNING
Warning indicates a hazardous situation which, if not avoid, may
result in death or serious injury.
CAUTION
Caution indicates a hazardous situation which, if not a avoided, may
result in minor or moderate injury.
Dr.MUSIC 3s (Model: DM-VME03S)
SMD-IFUE-DMV-001 Rev 0 3
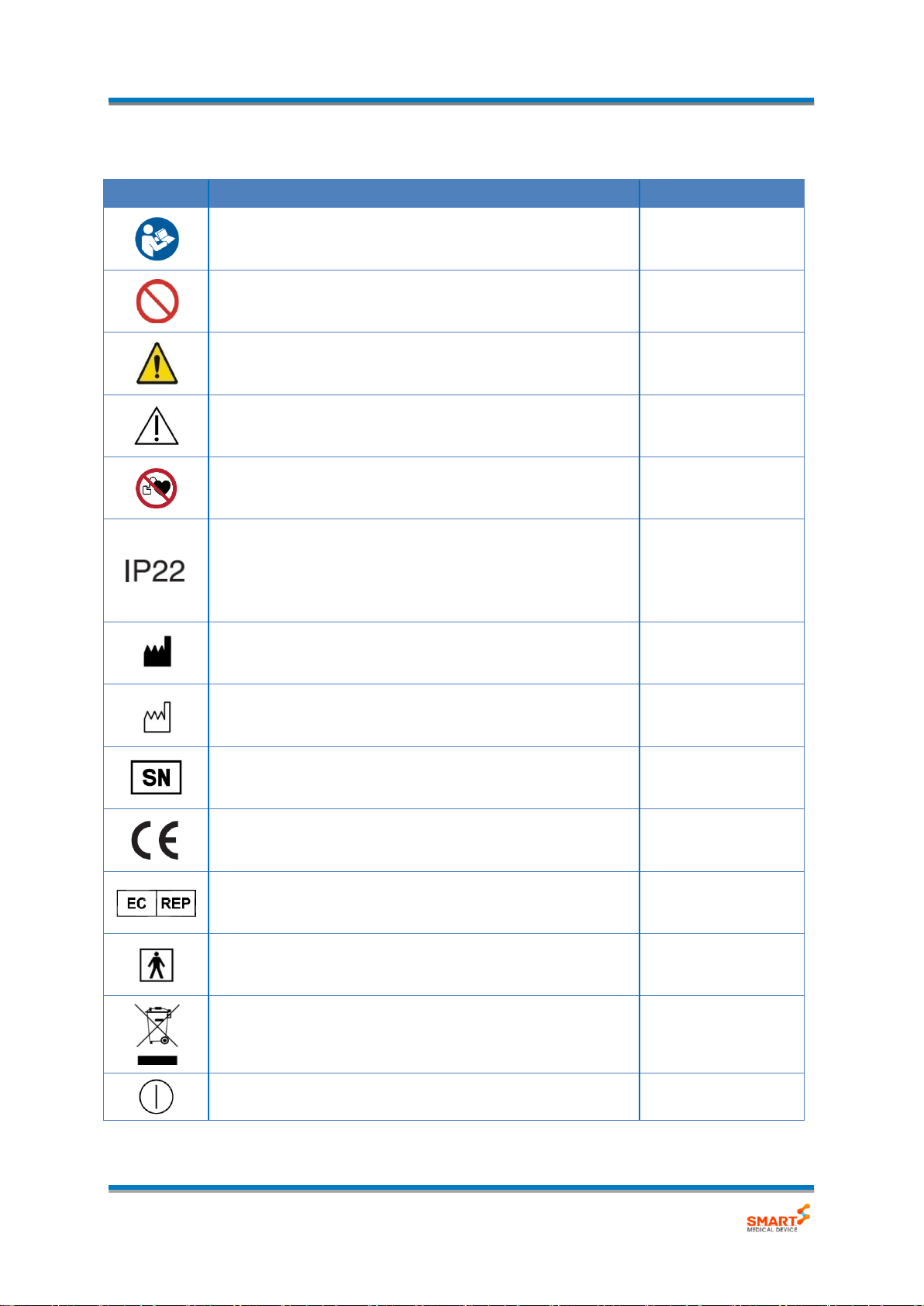
Symbol
Symbol
Description / Function
Reference
Refer to instruction manual/ booklet.
ISO 7010:2012-M002
General prohibition sign
ISO 7010:2012-P001
EU-Warning: This symbol indicates hazard.
