
DNA Size Selection System
Operations Manual

● ●
PippinHT Operations Manual ii
460005 Rev G
Sage Science Inc.
Suite 2400
500 Cummings Center
Beverly, MA. 01915
© 2018 Sage Science, Inc. All rights reserved. Sage Science and PippinHT are
trademarks of Sage Science, Inc.
All other brands and name mentioned herein are property of their owners.
The PippinHT instrument and gel cassettes are covered by U.S. patents
8,361,298 and 8,361,299. For research use only.

● ●
PippinHT Operations Manual iii
460005 Rev G
TABLE OF CONTENTS
1 INTRODUCTION …………………………………………………………..……………………..……….1-1
1.1 System Overview ....................................................................................................................... 1-1
1.2 How the system works .............................................................................................................. 1-2
1.3 Collection Strategies ................................................................................................................. 1-3
1.4 Collection Types ........................................................................................................................ 1-4
2 KEYS TO SUCCESSFUL COLLECTIONS ...................................................................... 2-1
2.1 Common Misconceptions ........................................................................................................ 2-1
2.2 Improving Sample Yield ........................................................................................................... 2-3
2.3 Improving Sample Yield for HMW DNA (High-Pass Protocols) ............................................ 2-3
3 SAFETY AND PRECAUTIONS ....................................................................................... 3-1
4 UNPACKING AND INSTALLATION ................................................................................ 4-1
4.1 Unpacking the PippinHT ........................................................................................................... 4-1
4.2 Setting up the PippinHT ............................................................................................................ 4-1
4.3 Unpacking Gel Cassette Kits .................................................................................................... 4-3
4.3.1 Storage Conditions for Cassettes and Reagents................................................................ 4-4
5 WORKFLOW SUMMARY ................................................................................................ 5-5
6 SAMPLE PREPARATION ............................................................................................... 6-1
6.1 Input Sample Characteristics .................................................................................................. 6-1
6.2 Preparing DNA Samples for the PippinHT ............................................................................. 6-1
6.2.1 Sample preparation for use with an internal standard ....................................................... 6-1
6.2.2 Sample preparation for use with an external marker ........................................................ 6-2
6.3 Recommended sample Load Guidelines ................................................................................. 6-2
7 PROGRAMMING A PROTOCOL ................................ .................................................... 7-1
7.1 Overview .................................................................................................................................... 7-1
7.1.1 Protocol Parameters Detail ................................................................................................ 7-2
7.2 Writing a Size Selection Protocol ......................................................................................... 7-4
7.3 Using an External Marker ....................................................................................................... 7-9

● ●
