SK Medical SK-600i Operator's Manual

SK-600Ⅰ
ⅠⅠ
Ⅰ
(This Operator’s Manual is also applicable for SK-600ⅠⅠⅠⅠB
infusion Pump)
Infusion Pump
Operator’s Manual


I
© 2011-2012 Shenzhen Shenke Medical Instrument Technical Development Co., Ltd.
All rights reserved.
For this Operator’s Manual, the issue date is April 2012.

II
Intellectual Property Statement
SHENZHEN SHENKE MEDICAL INSTRUMENT TECHNICAL DEVELOPMENT CO.,
LTD (hereinafter called SK Medical) owns the intellectual property rights to this SK
product and this manual. This manual may refer to information protected by copyright
or patents and does not convey any license under the patent rights or copyright of SK
Medical, or of others.
SK Medical intends to maintain the contents of this manual as confidential information.
Disclosure of the information in this manual in any manner whatsoever without the
written permission of SK Medical is strictly forbidden.
Release, amendment, reproduction, distribution, rental, adaptation, translation or any
other derivative work of this manual in any manner whatsoever without the written
permission of SK Medical is strictly forbidden.
, and are the trademarks, registered or
otherwise, of SK Medical in China and other countries. All other trademarks that
appear in this manual are used only for informational or editorial purposes. They are
the property of their respective owners.
III
Responsibility on the Manufacturer
Party
SK Medical reserves the final interpretation for this Operator’s Manual.
SK Medical reserves the rights to modify the Operator’s Manual without further notice.
The modified parts will be present in the new edition of Operator’s Manual.
SK Medical is not responsible for any software and devices provided by non SK
Medical and distributors.
SK Medical is responsible for the product safety, reliability and performance on
condition that all the following conditions are satisfied:
Installation, expansion, readjustment, improvement and maintenance must be
operated by professionals authorized by SK Medical.
All maintenance involving replacement of spare parts and its accessories,
consumables should use the original sets or sets authorized by SK Medical.
Relevant electrical equipment meets national standards and requirements of this
Operator’s Manual.
Please operate the product as per the Operator’s Manual.
WARNING
The device must be operated by professional clinicians or under the
guidance of professional clinicians. The users must receive adequate
product training. No unauthorized or untrained personnel should carry out
any operation.

IV
Warranty
THIS WARRANTY IS EXCLUSIVE AND IS IN LIEU OF ALL OTHER WARRANTIES,
EXPRESSED OR IMPLIED, INCLUDING WARRANTIES OF MERCHANTABILITY
OR FITNESS FOR ANY PARTICULAR PURPOSE.
Exemptions
SK Medical's obligation or liability under this warranty does not include any
transportation or other charges or liability for direct, indirect or consequential
damages or delay resulting from the improper use or application of the product or the
use of parts or accessories not approved by SK Medical or repairs by people other
than SK Medical authorized personnel.
This warranty shall not extend to:
Malfunction or damage caused by improper use or man-made failure.
Malfunction or damage caused by unstable or out-of-range power input.
Malfunction or damage caused by force majeure such as fire and earthquake.
Malfunction or damage caused by improper operation or repair by unqualified
orunauthorized service people.
Malfunction of the instrument or part whose serial number is not legible enough.
Others not caused by instrument or part itself.

V
Company Contact
Manufacturer: Shenzhen Shenke Medical Instrument Technical
Development Co., Ltd
E-mail Address:
http://www.skmedica.com
Service Hotline: +86 400 628 8806
Tel: +86 755 82402696
Fax: +86 755 82438567
EC-Representative:
Shanghai International Holding Corp. GmbH
(Europe)
Address:
Eiffestrasse 80, 20537 Hamburg,,,,Germany
Tel:
+49-40-2513175
Fax:
+49-40-255726

VI
Preface
Manual Purpose
This Operator’s Manual describes the product’s application, function and operation in
details. Please read this Operator’s Manual carefully and understand the content
before use to ensure the proper usage and guarantee the safety of the patient and
the user.
This Operator’s Manual describes the product as per the most complete configuration.
Some content of this manual may not be applicable for the product on your hand.
Please contact us for any questions.
Please keep this Operator’s Manual beside the infusion pump in order to consult it
conveniently.
Intended Audience
This Operator’s Manual is only applicable for well-trained clinical people.
Illustrations
All illustrations in this Operator’s Manual are used for reference only. Its settings or
data may be not entirely consistent with the actual displayed info on the product.
Conventions
Italic text is used in this manual to quote the referenced chapters or sections.
The terms danger, warning, and caution are used throughout this manual to point
out hazards and to designate a degree or level of severity.