If not avoided, the hazard can result death or serious injury.
ISO 7010:2012-W001
EU- Caution: This symbol indicates hazard.
Minor personal injury or product damage.
ISO 7000:2014
No access for people with active implanted cardiac devices.
ISO 7010-P007
This symbol on the device means: Protected against solid
foreign objects of 12,5 mm φand greater and
against vertically falling water drops when tilted up
to 15 degrees.
IEC60529
Manufacturer information: This symbol is followed by the
name and address of the device manufacturer.
ISO 15223-1:2012
Manufacture Date: This symbol is followed by the device
manufacture date in the form YYYY-MM.
ISO 15223-1:2012
Serial Number: This symbol is followed by the device serial
number.
ISO 15223-1:2012
This symbol means: Conforms to EC Directives.
CE stands for ‘Conformité Européenne’.
-
Authorized representative in the European community.
EN ISO 15223-1:2012
Electrical protection: Insulated patient application(TYPE BF)
IEC 60417-1:2002
EU-Electronics and Battery Disposal Information:
This symbol means that the product and battery should be
recycled separately from household waste.”
-
“ON/OFF” (power)
IEC 60417-1:2002
Dr.MUSIC 3s (Model: DM-VME03S)
SMD-IFUE-DMV-001 Rev 0 4
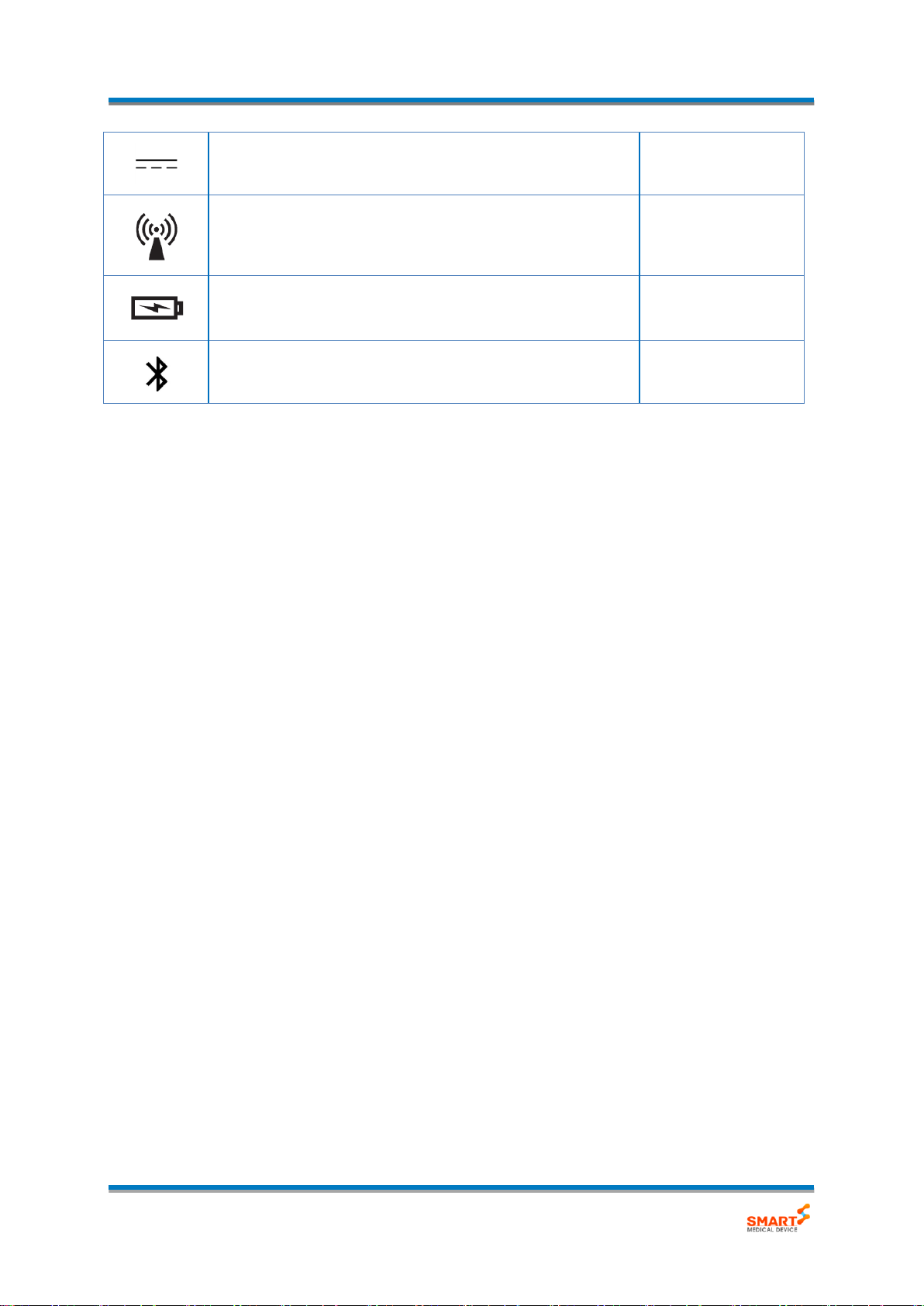
Direct current
IEC 60417-1:2002
This symbol means that this device emits non-ionising
radiation.
