Page 1
User Manual
FetalDoppler
Model:JPD-200S
Manual Ver.: 1.0
Thank you for purchasing the Fetal Doppler JPD-200S made by Jumper Medical. Before using the product,
read this manual carefully and operate the product as specified in this manual.
SECTION 1: INTRODUCTION
1.1 PACKING LIST
Main unit X 1;
Earphone X 1;
Audio cable for recording X 1;
User manual X 1.
1.2 PRODUCT DESCRIPTION
The product is a lightweight, portable detector. It is designed to meet your detecting and hearing needs by
providing advanced detecting functions and a full range of sound of the fetal heartbeat.
1.3 INTENDED USE
The product is mainly intended used to detect the sound of the fetal heartbeat (SFH).
The growth and development of a fetus can be found out through examination of these indices. It is
applicable for department of gynecology and obstetrics and clinic daily.
In accordance with classification criteria in Annex IX on “Medical Device Directive 93/42/EEC”, the product is
class IIa based on rule 10, “Devices for Direct Diagnosis or Detection on physiological process”.
The product is powered by an internal battery.
1.4 OPERATING PRINCIPLE
Fetal Doppler consists of probe (transmitter and receiver) and signal process unit.
Ultrasonic wave is transmitted from one piezoelectric ceramic at the front of the probe to the uterus of the
pregnant women. Echo is received by the other piezoelectric ceramic at the front of the probe when
ultrasonic wave reaches the fatal heart. Then it is converted into voltage. This Doppler signal is detected and
demodulated from the received signal. And the Doppler frequency is consistent with the rhythm of the fetal
systole and diastole. Once cardiac valves vibrate and a Doppler frequency excursion is formed. It is
transmitted an output signal of cardiac valves vibrating, and it is sent to the loudspeaker for getting a
rhythmical sound with the fetal heartbeat.
SECTION 2: SAFETY GUIDANCE
2.1 INDICATIONS FOR USE
The product is normally applied to fetus above 16 weeks growth, difference in pregnant mater.
● Listen to SFH: Operator can listen to the sound of fetal heartbeat from the loudspeaker or earphone.
● Audio record: The sound of fetal heartbeat can be recorded by a smartphone which is connected with
the product via audio cable.
2.2 CONTRAINDICATIONS FOR USE
Normally none, as a particular case, please consult your doctor.
2.3 NOTE FOR HOME USE
Please consult your doctor.
2.4 SAFETY TERMS AND CONDITIONS
The signal words shown below, left, identify the potential hazard categories. The definition of each category
is as follows:
DANGER: This alert identifies hazards that will cause serious personal injury or death.
Issuing Date: November,2016
WARNING: This alert identifies hazards that may cause serious personal injury or death.
CAUTION: This alert identifies hazards that may cause minor personal injury, product damage, or
property damage.
2.5 SAFETY ALERT DESCRIPTIONS
The following is a list of product safety alerts that appear in this section and throughout this manual. You
must read, understand, and pay heed to these safety alerts before attempting to operate the product.
DANGER: The product should not be used in life supporting or life sustaining applications.
DANGER: Fire and Explosion Hazard
Do not operate the Product in the presence of flammable gases to avoid possible explosion or fire
hazard.
WARNING:Strangulation resulting from baby or child entanglement in monitoring cables.
WARNING: Do not modify this equipment without authorization of the manufacturer.
WARNING:Dust, light may affect the safety and performance of the instrument.
WARNING:Degraded sensors and electrodes, or loosened electrodes, that can degrade performance
or cause other problems.
WARNING:The effects caused by pets, pests or children
WARNING: Use only Approved accessories
Do not use other accessories that are not approved by the manufacturer.suck as batteries, gel,
cables, or optional equipment.Otherwise it will cause the product function is not normal.
WARNING: Adjacent and/or Stacked Equipment
The Product should not be used adjacent to or stacked with other equipment. If adjacent or
stacked use is necessary, the Product should be observed to verify normal operation in the
configuration in which it will be used.
CAUTION:Temperature/Humidity/Pressure xtremes
Exposing the Product to extreme environmental conditions outside of its operating parameters may
compromise the ability of the Product to function properly.
