Sanofi-aventis Lantus SoloSTAR User Manual
QUICK REFERENCE GUIDE
4
3
5
6
10 secs
2
1
prescribed
insulin
a
#
1
These instructions are supplied as a guide only. Read the full
instruction leaflet accompanying the pen before you use Lantus
SoloSTAR®for the first time. If you have any questions, ask your
healthcare professional or call the 24-hour support line shown below.
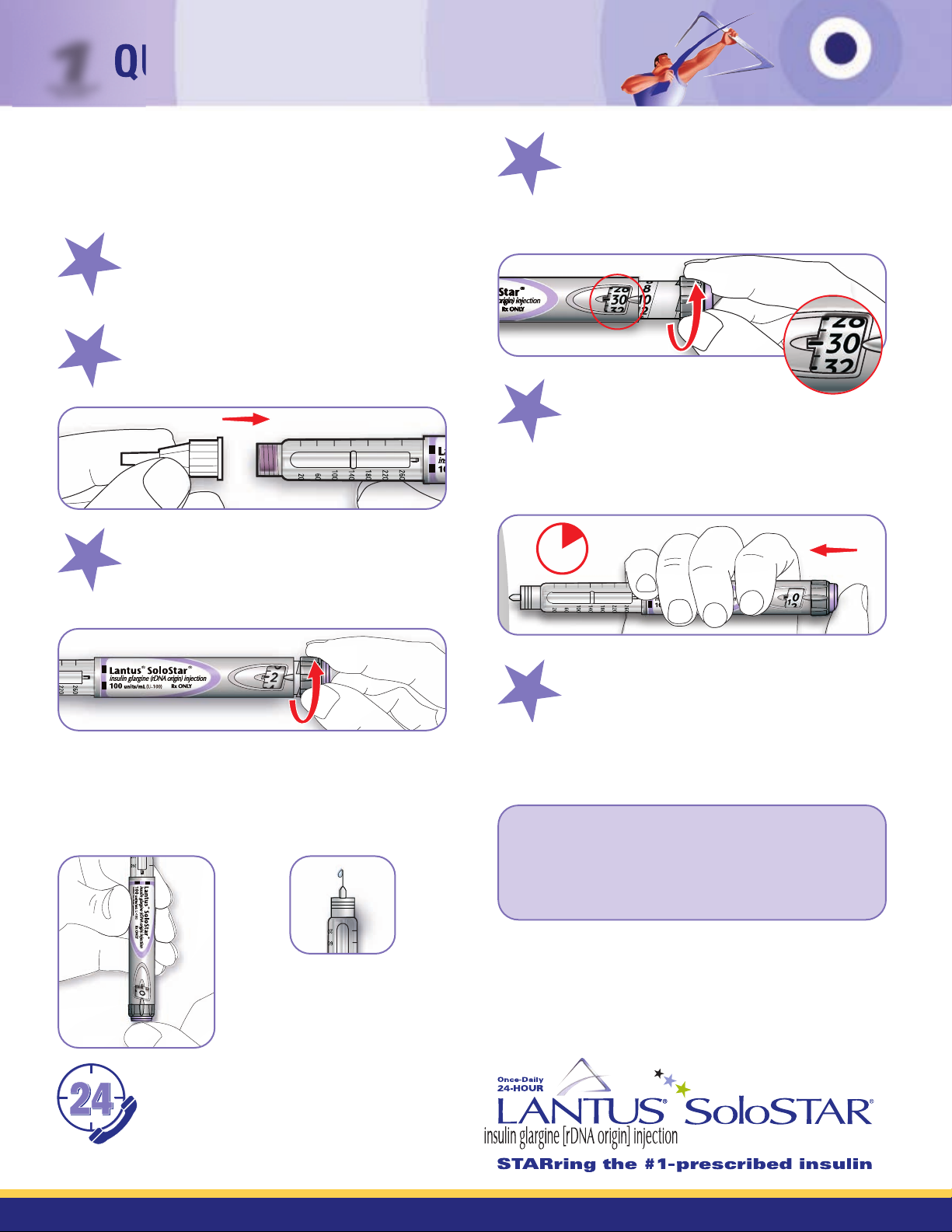
PREPARE FOR AN INJECTION
Before beginning, check the label on the insulin
pen to ensure you are using the correct insulin.