-
This is the battery symbol. It appears next to the battery
indicator.
-
Bluetooth
-
This device conforms to the following international standards:
• IEC 60601-1:2012 Electrical medical equipment
• IEC 60601-1-2:2014 Electromagnetic compatibility
• IEC 60601-1-6:2013& IEC 62366:2014 Usability safety
• IEC 60601-1-11:2010 Home Healthcare Environment
• IEC 60601-2-10:2012 Nerve and muscle stimulators
• IEC 62304:2006 Software life cycle processes
Dr.MUSIC 3s (Model: DM-VME03S)
SMD-IFUE-DMV-001 Rev 0 5
Contents
1. SAFETY INFORMATION ......................................................................................................... 7
1.1. CONTRAINDICATIONS .............................................................................................................. 7
1.2. GENERAL SAFETY ..................................................................................................................... 8
2. DR.MUSIC 3S INTRODUCTION .......................................................................................... 11
2.1. OPERATING PRINCIPLE ........................................................................................................... 11
2.2. INTENDED USE ....................................................................................................................... 11
2.3. PRODUCT COMPONENTS ....................................................................................................... 12
2.4. EXTERIOR AND FUNCTION ..................................................................................................... 13
2.5. LAYOUT OF DR.MUSIC 3S APP ........................................................................................... 15
3. OPERATING THE DR.MUSIC 3S ......................................................................................... 17
3.1. PRECAUTIONS BEFORE USING ................................................................................................ 17
3.2. USING THE DEVICE ALONE ..................................................................................................... 20
3.3. USING THE DEVICE WITH MOBILE APP ................................................................................. 23
3.4. CHARGING THE DEVICE .......................................................................................................... 32
4. MAINTENANCE AND INFORMATION OF DR.MUSIC 3S .............................................. 35
4.1. SPECIFICATION OF DR.MUSIC 3S ......................................................................................... 35
