Sanyo STERISONIC GXP User Manual

Sterisonic™ GxP
Series Cell Culture
Incubators
CO2 Incubators
MCO-19AIC(UVH)
MCO-19AIC(UV)
MCO-19AIC
CO
/ O2 Incubators
2
MCO-19M(UVH)
MCO-19M(UV)
MCO-19M
Features:
• The industry’s most complete cell culture
solution for highly regulated applications or
conventional incubation.
• Safe, effective and documented two-hour in situ
sterilization for fastest decontamination.
H
2O2
www.sanyobiomedical.com
My Life. My Work. My Choice.
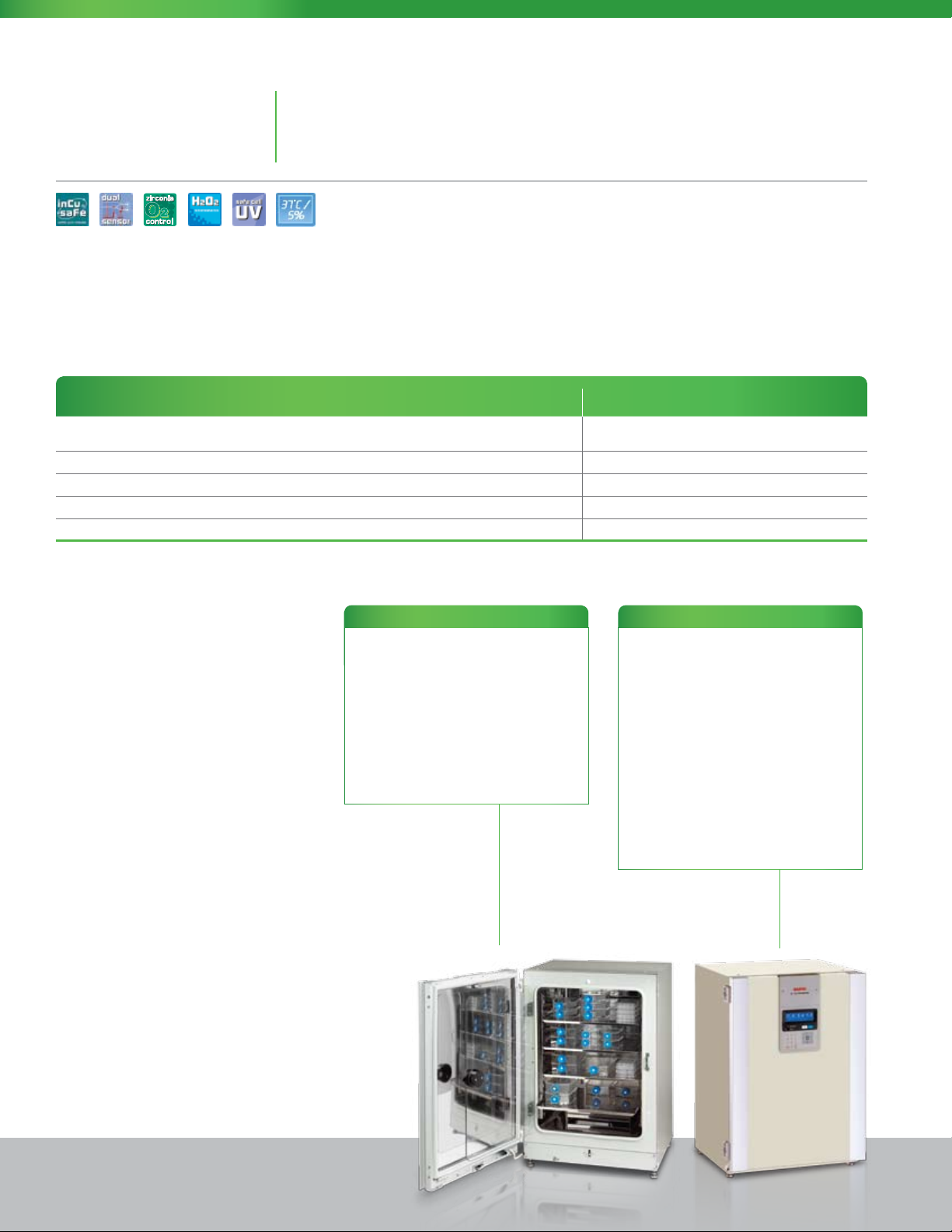
MCO-19AIC
MCO-19M
MCO-19AIC(UV)
MCO-19AIC(UVH)
MCO-19M(UV)
MCO-19M(UVH)
Sterisonic™GxP Series Cell Culture CO2 and CO2 / O2 Incubators
The industry’s most complete cell culture solution for highly regulated applications or conventional
incubation. Now with safe, effective and documented two-hour in situ H
decontamination turn-around and maximum availability.