CAUTION: Battery Disposal
Recycle or dispose of the battery in accordance with all federal, state and local laws. To avoid fire
and explosion hazard, do not burn or incinerate the battery.
CAUTION: Radio Frequency (RF) Electromagnetic interference
RF susceptibility from cellular phones, CB radios and FM 2-way radio may cause interference with
the product. Do not operate radiotelephones in the vicinity of the Product – turn off the
radiotelephone and other wireless equipment near the Product.
CAUTION: Systems Statement
Equipment connected to the product must be certified to the respective IEC Standards (i.e. IEC
60950 for data processing equipment and IEC 60601-1 for medical equipment).
CAUTION: Cleaning Solutions
When disinfecting the case, use a non-oxidizing disinfectant, such as ammonium salts or
glutaraldehyde based cleaning solution, to avoid damage to the metal connectors.
CAUTION: Environment of use
The product is designed for indoor use. Operator must confirm that the environment of use meets
the required operating environmental specifications before using.
CAUTION: Cold Environments
If the product is stored in an environment with a temperature below the operating temperature, the
product should be allowed to warm up to the needed operating temperature before using.
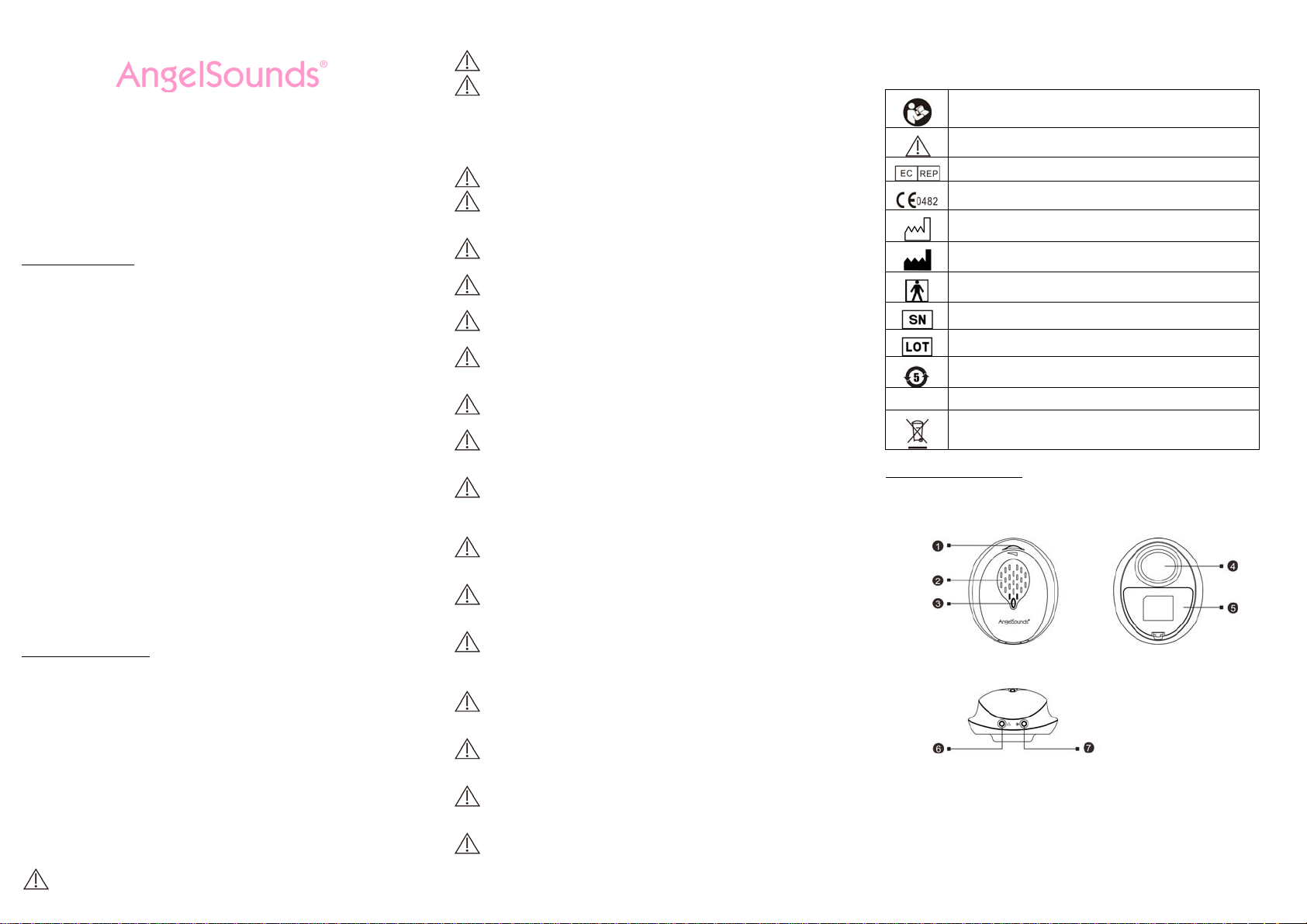
2.6 SYMBOL DESCRIPTIONS
The following symbols may appear in this manual, on the product, or on its accessories. Some of the
symbols represent standards and compliances associated with the product and its use.