4.2. SPECIFICATION OF EXTERNAL DEVICE CONNECTION .............................................................. 36
4.3. QUESTIONS ABOUT EQUIPMENT ............................................................................................ 39
4.4. MAINTENANCE ...................................................................................................................... 40
4.5. EMC(ELECTRO-MAGNETIC COMPATIBILITY) .......................................................................... 42
4.6. FCC(FEDERAL COMMUNICATIONS COMMISSION) ................................................................. 45
4.7. MARKING PLATE .................................................................................................................... 46
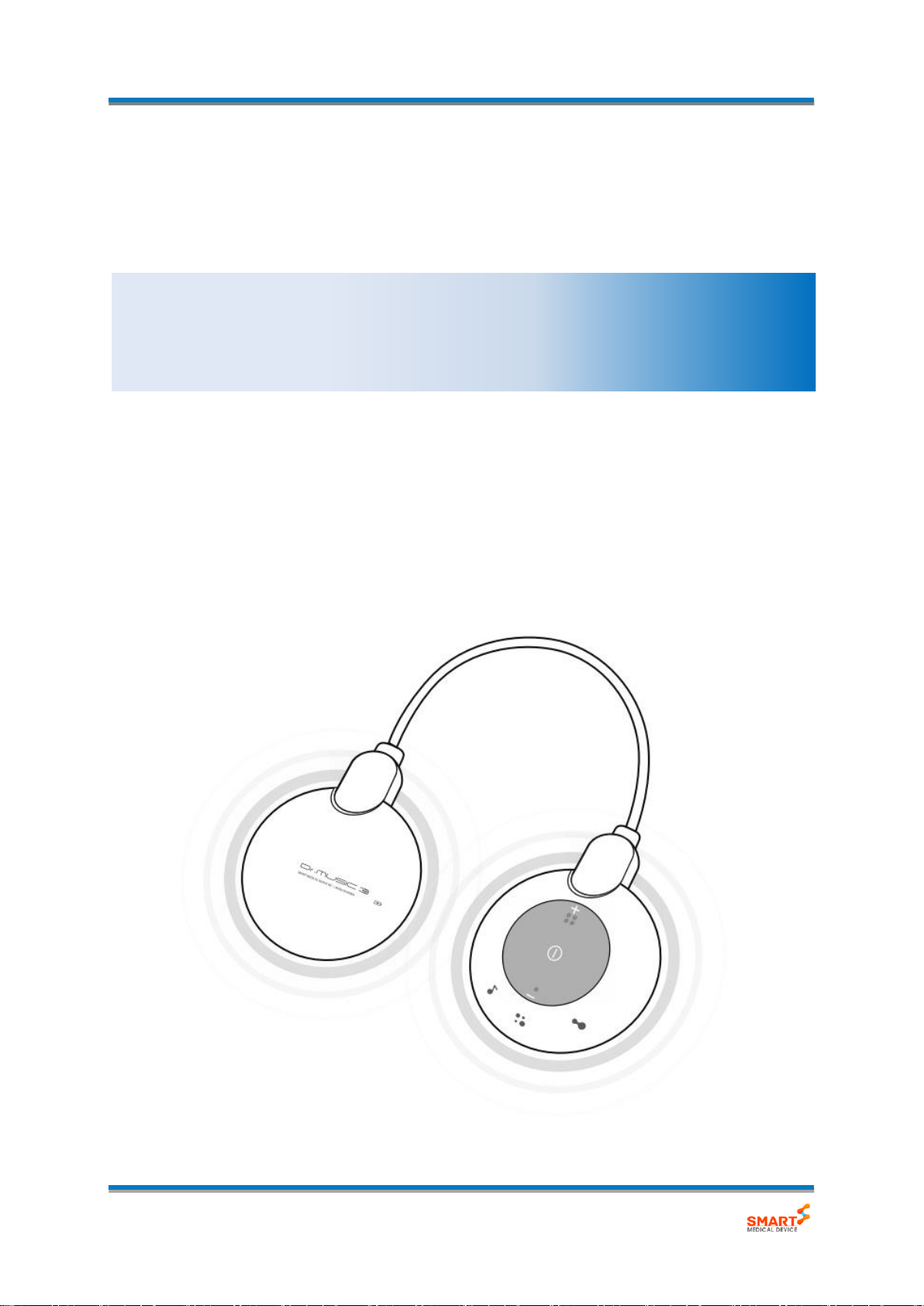
Dr.MUSIC 3s (Model: DM-VME03S)
SMD-IFUE-DMV-001 Rev 0 6
1.1 Contraindications
1.2 General safety
Chapter 1.
Safety Information

Dr.MUSIC 3s (Model: DM-VME03S)
SMD-IFUE-DMV-001 Rev 0 7
1. Safety Information
1.1. Contraindications
Never use this product in combination with following medical electronic device:
• Active implantable medical devices such as pacemakers.
• Electronic life support system such as an artificial heart/lung.
• Portable electronic medical device such as an electrocardiograph.
Never use person that has problem as below:
• This device is not designed for use on infant or children.
• You are pregnant woman or people with the possibility of pregnancy.
• You have cardiac disorder and cardiac lesion.
• You are physically and mentally debilitating.
• You have a problem for a blood pressure.
• People with high body temperature.
• A person who uses high-frequency medical devices.
• A person who visit the hospital regularly due to venous thrombosis.
• A person with much of menstrual bleed volume.
• If after inserting the (IUD, for example; coils, rings, etc.) within one month
contraceptive in the uterus.
• Do not use the site on scar wounds, burns, infected, acne, problems related to
blood clots, other vascular (for example; venous varices), or in limited parts of the
body.
• People deemed inappropriate by a doctor.

Dr.MUSIC 3s (Model: DM-VME03S)
SMD-IFUE-DMV-001 Rev 0 8
Patient with problem as below uses carefully and contacts a doctor:
People with acute disease.
People with malignant tumors.
People with a high fever.
People with problem on the skin of application site.
People who recently operated on.
People who are administered the insulin to treat diabetes.