Sterisonic™ GxP Selection
Feature
Vapor Sterilization Standard Optional Optional Standard Optional Optional
H
2O2
™
SafeCell
UV Decontamination Standard Standard Optional Standard Standard Optional
Single Beam, Dual Detector IR CO
Oxygen Concentration Control, Zirconia Control N/A N/A N/A Standard Standard Standard
Sensor Standard Standard Standard Standard Standard Standard
2
MCO-
19AIC(UVH)
CO2 Incubators CO2/O2 Incubators
MCO-
19AIC(UV)
MCO19AIC
sterilization for fastest
2O2
MCO-
19M(UVH)
MCO-
19M(UV)
MCO-
19M
Product Applications
The combination of Sterisonic™ GxP
incubator performance functions permit
use with condence in high-value cell
protocols among hard-to-grow cell lines,
cells highly sensitive to contamination,
ultra-sensitive media and reagents, or
protocols that require a strict isolation
and sterilization between processes.
These include but are not limited to:
Stem cell research•
Autologous tissue regeneration •
and regenerative medicine
• In vitro fertilization
1
Genomic and proteomic expression•
Esoteric plant and amphibian •
cell culture
Hypersensitive and transgenic •
cell culture
Low media volume microplate work•
1
501(k) clearance applied for. Contact SANYO for status. MCO-19AIC
and MCO-20AIC CO2 incubators have received U.S. Food and Drug
Administration 510(k) clearance for in vitro fertilization applications in
accordance with the FDA Safe Medical Devices Act of 1990 and the Medical
Device Amendments of 1992. Reference: Number K013703. Regulation
Number: 21 CFR 884-6120, Assisted Reproduction Accessories, Regulatory
Class II, Product Code 85MOG, October 30, 2001
Designed for use with a variety
of standard cell culture vessels
and protocols.
Interior components and adjustable •
shelves are congured for easy access,
in situ sterilization and exible arrangement for a variety of applications.
Four adjustable shelves are included, •
standard; maximum shelf capacity
is 15 shelves.
MCO-19AIC(UVH)
MC O-19M
Multi-Gas Incubator, Oxygen
and CO2 Control
Ideal for • in vitro fertilization (IVF), genetic
research, regenerative medicine and
other protocols that require CO
sub-ambient (hypoxic) or above-ambient
oxygen control.
Cabinet based on MCO-19 platform •
with inCu safe
stainless steel chamber, continuous
contamination control, patented D.H.A.
multi-point, air-jacketed temperature
control system, elevated relative humidity
with integral water-level sensor.
™
copper-enriched
and
2
2 Sterisonic™ GxP Series Cell Culture Incubators

Sterisonc™ GxP Features and Benefits
The SANYO Sterisonic™ GxP is designed for a wide array of demanding and highly regulated applications in the biomedical, pharmaceutical,
medical research and clinical laboratory. Representing years of research, development and component testing, the Sterisonic™ GxP incorporates a
collective of mutually functional systems and design attributes to offer a holistic solution to cell culture protocols, from the most sophisticated to
more familiar and conventional processes.
Sterilization and Decontamination
The unique Sterisonic•
sterilization system limits downtime
to less than three hours when total
chamber sterilization with verication
is desired.
All interior components and CO•
sampling loop are sterilized in situ;
no need for removal and autoclaving.
Active Background Contamination •
™
Control
ghts contamination while
cell culture protocols are in process.
The patented SafeCell
system scrubs interior airow
to destroy airborne and humidity pan
contaminants.