1
Contents
1 Safety .....................................................................................................................1-1
1.1 Safety Information ...............................................................................................1-1
1.1.1 Dangers ...................................................................................................1-1
1.1.2 Warnings .................................................................................................1-2
1.1.3 Cautions ..................................................................................................1-4
1.1.4 Notes .......................................................................................................1-5
1.2 Equipment Symbols ............................................................................................1-6
2 The Basics .............................................................................................................2-1
2.1 Product Introduction ............................................................................................2-1
2.1.1 Application Scope ....................................................................................2-1
2.1.2 Contraindications .....................................................................................2-1
2.1.3 Product Structure, Composition and Performance .................................2-1
2.2 Appearance .........................................................................................................2-3
2.2.1 Front Panel ..............................................................................................2-3
2.2.2 Back Panel ..............................................................................................2-5
2.3 Screen Displaying................................................................................................2-6
2.4 Battery .................................................................................................................2-6
2.4.1 Overview..................................................................................................2-6
2.4.2 Battery Guidelines ...................................................................................2-7
2.4.3 Battery Maintenance ...............................................................................2-8
2.4.3.1 Conditioning a Battery ............................................................. 2-8
2.4.3.2 Checking a Battery .................................................................. 2-9
2.4.4 Battery Recycling ....................................................................................2-9
3 Installation and Maintenance ..............................................................................3-1
3.1 Installation............................................................................................................3-1
3.1.1 Out of Box Audit(OOBA) .........................................................................3-1
3.1.2 Environmental Requirements ..................................................................3-2
3.1.3 Power Supply Requirements ...................................................................3-2
3.1.4 Fix Infusion Pump ....................................................................................3-3
3.1.5 Install Power Cord ...................................................................................3-4
3.1.6 Installation of Drop Sensor (Optional) .....................................................3-4
3.2 Maintenance ........................................................................................................3-6
3.2.1 Inspection ................................................................................................3-6
3.2.2 Cleaning ..................................................................................................3-6
3.2.3 Preventive Maintenance ..........................................................................3-8
3.2.4 Pollution-Free Treatment and Recycling .................................................3-8
4 Operation Guide ...................................................................................................4-1
4.1 Operation Flow Chart ..........................................................................................4-1