Consult instructions for use of the product and/or its accessories.
Warning Information
Authorized Representative in the European Community
CE Mark: The Product system conforms to essential requirements of the
Medical Device Directive 93/42/EEC.
Date of manufacture.
Manufacturer information.
Type BF applied part
Specifies serial number of the Product
Batch code
The environmental protection use period is 5 years.
IP22
SECTION 3: USING THE PRODUCT
This section provides the description for operation.
3.1 PRODUCT STRUCTURE
1. Power on/off & Volume Knob
2. Loudspeaker
3. Working indicator light
4. Transducer
5. Battery compartment cover
6. Earphone socket
7. Recording socket
Degree of protection against ingress of water and particulate matter.
It indicates that the equipment should be sent to the special agencies according
to local regulation for separate collection after its useful life.
Page 2
A
A
A
A
g (
p
3.2 OPERATE KNOB AND INDICATOR LIGHT
3.2.1 POWER ON
When the product is not in use, turn the 'power on/off/volume knob (1)' to right for switching on the device.
Indicator light (3) is on.
3.2.2 POWER OFF
When in use, turn the 'power on/off/volume knob (1)' to the end of left for switching off the device. Indicator
light (3) is off.
3.2.3 VOLUME ADJUSTMENT
Turn the 'power on/off/volume knob (1)' to right; the sound volume will increase. Contrary, Turn the ' power
on/off/volume knob (1)' to left, the sound volume will decrease.
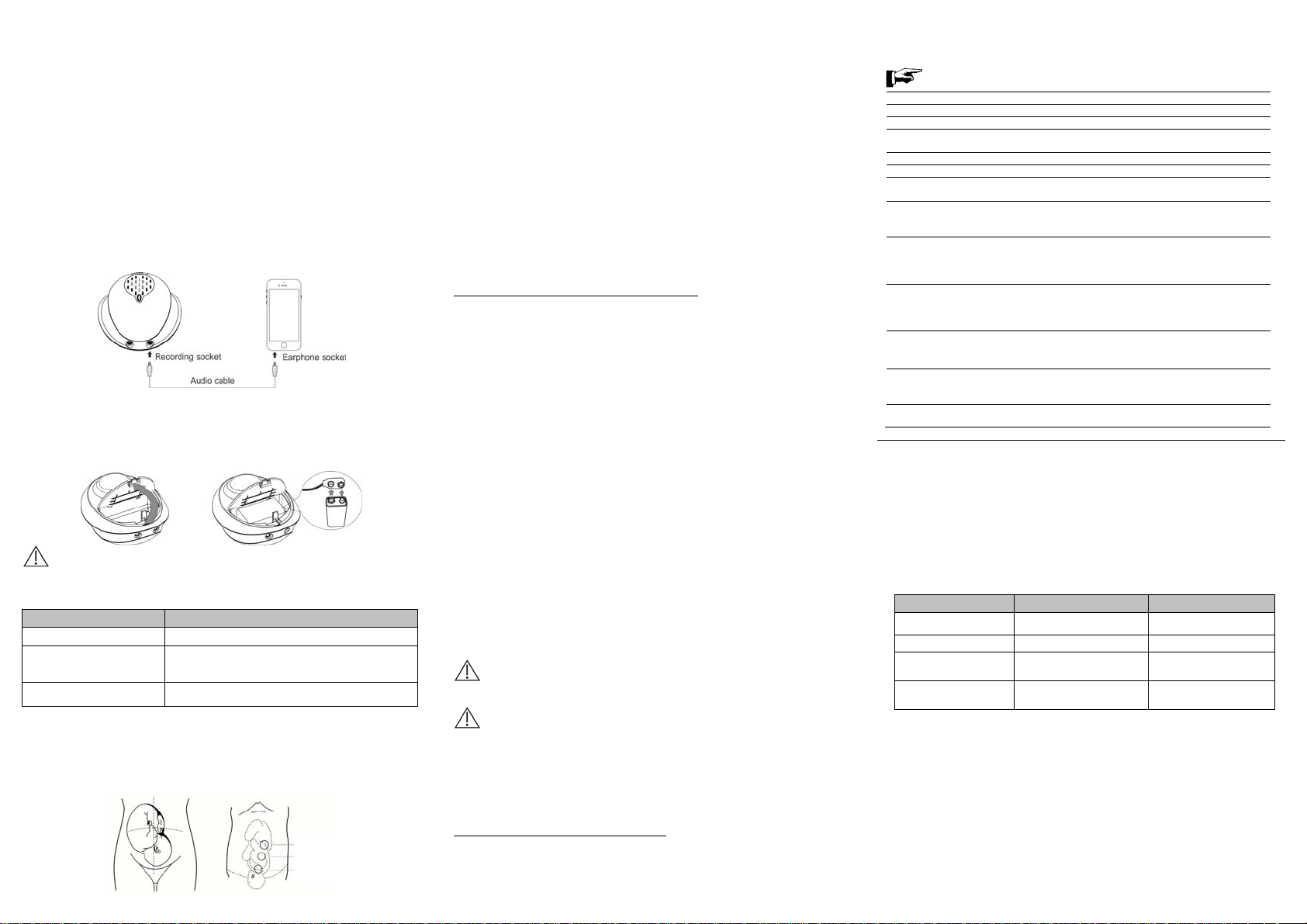
3.3 SOCKET INTRODUCTIONS
3.3.1 Earphone socket
The earphone socket(6) for audio output can be connected with a earphone.
3.3.2 Recording socket
The recording socket(7) for audio output can be connected with a smartphone via a audio cable .
Gestation
Antepartum
Parturition
3.4 INSTALLING THE BATTERY