Exclusive inCu saFe
enriched stainless steel interior
surfaces assure constant germicidal
protection.
™
GxP H2O2
2
™
UV
™
copper-
Shelf brackets are formed
with an exaggerated angle
to minimize surface contact
with flat shelves*.
Control and Monitoring
The Sterisonic•
information center includes an intuitive
pop-up menu, high resolution LCD
for inputs, outputs and performance
at-a-glance.
Multi-point data logging offers •
push-button graphical display. An
optional PC interface permits remote
transmission for GMP/GLP protocols
as required.
Precise PID logic controls and •
adjusts to all temperature, CO
points and alarm parameters.
™
GxP control and
set-
2
CO2 Control
SANYO proprietary single-beam,
dual detector infrared (IR2) CO
sensor delivers precise CO
control,
2
quick recovery following door openings,
and auto sampling with no moving parts.
Continuous zero calibration is standard.•
An optional semi-automatic, one-point •
calibration system is available. Catalog
No. MCO-SG; see Accessories.
2
Zirconia O2 Control System,
MCO-19M Series
A zirconia oxygen sensor main-
tains sub-ambient O
1% to 18%. Additionally, enriched O
levels from 22% to 80% are enabled
with proper safety precautions.
Concurrently the MCO-19M permits •
a CO
range 0% to 20% via infrared
2
sensor.
Nitrogen gas bubbler accelerates •
recovery of chamber humidity levels
following door openings.
An electronic P.I.D. control maintains •
accurate temperature and gas setpoints over the entire system range.
The MCO-19M includes an automatic •
gas switchover system that changes
from the primary to a secondary gas
cylinder for either oxygen or nitrogen;
an optional second gas switchover
system is available for CO
Cabinet based on MCO-19 platform •
with inCu saFe
™
copper-enriched
stainless steel chamber, continuous
contamination control, patented D.H.A.
multi-point, air-jacketed temperature
control system, elevated relative humidity with integral water-level sensor.
levels from
2
.
2
2
MC O-19M
MCO-19M shown with
four separate inner
doors with gaskets.
3
Sterisonc™ GxP Features and Benefits
Temperature
and Humidity Control
The patented Direct Heat and Air•
™
conditioning system manages setpoint
temperature through multiple, variable
warming points under microprocessor
control.
The humidity pan is easy to ll, easy •
to clean; the automatic optical sensor
advises of low water level.
H2O2 Sterilization
The use of H2O2 sterilization in
biological safety cabinets and barrier isolators is a popular alternative to ethylene
oxide (EtO) as a safer, more efcient
decontamination method. H
been widely used in the pharmaceutical
industry. In aerospace research, H
used to sterilize satellites.
The FDA has recently granted 510(k)
clearance to use H
in individual medi-
2O2
cal device manufacturing applications.
EtO criteria outlined in ANSI/AAMI/ISO
has long
2O2
2O2
is
14937 may be used as a validation guideline. For references online visit
www.sanyobiomedical.com/sterisonic.
Unlike conventional incubators, unique
features of the SANYO Sterisonic
GxP incubator permit use of the H
process in situ with complete safety,
zero impact on adjacent equipment or
the environment, and speed to return
the incubator to service.
The • H
tions with the patented SANYO
SafeCell
sterilization process func-
2O2
™
UV system. Following a
seven-minute H
circulation and dwell cycle, vaporization
is stopped and the SafeCell
turned ON for up to ninety minutes.
When exposed to UV light, the • H
vapor breaks down into water and
oxygen, leaving only traces of water
droplets. These droplets automatically condense onto a naturally cooler
section of the interior oor for easy
wipe-up.
Throughout the entire cycle the •
Sterisonic
™
GxP airow system continues to gently circulate interior air
assuring 100% vapor contact with all
interior surfaces, ultimately creating
a serial dilution of H
over the UV lamp.
vaporization,
2O2
as it passes
2O2
™
2O2
™
UV lamp
2O2
Orientation of interior sample ports •
of the single beam, dual detector IR
CO
sensor creates a slight Venturi
2
ow through the sample chamber,
permitting total sterilization of the
CO
system at the same time.