2
4.2 Operation Steps ...................................................................................................4-2
4.2.1 Start Infusion Pump .................................................................................4-2
4.2.2 Install the Infusion Tube ..........................................................................4-3
4.2.3 Setting Infusion Parameters ....................................................................4-4
4.2.4 Clearing Accumulated Volume ................................................................4-4
4.2.5 Starting Infusion .......................................................................................4-5
4.2.6 Infusion Over ...........................................................................................4-5
4.2.7 Shutdown .................................................................................................4-5
5 Function and Interface .........................................................................................5-1
5.1 System Function Setting .....................................................................................5-1
5.1.1 Setting Flow Rate ....................................................................................5-1
5.1.2 Setting Volume Limit ...............................................................................5-1
5.1.3 Setting Bed No. .......................................................................................5-2
5.2 Starting Bolus Function .......................................................................................5-3
5.3 Pressure calibration .............................................................................................5-3
5.4 Changing Brand of Infusion Tube ........................................................................5-4
5.4.1 Selection of infusion tube ........................................................................5-4
5.4.2 Calibrate the Accuracy ............................................................................5-5
5.4.3 Setting Occlusion Level ...........................................................................5-7
5.4.4 Setting Air Bubble Filter Level .................................................................5-7
5.5 Drop Rate Function (Optional) ............................................................................5-8
5.5.1 Open and Close Drop Rate Function ......................................................5-8
5.5.2 Setting Drop Rate for Infusion Tube ........................................................5-9
5.5.3 Convert the Drop Rate Unit .................................................................. 5-10
6 Alarms ....................................................................................................................6-1
6.1 Overview ..............................................................................................................6-1
6.2 Alarm Type ...........................................................................................................6-1
6.2.1 Audible Alarm ..........................................................................................6-1
6.2.2 Alarm Information ....................................................................................6-1
6.3 Alarm Countermeasures ......................................................................................6-2
A Product Specification ......................................................................................... A-1
A.1 Safety Specification ............................................................................................ A-1
A.2 Environmental Specification ............................................................................... A-1
A.3 Power Supply Specification ............................................................................... A-1
A.4 Hardware Specification ...................................................................................... A-2
A.5 Basic Parameters of Infusion Pump .................................................................. A-2
A.6 Pressures that trigger a occlusion alarm, maximum alarm delays, and permissible
maximum volumes per infusion ................................................................................ A-4
A.7 Infusion accuracy table ...................................................................................... A-5
B EMC GUIDANCE AND MANUFACTURER’S DECLARATION .......................... B-1

3
C Alarm information ............................................................................................... C-1
C.1 Alarm information ............................................................................................... C-1
C.2 Prompt Message ................................................................................................ C-4
D Symbols and Terminology ................................................................................. D-1
D.1 Units ................................................................................................................... D-1
D.2 Terminology ........................................................................................................ D-2

4
FOR YOUR NOTES
1-1
1
Safety
1.1 Safety Information
The safety statements presented in this chapter refer to the basic safety information
that the operator shall pay attention to and abide by. There are additional safety
statements in other chapters or sections, which may be the same as or similar to the
followings, or specific to the operations.
DANGER
Indicates an imminent hazard that, if not avoided, will result in death or
serious injury.
WARNING
Indicates a potential hazard or unsafe practice that, if not avoided, could
result in death or serious injury.
CAUTION
Indicates a potential hazard or unsafe practice that, if not avoided, could
result in minor personal injury or product/property damage.
NOTE
Provides application tips or other useful information to ensure that you get
the most from your product.
1.1.1 Dangers
There are no dangers that refer to the product in general. Specific “Danger”
statements may be given in the respective sections of this manual.