People who have symptom side effects such as hypersensitivity, Inflammation, skin
disorders, etc.
People with problems in the muscles or joints.
Within six weeks after the labour.
People within three months after a caesarean section.
People who want to use this device as a part of rehabilitation.
People who received medical treatment by doctor.
People with serious illnesses what damage has not been mentioned in this document.
1.2. General safety
Do not modify this product without authorization of SmartMedicalDeivce Co.,Ltd.
Pregnant women need to consult their healthcare provider before use.
Use the product only for its intended use as described in this manual.
Keep the user’s manual with the equipment at all times.
Individuals with any kind of contagious disease or injury must not use or contact with this
product.
Always operate this product within prescribed ranges of temperature, humidity and pressure.
Operating in other environments may affect the operation of this product, and may cause

Dr.MUSIC 3s (Model: DM-VME03S)
SMD-IFUE-DMV-001 Rev 0 9
malfunction.
Be careful not to spill or drop beverages or any other liquid on this product. It may cause
serious damage to the electronic components.
Stop using the device and consult your doctor if you experience adverse reactions from the
device.
Do not use concurrently with other stimulator.
Strong magnetic line of force or electromagnetic radiation can cause failure or malfunction.
Keep hands away from where children and pets.
It may interfere with the normal operation of these devices when used in the presence of
electronic monitoring devices (heart monitor, ECG alarms, etc.).
Patients that are connected to the high frequency surgery equipment is prohibited to use.
Skin may cause burns and the device may damage the equipment.
When used within 1m from a short-wave or microwave medical equipment, Output of the
device may become unstable.
Portable RF communications equipment (including peripherals such as antenna cables and
external antennas) should be used no closer than 30 cm(12 inches) to any part of the device
including cables specified by the manufacturer. Otherwise, degradation of the performance
of this equipment could result.
When used the device in environments such as bathrooms, showers and swimming pools, it
may cause a risk of electric shock and burns.
Dr.MUSIC 3s (Model: DM-VME03S)
SMD-IFUE-DMV-001 Rev 0 10
2.1 Dr.MUSIC 3s 소개
2.1 Operating principle
2.2 Intended use
2.3 Product Components
2.4 Exterior and Function
2.5 Layout of Dr.MUSIC 3s APP
Chapter 2.
Dr.MUSIC 3s Introduc tions

Dr.MUSIC 3s (Model: DM-VME03S)
SMD-IFUE-DMV-001 Rev 0 11
2. Dr.MUSIC 3s Introduction
2.1. Operating principle
Transcutaneous electrical nerve stimulation (TENS) is a non-invasive pain relief method
for which clinical proof exists that it can help relieve pain. TENS treatment passes
electrical pulses across the intact surface of the skin to activate the underlying nerves.
The device uses a rechargeable battery to generate pulses. These pulses are applied to
the skin through self-adhesive hydrogel electrodes. You can choose the stimulation
programs with different pulse settings using Mobile App, or Device itself.
2.2. Intended use
DM-VME03S is intended to be used by adults for temporary relief of pain associated with
sore/aching muscles in the shoulder, waist, back, neck, upper extremities(arm) and lower
extremities(leg) due to strain from exercise or normal household work activities and
suitable for home use.
2.2.1. Intended user profile
Considerations
Requirement Description
Education
• Understand the instruction for the product
Knowledge
Understand Symbols on User’s manual and App
Read and understand User’s manual.
Language
• The display language depends on the language settings menu of
the Mobile phone.
Experience
• Able to use based android or iOS software.
• Who read through the user’s manual how to use it.
Permissible
impairments
• Mild visual impairment.
• Average degree of aging-related short term memory impairment.
This progress is required to accompany a guardian for the other users
Dr.MUSIC 3s (Model: DM-VME03S)
SMD-IFUE-DMV-001 Rev 0 12
2.2.2. Intended patient population
Considerations
Requirement Description
Age
Adult
Gender
male and female
Health
Do not use patient that operated active implantable medical
devices (e.g., Implanted pace-maker).
Do not use on infants or patient who cannot properly expresses.
Refer to Chapter 1.1 Contraindications in this user’s manual.