PippinHT Operations Manual iv
460005 Rev G
CONTENTS (Cont'd)
8 OPTICAL CALIBRATION ................................................................................................ 8-1
9 PREPARING A CASSETTE ............................................................................................ 9-1
10 CONTINUITY TEST ....................................................................................................... 10-1
10.1 Continuity Test Failure and Troubleshooting ..................................................................... 10-2
11 LOADING SAMPLES ................................................................................................... 11-1
11.1 Sample loading best practices ........................................................................................... 11-1
11.2 Sample loading procedure .................................................................................................. 11-2
11.3 Loading less than 12 samples, and reusing a gel cassette ............................................. 11-2
12 RUNNING A PROTOCOL ............................................................................................. 12-1
12.1 Overview ............................................................................................................................... 12-1
12.2 Sample Tracking .................................................................................................................. 12-2
12.2.1 Gel cassette barcodes ................................................................................................ 12-2
12.2.2 Sample ID entry .......................................................................................................... 12-3
12.2.3 Importing Sample IDs ................................................................................................. 12-3
12.2.4 Using the Sample ID Template ................................................................................... 12-5
12.2.5 Log Files ...................................................................................................................... 12-6
12.2.6 Log File Name Format ................................................................................................ 12-7
12.2.7 Adding a Prefix to Log File Names ............................................................................. 12-7
12.3 Starting, Pausing and Run Completion ............................................................................. 12-8
12.4 Monitoring a Run .............................................................................................................. 12-10
12.4.1 Run Status Indicators ............................................................................................... 12-10
12.4.2 Lane Status Indicators ............................................................................................. 12-11
12.4.3 Marker Detection Failure Alert ................................................................................. 12-12
12.5 Manual Mode ...................................................................................................................... 12-14
13 LOG REVIEW ............................................................................................................ 13-1
13.1 Overview .............................................................................................................................. 13-1
13.2 Loading a file ....................................................................................................................... 13-2
14 MANAGING FILES .................................................................................................... 14-1
14.1 Overview ............................................................................................................................. 14-1
14.2 File Types .......................................................................................................................... 14-2