1. Open the battery cover. The rear panel is upturned. First, open the cover of battery compartment.
2. Install the battery. Take out the battery connector. Then plug the battery to connector, after that put them
into the battery compartment.
3. Close the battery cover. First, along the left of battery compartment latch, put the cover at the right place.
Then close the cover .
CAUTION: Replace the battery if the device is not likely to be used for some time.It indicates that the
battery is low.
3.5 DESCRIPTIONS OF THE INDICATOR LIGHT STATES
Indicator color and state Device working state
Steady green The device is powered on
Flashing green light with the fetal
heart signal frequency
The device is in the working state, and the ultrasonic probe
captures a fetal heart signal.
Flashing orange evenly The device is in a low voltage state.
3.6 USING PRODUCT TO DETECT
Locate the position of the fetus by hand touching, firstly to find out the best direction to the fetal heart. Place
the faceplate of probe at the best position for detecting fetal heartbeat. Adjust the transducer to obtain an
optimum audio signal ideally by angling the transducer around. Generally, the site of heart of fetus is 1/3
below of navel line at its earlier stage, it then moves upward with increasing of gestational period, and the
site of heart of fetus will be a little deviation to left or right with different fetus. Pls. make sure that the surface
of the probe should be contacted fully with the skin. After the sound become clear, it is the proper functioning.
If no coupling gel, water can be used.
3.7 USE OF THE FETAL DOPPLER ALONE
1. Insert the earphone into the earphone socket of the device, and wear the earphone.
2. Turn on the device.
3. Coat the coupling agent on the ultrasonic probe, and evenly coat the coupling agent on the surface of
the probe.