1-2
1.1.2 Warnings
WARNING
This infusion pump is used for clinic infusion, do not for intestinal or gastric
nutritional feeding solution. It can only be used by professional clinicians,
medical electrical experts, or well- trained nurses on specific occasions.
Infusion pump and its accessories must be inspected before use to
guarantee its normal and safe operation.
Avoid using this infusion pump in the flammable or explosive atmosphere
in case of fire outbreak or explosion.
Infusion alarms must be set based on the actual situation of the patients. Do
not rely too much on the audible alarm system in the infusion supervision.
Pay close attention to the actual clinical situation of the patient.
Keep observing the remained liquid volum in infusion bags (or infusion
bottles) and ckeck if there is any air bobble in infusion tubes during the
infusion. Do not rely on the alarm function of the infusion pump only.
The pressure detector may not work normally in high-pressure
environment, especially in hyperbaric oxygen therapy.
Making sure the blood vessel is well protected before infusion.
In the infusion tube, the occlusion caused by tube knot and filter
coagulation or intubations may lead to the rise of the inner pressure of the
infusion tube. At this moment, the effort to eliminate the occlusion may
cause too much liquid to be infused into the patient’s body with a large
dose. Proper measures should be taken to prevent this phenomenon. For
example, to clamp the infusion tube before occlusion elimination.
This infusion pump should be used 120 CM above or below the patient’s
heart.
Avoid using the infusion pump when there is any alarm.
When another set of infusion system or accessories is connected to the
infusion tube used in this infusion pump, the operation of this pump may
not meet its specifications.
Only standard components, connectors and disposable products can be
used with this pump. Subsidiary items are not allowed to be attached to the
pump and its accessories. Reconstruction of the pumps is not allowed.
The accuracy will not be maintained when the pump is used with the
non-standard infusion tube or the parameters of the infusion tube are not
set accurately. The maximum deviation may reach 40% or above.
1-3
Disposable accessories must be disposed after use in accordance with the
relavant regulations of the hospital.
This infusion pump belongs to Class II (type of electric shock protection),
the supplied Type I power cord PE earth terminal should not be used as
ground protection and functional earthing.
Do not open the case of the infusion pump, otherwise there might be
electric shock. The infusion pump must be maintained or updated by
maintenance staff trained and authorized by our company.
Packing materials must be disposed in accordance with the relavant local
statutes or the waste disposal regulations of the hospital. They must be
kept out of the reach of children.
The double thickness of the infusion tube should be between
0.8mm-1.2mm. Outer diameter should be between 3.5mm-4.5mm.
Otherwise, its accuracy can not be guaranteed, which may cause severe
injury to patients.
1-4
1.1.3 Cautions
CAUTION
Please use the accessories specified in this Operator’s Manual to
gurantee the safety of the patient.
Cables must be connected carefully to reduce the possibility of the
patient getting intertwined or choked.
Disposable accessories can only be used once. Repeated use may lead
to declined performance or cross-infection.
When installation of the infusion tube is Over, please observe whether
there is leakage before the infusion. If any leakage the machine should
be examined and processed immediately.
Adjust the fixing place of the infusion tube every 4 hours after infusion
begins to guarantee the acccuracy. Replace the infusion tube after the
infusion lasts for 24 hours.
This infusion pump or its accessories must be disposed in accordance
with local statutes or hospital regulations after its operating life. Please
contact the distributor that sells the product to you or the manufacturer
if there is any inquiry.
Electromagnetic field may influence the performance of the infusion
pump. Therefore, equipments or devices used near the infusion pump
must meet the EMC standard. Mobile phones, X ray or MRI equipments
are all potential interference sources because of their high-intensive
electromagnetic radiation.
Avoid the direct sunshine, high temperature or humidity.
Avoid exposing this infusion pump to high-pressure sterilization or
chemical materials.
Check the built-in battery before use to make sure the power is enough.
Recharge the battery if necessory.
Before the infusion pump is connected to the power supply, make sure
the voltage and frequency of the power supply meet the label of the
pump or the specific requirments in this Operator’s Manual.
Please install and carry the infusion pump appropriately to protect the
pump from drop, impact, strong oscillation or other damage caused by
machinery external force.
Use a piece of wet soft cloth with warm water to wipe the surface of the
infusion pump when there is any liquid on.