Nationality
Multiple
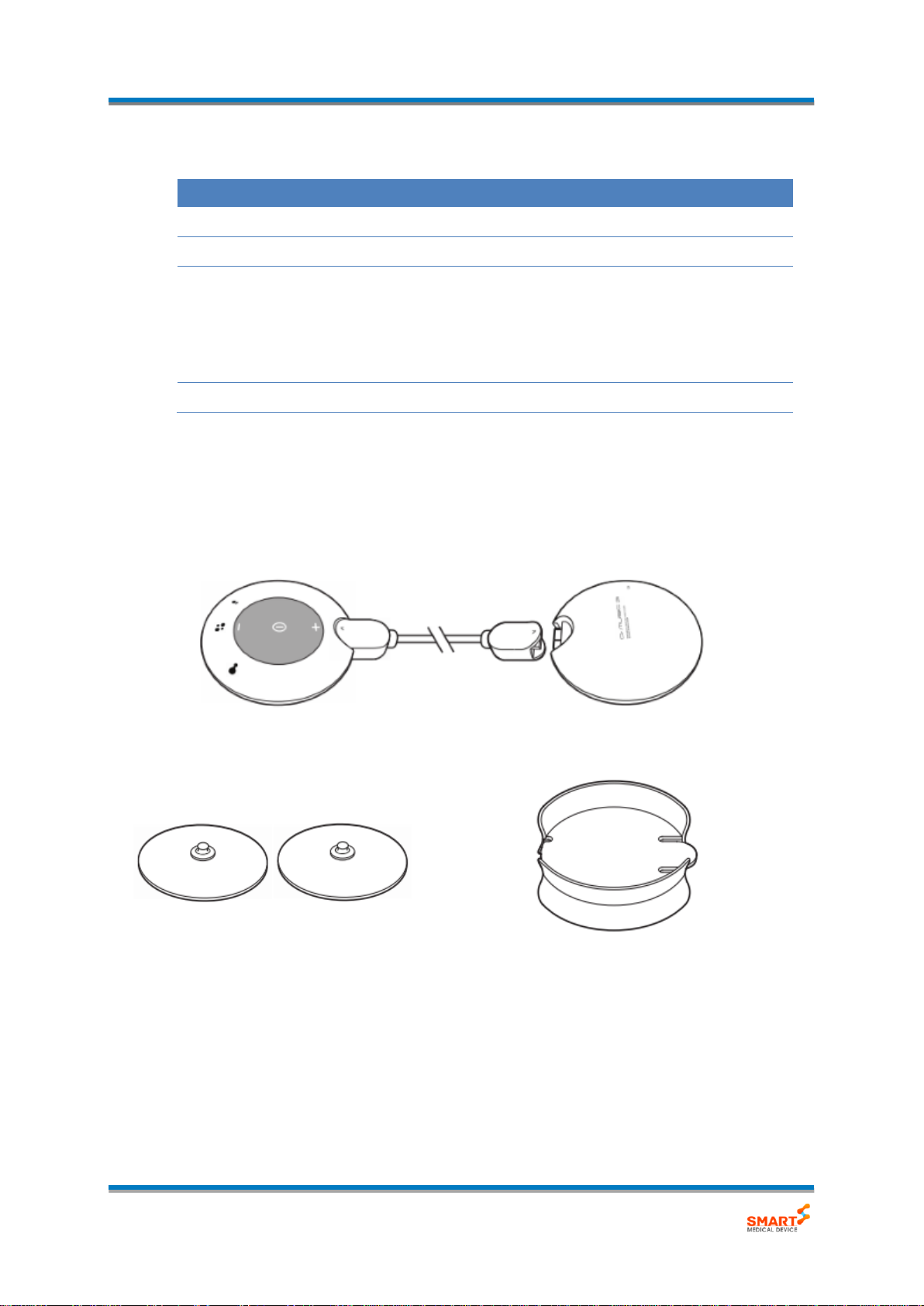
2.3. Product Components
The Dr.MUSIC 3s consists of the following components.
Main Unit
Control part
Battery part
Electrode
Holder
Please inspect defects of each component prior to installation.