4. Close the probe to the belly of a pregnant woman and move the probe slowly to search for the location
of the fetal heart until hearing the fetal heart sounds. When the fetus sounds are not clear or very low,
increase the volume until hearing clear fetal heart sounds.
5. Start to count the fetal heart beats per minute, that is, the fetal heart rate.
6. After finishing using the device, turn off the device.
3.8 CONNECTING THE DEVICE TO THE SMARTPHONE VIA AUDIO CABLE
Software Downloading:
1. Download and install the mobile phone APP software "AngelSounds" by scanning the QR code on the
packing box or searching for the APP in application stores such as APP Store/Google Market.
2. This software supports iOS 7.0 and later versions, and Android 4.3 and later versions. In addition,
hardware of the smartphone needs to support Bluetooth 4.0.
Audio cabel connection:
According to the figure on the left.
Software Usage:
Detailed see software operation manual.
SECTION 4: MAINTENANCE & CLEANING AND DISINFECTION
4.1 MAINTENANCE
4.1.1 The transducer acoustic surface is frangible and must be handle with care .Gel must be wiped off from
the transducer after use. These precautions will prolong the life of the unit.
4.1.2The user must check that the equipment does not have visible evidence of damage that may affect
patient safety or product’s capability before use .The recommended inspection interval is once per month or
less. If damage is evident, replacement is recommended before use.
4.1.3 To ensure the product is always functional when required, the following maintenance shall be
performed.
● Visual Inspection
● Cleaning the product and its accessories
● Check the battery fuel gauge
● Testing product performance
● The product requires no calibration.
● If the product is not been used more than three months, remove the battery .
4.2 CLEANING PRODUCT AND ACCESSORIES
The following cleaning products may be used to clean the exterior surfaces of the product as well as the
batteries.
Isopropyl alcohol (70% solution in water)
Mild soap and water
Sodium hypochlorite (chlorine bleach) (3% solution in water).
Quaternary ammonium compounds (such as Lysol) (10% solution in water).
WARNING:Do not use abrasive cleaners or strong solvents such as acetone or acetone-based cleaners.
WARNING:Do not use mixing disinfecting solutions (such as bleach and ammonia) as hazardous gases may
result.
WARNING:Do not clean electrical contacts or connectors with bleach.
4.3 CLEANING INSTRUCTIONS
1. Before cleaning the product, turn off the device.
2. Before cleaning, remove all adherent soil (tissue, fluids, etc.) and wipe thoroughly with a cloth
dampened with water before applying the cleaning solution.
3. When cleaning, do not immerse. Keep the exterior surface of the device clean and free of dust and dirt,
clean exterior surface of the unit with a dry, soft cloth .if necessary, clean it with a soft cloth soaked in a
solution of soap and wipe dry with a clean cloth immediately.Wipe the transducer body with soft cloth to
remove any remaining coupling gel .Clean with soap only.
CAUTION: To prevent damage to equipment, do not clean any part of the Product or Accessories
with phenolic compounds. Do not use abrasive or flammable cleaning agents. Do not steam, autoclave, or
gas-sterilize the Product or accessories.
CAUTION: Cleaning liquids: do not submerge the device in liquids or pour cleaning liquids over, into
or onto the device.
4.4 DISINFECTIONS
Cleaning the unit surface and the transducer as the above mentioned, then wipe the surface of transducer
with 75% ethanol or alcohol, clean the transducer surface with a dry, soft cloth.
WARNING: Don’t use low temperature steam sterilization or other way to sterilize.
WARNING: Don’t use high temperature sterilizing process.
SECTION 5: SPECIFICATIONS & TROUBLESHOOTING
This section presents the specifications and safety standards of the Product.
5.1 SPECIFICATIONS
NOTE: The following specifications are subject to change and are only noted as a point of
reference.