1-5
If the surface tention, proportion and viscosity of the infusion solution is
different from saline (for example, a kind of solusion mixed with surface
activiting agent), the infusion accuracy may be different from the
accuracy listed in the specifications table.
When the infusion rate is high (≥ 1000ml/h), high-quality silicone tubes
with 0.9mm transfusion needles must be used with the pump to keep the
infusion accuracy.
If the infusion pump fails to work as specified in the Operator’s Manual
due to any uncertainty, please stop infusion, and report the situation
(including infusion accessories used with the pump, infusion volume,
infusion rate, SN No., liquid type, etc.) to your supplier or our company.
The drop sensor is not applicable for light-proof medicine infusion.
Adopting light-proof IV sets on the pumps might cause failure operating
of drop sensor and sever damage to the patients.
1.1.4 Notes
NOTES
Please keep this Operator’s Manual along with the infusion pump for the
convinent and timely reference.
Please install the infusion pump to the place convinient for observation,
operation and maintenance.
This Operator’s Manual describes all the configuration and functions of
the infusion pump. The infusion pump you buy may not have some of
the configuration or functions.
Please do not insert devices which are not specified by our company to
the data interface.
The SN No. of this infusion pump has been set. Users are not allowed to
change it.
1-6
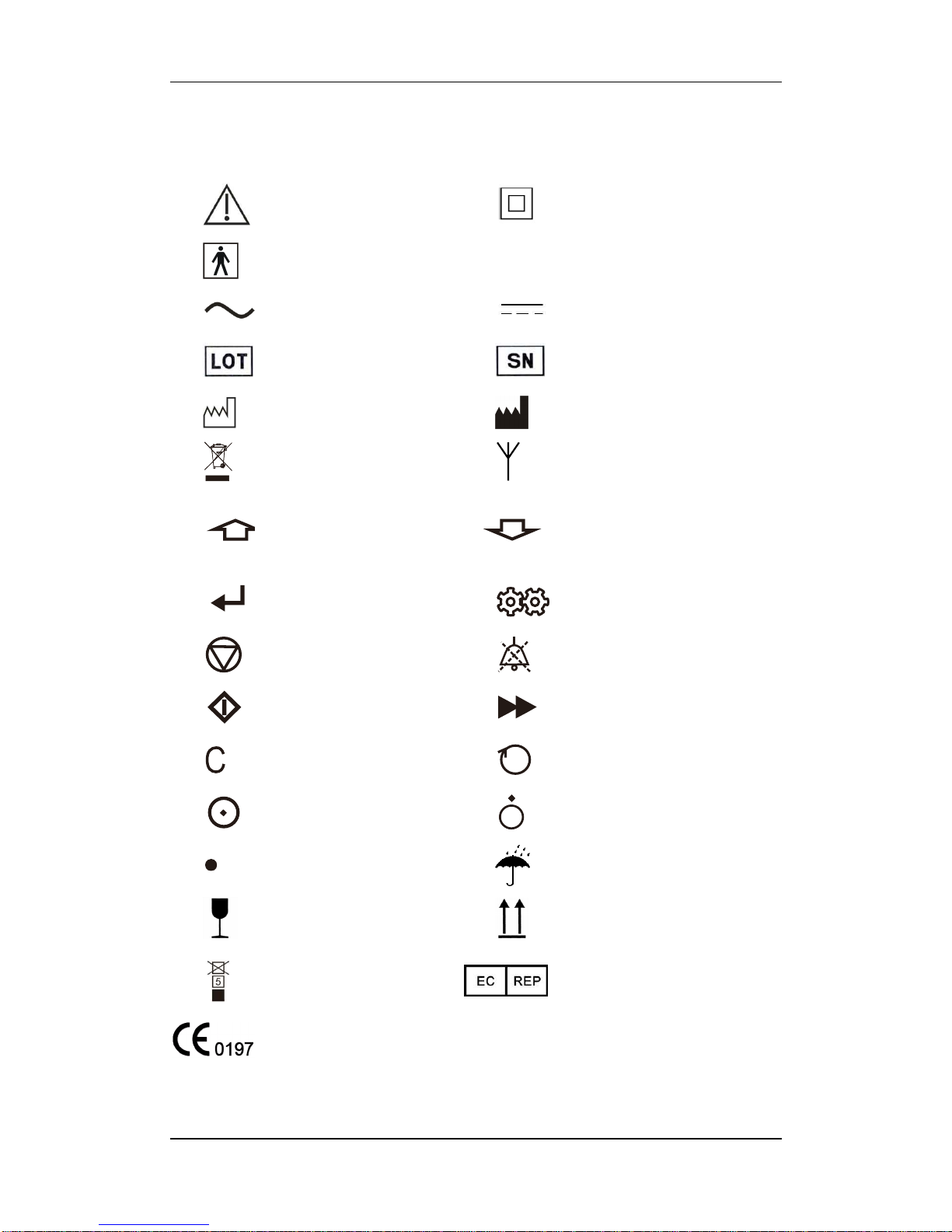
1.2 Equipment Symbols
Note! Please refer to the
Operator’s Manual
Class II Equipment
Type BF applied parts
IP21
Splash-proof
Alternating Current Power
Supply (AC)
Direct Current Power Supply
(DC)
Batch No.
Serial No.
Date of Production
Manufacturer
Pollution-Free Treatment
Wireless transceiver
Upward or Add Value
Downward or Reduce Value
Confirm
Setting
Stop
Alarm silence
Start
Bolus
Clear
Select
Turn on
Turn off
Decimal point
Transport package fear of rain
Fragile items, handle with
care
Transport should be straight up
The same packing stacked
up to 5-layers
Authorised Representative in
the European Community.
This product meets the EU Medical Device Directive 93/42/EEC and the
basic requirements in Directive Appendix I, hence the CE mark.
2-1
2
The Basics
2.1 Product Introduction
2.1.1 Application Scope
This infusion pump is used in wards, operation rooms, and observation rooms for
accurate and continuous infusion to patients. Do not for intestinal or gastric nutritional
feeding solution.
Any institutes or units, such as hospital outpatient, emergency rooms, wards,
operation rooms, observation rooms, clinics, nursing home, etc., capable enough to
provide health care, is expected to use this infusion pump.
WARNING
Check the infusion pump and its accessories before use to ensure its
normal and safe operation.
CAUTION
The operation environment and power supply of this infusion pump
must meet the requirments in A. Product Specification.
2.1.2 Contraindications
None
2.1.3 Product Structure, Composition and Performance
SK-600Ⅰ/ 600ⅠB Infusion Pump consists of the case, pump device, the board card
and battery, etc.
SK-600Ⅰ/ 600ⅠB Infusion Pump contains the following parts:
Microcomputer System: the core of the whole system, which gives
intellectualized control and management over the whole system and processes
detection signals. In this system, two single-chip Micyoco (SCM) systems are
adopted for mutual backup copy and supervision. When one SCM goes wrong,
the other one will give a timely warning signal and cut the power of the host
computer to stop the pump with the purpose to ensure the patient’s safety.