14.3 Transferring files ............................................................................................................... 14-3
15 SYSTEM OPTIONS.................................................................................................... 15-1

● ●
PippinHT Operations Manual v
460005 Rev G
CONTENTS (Cont'd)
16 UPGRADING PIPPINHT SOFTWARE ....................................................................... 16-3
16.1 Extracting the Files to a USB flash drive....................................................................... 16-3
16.2 Upgrading the Pippin Instrument Software.................................................................. 16-4
17 PREVENTATIVE MAINTENANCE – RINSING ELECTRODES ................................. 17-7
17.1 Electrode Rinse Procedure ............................................................................................... 17-7
18 WARRANTY AND SERVICE ..................................................................................... 18-1
19 INSTRUMENT SPECIFICATIONS ............................................................................. 19-1
20 GEL CASSETTES SPECIFICATIONS ....................................................................... 20-1
20.1 3% Agarose, 100 bp-250 bp, Marker 30G....................................................................... 20-2
20.2 2% Agarose, 100 bp-600 bp, Marker 20B ........................................................................ 20-3
20.3 1.5% Agarose, 250 bp-1.5 kb, Marker 15C ...................................................................... 20-4
20.4 1.5% Agarose, High-Pass, >300bp - 1.5kb, Marker 15C…………………………………..20-5
20.5 0.75% Agarose, High-Pass >6-10 kb, Marker 75D........................................................... 20-6
20.6 0.75% Agarose, High-Pass >15-20 kb, Marker 75E ........................................................ 20-7
20.7 0.75% Agarose, High-Pass >30-40 kb, Marker 75F ........................................................ 20-8

● ●
PippinHT Operations Manual 1-1
460005 Rev G
1 Introduction
Thank you for purchasing the PippinHT from Sage Science. The PippinHT is an
automated preparative gel electrophoresis system.
We urge you to read this manual to familiarize yourself with the system’s capabilities and
precautions.
1.1 System Overview
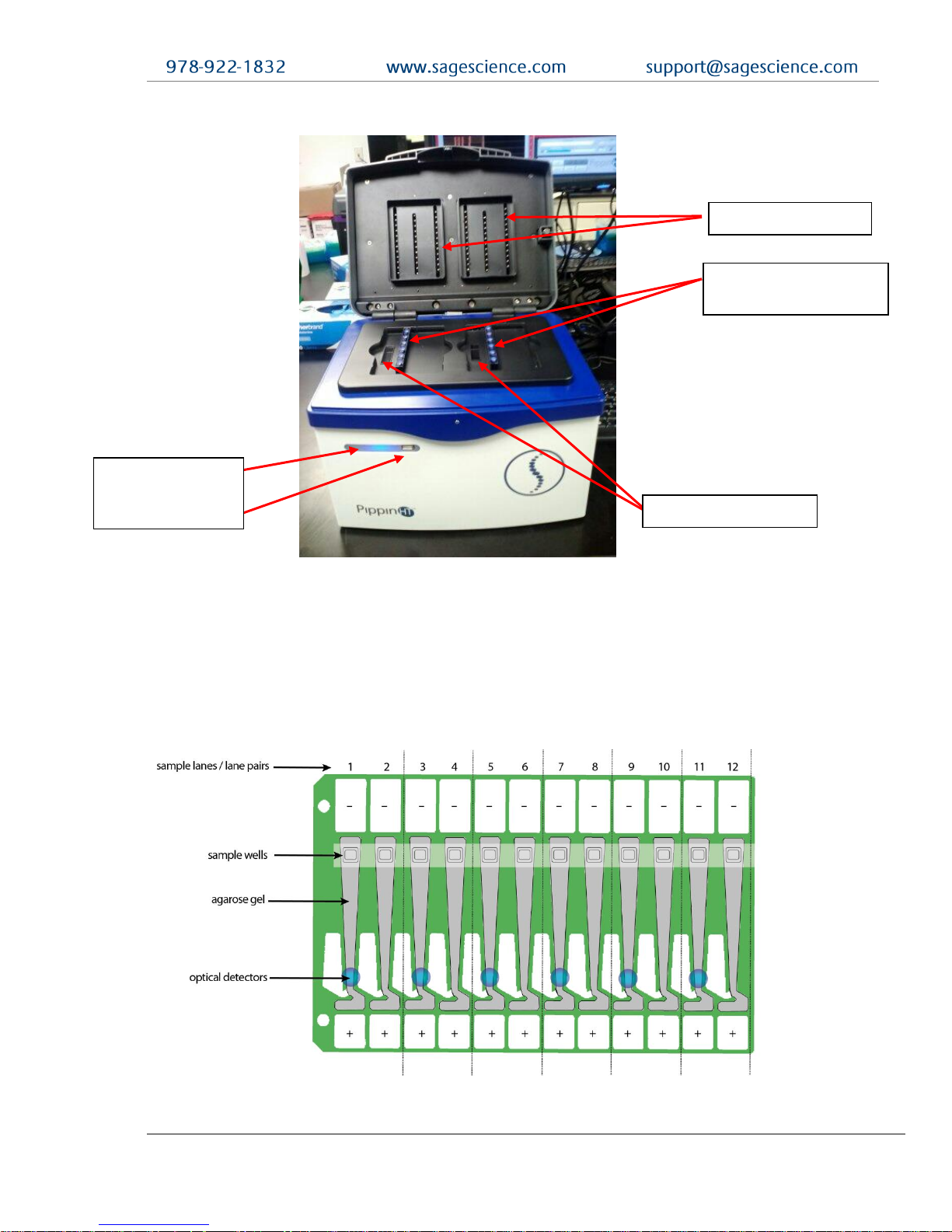
There are three components to the PippinHT system:
• Instrument – The instrument features two epifluorescent detector arrays, two
barcode readers, an electrophoresis power supply, two electrode arrays, and a
single-board computer. The system computer is accessed by an external LCD
monitor, mouse, and keyboard. (Figure 1-1)
• Software – System software allows the user to:
o Enter parameters that define the DNA fragment size ranges that are to
be collected, and enter sample identifications.
o Run the instrument and monitor operations.
o View previous runs with a review screen, and track run data with saved
run log files.
• Gel Cassettes – Pre-cast, disposable agarose cassettes are manufactured by
Sage Science. Cassettes are supplied in kits that contain 10 cassettes and
sufficient reagents to complete all collections. Kits have been developed to
address DNA size range collections at various size ranges.
● ●
PippinHT Operations Manual 1-2
460005 Rev G
Figure 1.1. Layout of the PippinHT instrument
1.2 How the system works
The schematic in Figure 1.2 Illustrates agarose gel columns during separation of DNA
fragments. Note that the optical detectors monitor the odd numbered lanes only.
Accordingly, each lane-pair must run the same size selection protocol. If an external
marker strategy is used, the marker must be run on an odd-numbered lane.
Electrode arrays
Epifluorescent
Detectors
Barcode readers
Indicator lights
USB Port
Figure 1.2. A gel cassette during separation. Detectors monitor odd-numbered lanes only.