ULTRASOUND
Ultrasonic emitting frequency:
Ultrasonic emitting power:
Overall sensitivity at the distances 200mm from the face of
the transducer
Spatial-peak temporal-peak acoustic pressure:
Effective area of the ultrasonic transducer active element: 7.4cm2
The acoustic coupling medium for normal use:
UDIO OUTPUT
udio Output Power:
udio out socket:
BATTERY
Battery Voltage:
Type:
Typical operation time
SAFETY TYPE
Internally powered equipment
Waterproofing level:
Operating mode
DIMENSIONS
The acoustic output parameter meets the provision freedom from publication in IEC61157
Requirement for the declaration of the acoustic output of medical diagnostic ultrasonic equipment:
P_<1MPa; Iob<20mW/cm
Product service life: 5 years
Production date: See the label
ND WEIGHT
2
; Ispta<100mW/cm2
3MHz
<10mW
≥90dB
<0.1MPa
ph :5.5~8, Acoustic impedance:
6
1.5*10
~1.7*106Pa·s/m
<0.2 W
Φ3.5mm
9V
IEC6F22 9V alkaline
About 6 hours
Type BF applied part
IP22
Continuous operating
100mm×85mm×50mm
including battery)
Wt: 0.15k
The materials or ingredients contact with the patient or operator.:ABS
5.2 ENVIRONMENTAL REQUIREMENTS
OPERATING CONDITIONS
Temperature: 5C to 40C
Humidity: <80% RH, non-condensing
Atmospheric pressure: 86kPa to 106kPa
STORAGE AND SHIPPING CONDITIONS
Temperature: -20C to 55C
Humidity: 10% - 93% RH, non-condensing
Atmospheric pressure: 50kPa to 106kP
5.3 TROUBLESHOOTING
Symptom Possible cause Troubleshooting
Power-on failure Low battery level Replace new battery
No sound Low volume Increase the volume
Fetal heart cannot be found
Low sensitivity
●Low volume
●The coupling agent is not
coated
●Incorrect probe location
●The coupling agent is not
coated
●Increase the volume
●Coat the coupling agent or
water
●Adjust the probe location
●Coat a proper amount of
cou
ling agent
Page 3

SECTION 6: FCC/ISED CAUTION
This device complies with part 15 of the FCC Rules. Operation is subject to the following two conditions: (1)
This device may not cause harmful interference, and (2) this device must accept any interference received,
including interference that may cause undesired operation.
Note: This equipment has been tested and found to comply with the limits for a Class B digital device,
pursuant to part 15 of the FCC Rules. These limits are designed to provide reasonable protection against
harmful interference in a residential installation. This equipment generates uses and can radiate radio
frequency energy and, if not installed and used in accordance with the instructions, may cause harmful
interference to radio communications. However, there is no guarantee that interference will not occur in a
particular installation. If this equipment does cause harmful interference to radio or television reception,
which can be determined by turning the equipment off and on, the user is encouraged to try to correct the
interference by one or more of the following measures:
-Reorient or relocate the receiving antenna.
-Increase the separation between the equipment and receiver.
-Connect the equipment into an outlet on a circuit different from that to which the receiver is connected.
-Consult the dealer or an experienced radio/TV technician for help.
Any Changes or modifications not expressly approved by the party responsible for compliance could void the
user's authority to operate the equipment.
The device has been evaluated to meet general RF exposure requirement. The device can be used in portab
le exposure condition without restriction.
- English:
This device complies with Industry Canada licence-exempt RSS standard(s).
Operation is subject to the following two conditions: (1) This device may not cause interference, and (2) This
device must accept any interference, including interference that may cause undesired operation of the
device.
- French:
Le présent appareil est conforme aux CNR d'Industrie Canada applicables aux appareils radio exempts de
licence. L'exploitation est autorisée aux deux conditions suivantes:
(1) l'appareil ne doit pas produire de brouillage, et
(2) l'utilisateur de l'appareil doit accepter tout brouillage radioélectrique subi, même si le brouillage est
susceptible d'en compromettre le fonctionnement.
SECTION 6: CONTACT INFORMATION
Authorized European Representative:
Wellkang Ltd
Suite B, 29Harley Street, London W1G 9QR,UK
Shenzhen Jumper Medical Equipment Co., Ltd
Address: D Building, No. 71, Xintian Road, Fuyong Street, Baoan,Shenzhen,
Guangdong,China
Tel: +86-755-26696279 / 26692192
Fax: +86-755-26852025
Website: www.jumper-medical.com
E-mail: info@jumper-medical.com
 Loading...
Loading...