2-2
Pump Device: the power source of infusion,employs step motor to drive the
pump tablets continuously extruding upon infusion tube to materialize infusion.
Detection Device: the device mainly includes all kinds of sensors, like air bubble
sensor (detect air bubble inside the infusion tubes), pressure sensor (detect the
pressure inside the infusion tube), etc.
Alarm Device: the device mainly includes audible alarms and information
alarms, drawing the user’s attention to the correct operation.
Input and Display Device: the input device is in charge of setting infusion
parameters, such as flow rate, etc. While the display device is in charge of
displaying all the parameters and the current working status on the screen.
Built-in Battery: the battery sustains the operation of the infusion pump when
there is no AC power supply.
The performance of SK-600Ⅰ/ 600ⅠB Infusion Pump:
Accurate control of flow rate.
Accurate control of infusion volume.
Timely alarms for air bubble, over, occlusion, low battery, infusion tube
installation error, and control abnormal, etc.
2-3
2.2 Appearance
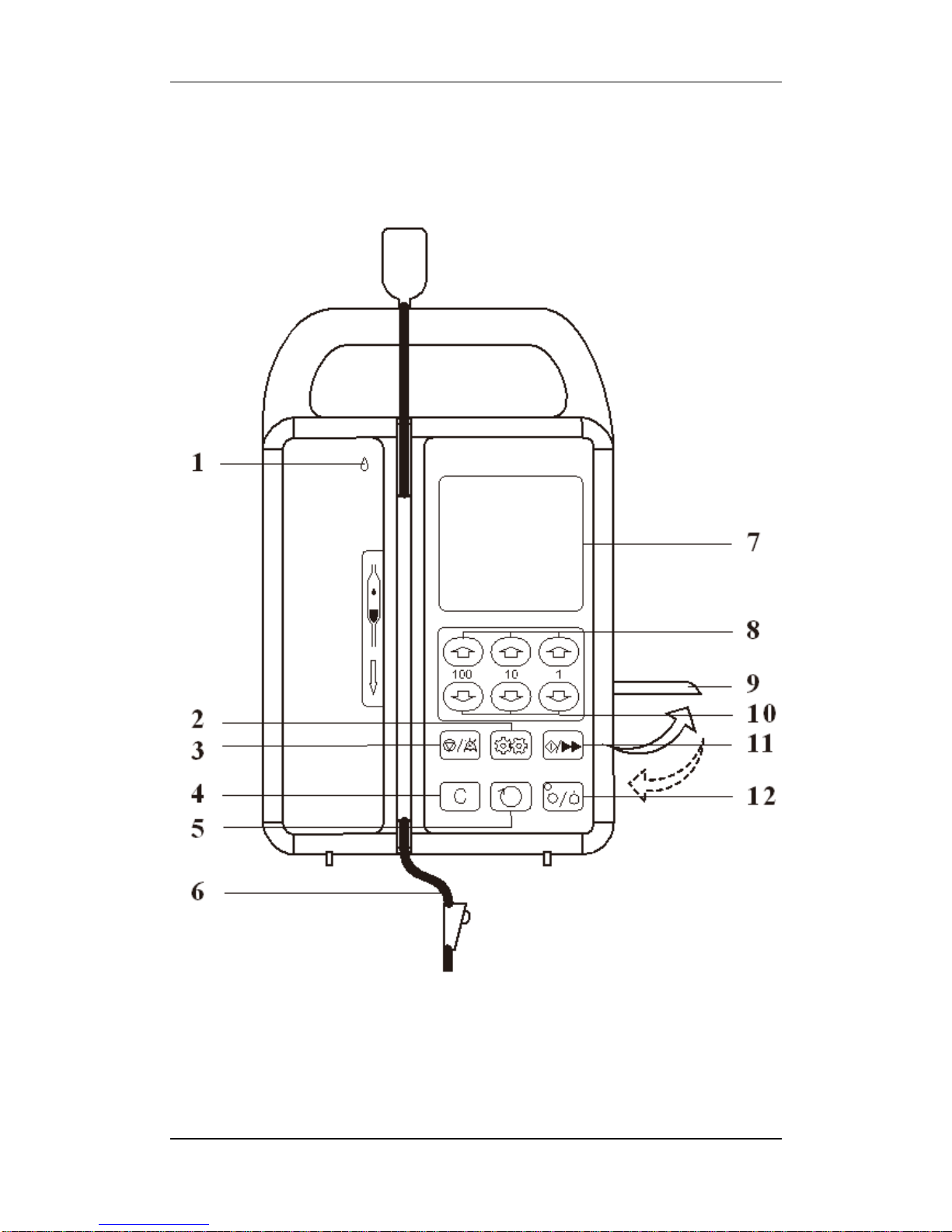
2.2.1 Front Panel
1.Running indicator light
The light is on and flashes during infusion when the infusion tube was properly
installed.