Dr.MUSIC 3s (Model: DM-VME03S)
SMD-IFUE-DMV-001 Rev 0 13
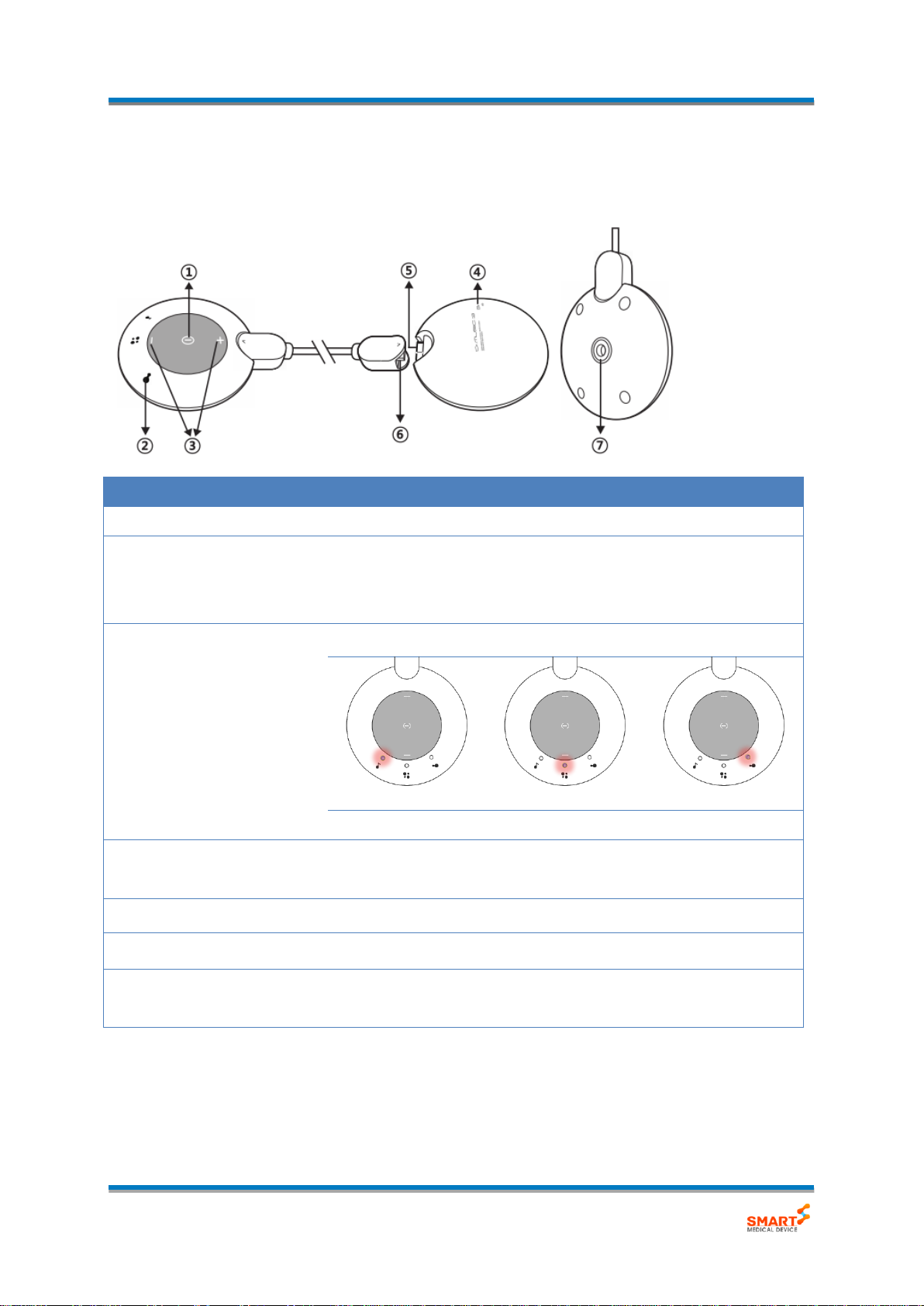
2.4. Exterior and Function
- Main Unit
No.
Name
Description
①
Power/Mode Button
Power ON/OFF, Change of Operating Mode.
②
Mode/Status indicator
Display mode and level, Battery status.
- Mode/Level/Output : Orange LED
- Battery: Blue LED
③
Intensity Button
Control of the level.
0 to 11 grades
12 to 23 grades
24 to 35 grades
④
Battery Indicator
Indicated the Status of the battery charging.
(Charging: Orange LED, Fully Charged: Green LED)
⑤
Micro-USB socket
Socket for the battery charging.
⑥
Detachable Connector
Connector for the cable of Micro USB adaptor.
⑦
Electrode connector
Connected output socket with electrode for Low frequency
stimulus.