2
Shape and location of interior compo-•
nents such as shelves, shelf brackets,
plenum covers and the humidity pan
permit the components to remain
in the chamber during the sterilization
process, conveniently bypassing the
need for a separate autoclave cycle.
Once the cycle is complete, the door •
locking system is released; the inner
door can be opened, interior components repositioned and the incubator
is returned to service.
Ergonomic Cabinet Design
With reversible inner and outer doors,
a single SANYO incubator offers the
industry's most exible installation
options available.
Low prole cabinet with door-mounted •
control panel permits easy access and
viewing.
The outer door latch and door heater •
cable is easily switched if a reverse
opening is required.
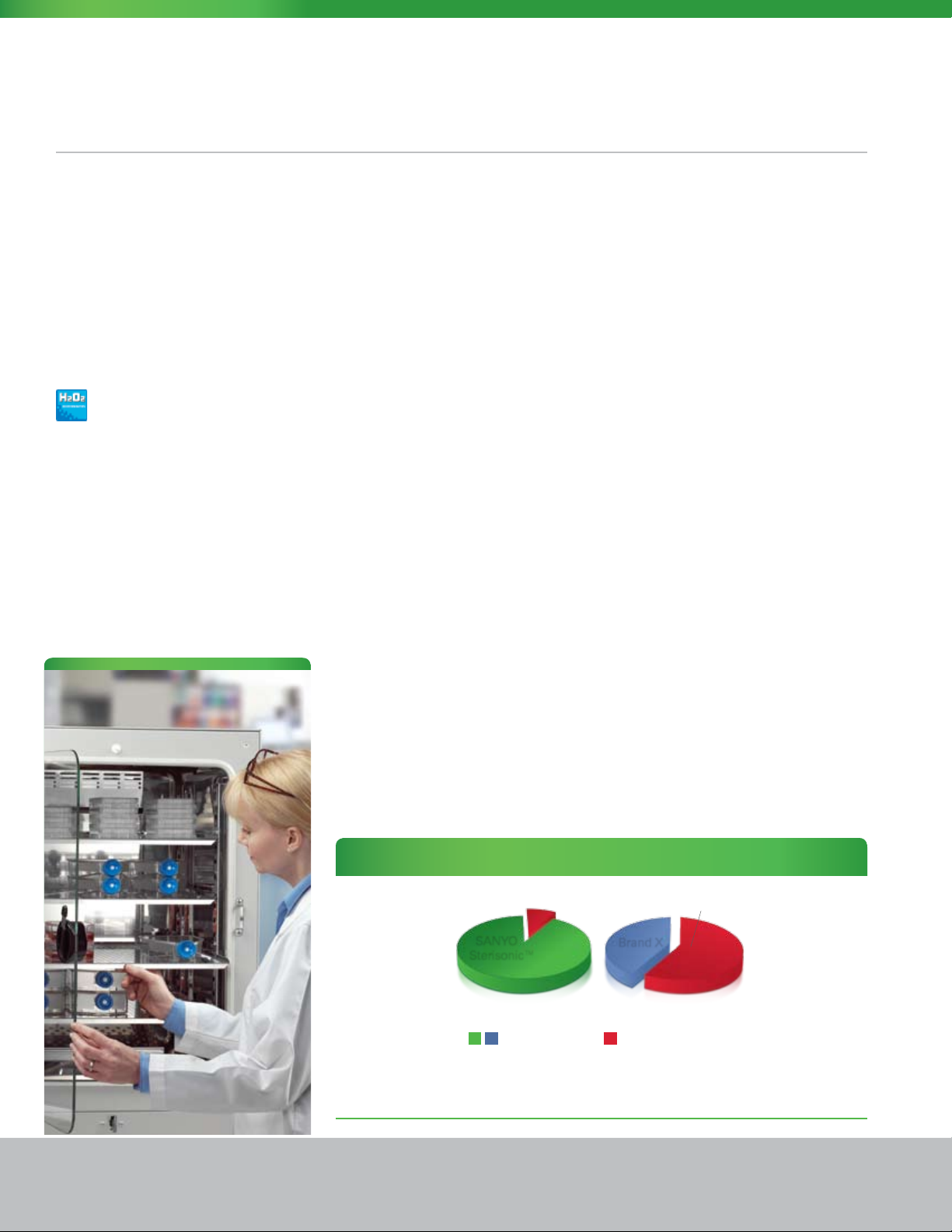
Sterisonic™ GxP Series H2O2 Sterilization Performance Value
2 Hours
SANYO
Sterisonic™
H2O
sterilization vs. high heat sterilization
2
= Uptime (Hours)
The documented two-hour in situ H2O2 sequence puts the fully sterilized Sterisonic™ GxP available and
ready for use quicker than any other incubator worldwide.