2-4
2.SET key
To set flow rate, volume limit, and Bed No.
3.STOP / SILENCE key
In running status, press this key to stop infusion. When the alarm is on,
press this key to silence the alarm (except low battery alarm).
In value inputting status, press this key to stop saving the value newly set
and quit.
4.CLEAR key
In Stop status, press this key to clear accumulated volume.
5.SELECT key
Select the needed parameter at setting interface.
6.nfusion tube
Please use infusion tube in accordance with standards.
7.Display screen
It displays working status and all parameters information.
8.Value-increase keys
Press the keys to increase values respectively by 100, 10, and 1.
9.HANDLE
Pull up the handle: to install or take off the infusion tube
Push down Handle: to tightly clamp the tube. The running indicator light is
on if infusion tube is well installed when the handle was pushed down.
10.Value-decrease keys
Press the keys to decrease values respectively by 100, 10, and 1.
11. START/ BOLUS
In stop status, if the infusion tube is correctly installed, press this key to start
infusion. During infusion, by keeping your finger on the key as long as you need
for bolus function, the pump shall start the bolus function after a few seconds
(when flow rate is ≤600ml/h). After removing your finger from the key, it will return
to its original infusion rate.
12. POWER key
Turn on the machine: Press the key and then release.
Turn off the machine: Press this key and then release.
Backlight: press it once to open or close display screen backlight
 Loading...
Loading...