ATTACH A NEW NEEDLE
Keep the needle straight as you attach it. Lantus
SoloSTAR®uses push-on or screw-on needles.
®
PERFORM A SAFETY TEST
This removes air bubbles and ensures that the pen and
needle are working properly. Select a dose of 2 units.
Always perform the safety test before each injection.
®
SELECT YOUR DOSE
Be sure the dose window shows “0” following
the safety test. Select your required dose. If you
need a dose larger than 80 units, use 2 or more
injections. (This example shows 30 units.)
INJECT YOUR DOSE
Using the method your healthcare professional showed
you, insert the needle into the skin in either your upper
arm, abdomen (stomach area), or thigh (upper leg). Press
the injection button in all the way. Hold the button in that
position, slowly count to 10, then withdraw the needle.
Take off the outer needle cap and keep it to remove the used
needle after your injection. Then take off the inner needle cap
and discard it. Hold the pen with the needle pointing upward.
Tap the reservoir gently so any air bubbles rise up to the
needle. Press the injection button all the way in. Check if
insulin comes out of the needle.
If insulin does not come out, check
for air bubbles and repeat the test
2 more times to remove them. If no
insulin comes out after the third
time, try again with a new needle.
24-HOUR SUPPORT
1-800-633-1610
REMOVE THE NEEDLE
Always remove the needle after each injection. Put
the outer needle cap back on the needle and use it to
unscrew the needle from the pen. Dispose of the needle
safely, as instructed by your healthcare provider (eg, in
a sharps container). Put the cap on the pen.
If you can’t dial to the dose you want, check if you have
enough insulin in the reservoir.
If you have any other problems with the pen, first try
changing the needle and repeating the safety test.
Each Lantus
Please see Important Safety Information for Lantus
on reverse side.
Please see accompanying additional important information.
a
Based on TRx data from IMS Health, NPATMMonthly database, time period from
May 2003 to January 2009.
®
SoloSTAR®is for use by one person only.
®
NDC# 0088-2220-60
1
prescribed
insulin
a
#
Lantus
®
SoloStar
®
1 box(5x3-mL)
prefilled disposablepens
Important Safety Information for Lantus
Prescription Lantus®is for adults with type 2 diabetes or adults and children (6 years and older) with type 1 diabetes who require long-acting
insulin for the control of high blood sugar.
®
Lantus
DO NOT DILUTE OR MIX LANTUS
sugar control, which could be serious. Do not change your insulin without talking with your doctor. The syringe must not contain any
other medication or residue. You should not use Lantus
day, at the same time each day. You must test your blood sugar levels while using an insulin such as Lantus
The most common side effect of insulin, including Lantus
include injection site reactions, including changes in fat tissue at the injection site, and allergic reactions, including itching and rash. In rare
cases, some allergic reactions may be life threatening. Tell your doctor about other medicines and supplements you are taking because they
can change the way insulin works.
SoloSTAR®is a disposable prefilled insulin pen. Needles and the Lantus®SoloSTAR®pen must not be shared.
®
WITH ANY OTHER INSULIN OR SOLUTION. It will not work as intended, and you may lose blood
®
if you are allergic to insulin. Lantus®is a long-acting insulin you inject just once a
®
®
®
.
, is hypoglycemia, which may be serious. Other possible side effects may
Please see accompanying additional important information.
a
© 2009 sanofi-aventis U.S. LLC US.GLA.09.03.043
Based on TRx data from IMS Health, NPATMMonthly database
time period from May 2003 to January 2009.
 Loading...
Loading...