4 Sterisonic™ GxP Series Cell Culture Incubators www.sanyobiomedical.com
Brand X
=
Downtime (Hours)
14 Hours

Sterisonc™ GxP Features and Benefits
Cabinet knock-outs are pre-drilled •
and tapped to eliminate drilling and to
simplify re-mounting of door hardware.
The outer door closes against a •
soft, easily cleaned magnetic gasket
designed to eliminate ambient air
shear across the glass inner door,
minimizing condensation.
A door ajar alarm provides an audible •
and visual warning if the outer door
is left open.
A center-mounted key lock is located •
beneath the door for added security;
model MCO-19AIC(UVH) only.
Shelves and Inventory
Management
Convenient space efcient inventory
management is simplied through a system of adjustable, extendable shelves.
Inventory shelves and brackets are •
formed from polished copper-enriched
stainless steel, removable without
tools, and can remain inside the incubator during the H
or autoclaved separately if desired.
sterilization cycle
2O2
Shelves are perforated to permit natu-•
ral vertical air convection through and
around labware.
Shelf brackets are formed with an •
exaggerated angle to minimize surface
contact with at shelves (patent pending). Brackets slip easily into vertical
supports that attach to interior chamber walls with clearance sufcient to
permit proper air circulation.
Inner Door and Gasket
The inner design is critical to successful
contamination control technique.
The inner gasket body forms an •
effective thermal transition between
the ambient air and warm, humidied
incubator atmosphere, minimizing
condensation and eliminating moisture
traps which can harbor contaminants.
The inner door gasket is a dual •
durometer extrusion (two levels of
softness) from closed-cell silicone
to inhibit contamination.
The gasket feather-edge allows •
the inner glass door to close gently
against the chamber opening for
a tight peripheral seal.
The entire inner door gasket is remov-•
able for cleaning and/or replacement
if required.
Radiant heat from the outer door, •
apportioned by the microprocessor
MCO-38AIC(UVH)
Stacking Doubles Capacity
in Same Footprint.
The Sterisonic•
designed for stacking, allowing one
unit to be positioned on top of
another, doubling interior volume
without additional oor space.
The combination of stacking and •
reversible doors offers the most
installation options possible.
An optional roller base is recom- •
mended for stacked installations
to permit mobility if required;
see Accessories.
™
GxP cabinet is
control as a function of the patented
Direct Heat and Air Jacket
™
, automatically warms the inner door glass
in proportion to total heat demand
and condensation control.
Field Reversible Door
The eld-reversible door allows
universal installation using the left-hand
hinge (standard) or a right-hand hinge
modication
The outer door includes a universal •
nger grip at each side.
Mounting holes for hinge hardware •
are pre-drilled and capped with easily
removable trim plugs.
The door heater cable plugs into the •
alternate connection to complete
the change.
Elevated Humidity,
Low Water Level Warning
To avoid cell culture desiccation, the
SANYO Sterisonic
maintains ~95% RH at 37°C.
Humidication is achieved by a com-•
bined forced-air and natural evaporation method enhanced by the Direct
Heat and Air Jacket
protected by an optical water level
indicator to warn of low water in the
removable humidity pan.
The humidity pan removes easily for •
regular cleaning and rell.
When lled, the pan slides into place. •
The optical sensor returns to position
and the SafeCell
any contaminants introduced during
the process.
For the H•
the humidity pan is repositioned
against the chamber wall to eliminate
the need for separate autoclaving.
™
GxP CO2 incubator
™
base heater, and
™
UV lamp destroys
in situ sterilization cycle
2O2
5
 Loading...
Loading...