Dr.MUSIC 3s (Model: DM-VME03S)
SMD-IFUE-DMV-001 Rev 0 14
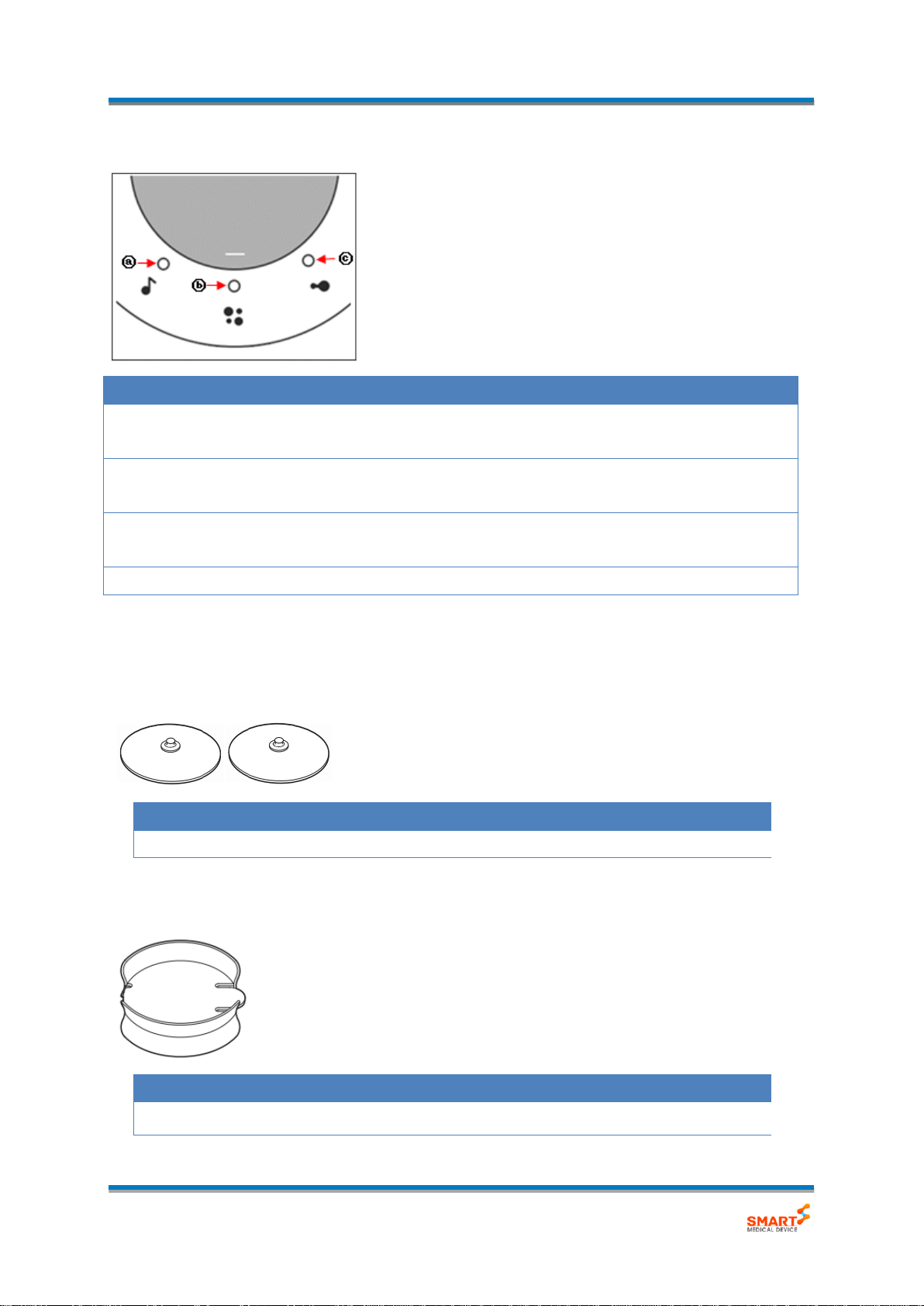
- Mode/Status Display LED
Name
Description
Mode indicator
Indicated each Status.
(ⓐ Music sync, ⓑ Tapping, ⓒ Massaging)
Intensity indicator
When adjusting the higher intensity, the LED is lighting from
left to right gradually.
output indicator
Each of LED is flashing during output.
(ⓐ Music sync, ⓑ Tapping, ⓒ Massaging)
Low battery indicator
When battery is low, ⓑ LED is flashing.
- Component
(1) Electrodes
Description
Electrodes are delivered low frequency pulse to body by attaching on the skin.
(2) Holder
Description
It is used to store the electrodes by attaching.
 Loading...
Loading...