● ●
PippinHT Operations Manual 1-3
460005 Rev G
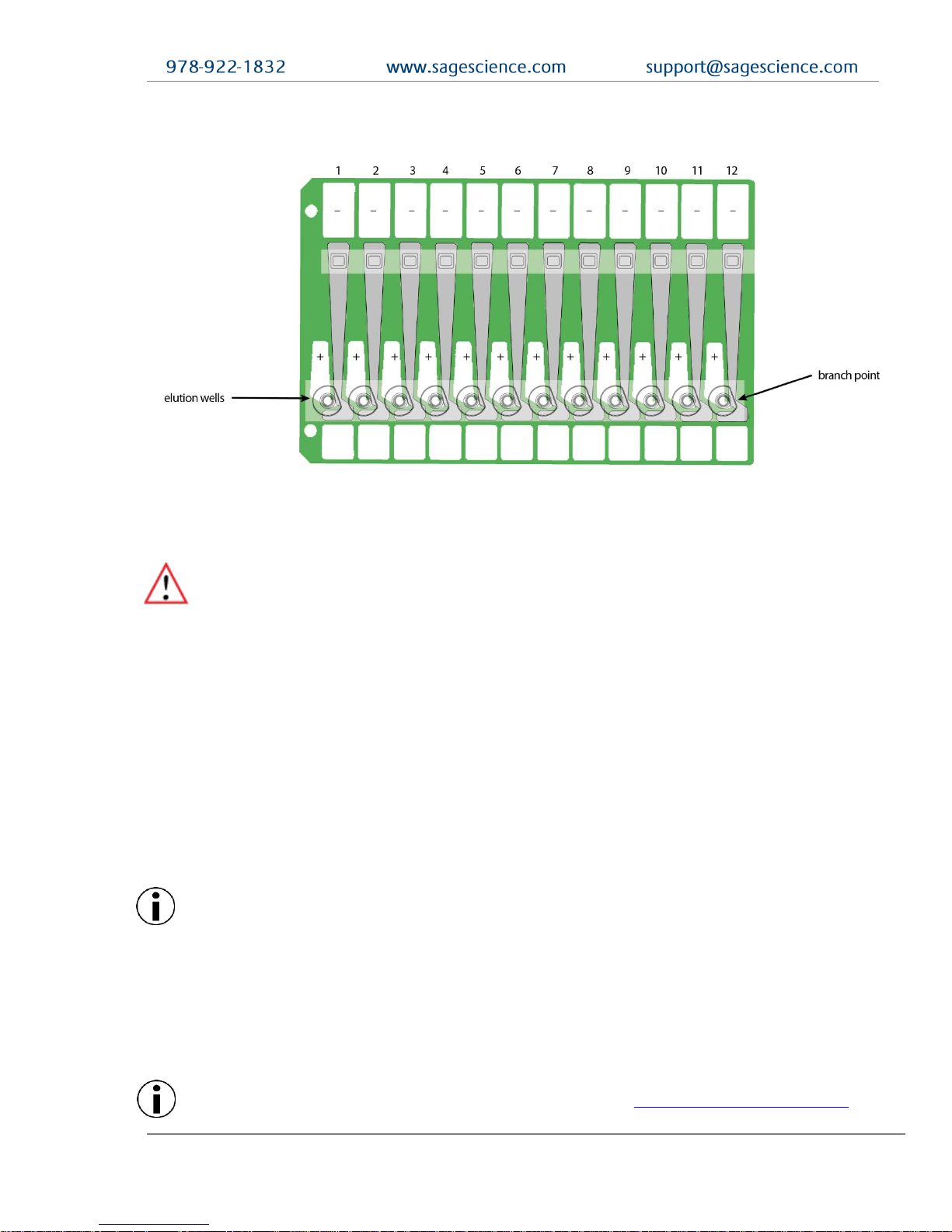
Figure 1.3 illustrates a gel cassette during elution of DNA fragments. Elution wells are
bound with membranes and collect DNA in electrophoresis buffer.
Important! Fail-Safe for Missed Peak Detection: Each cassette definition includes a time
value by which all peaks should be called. If the run reaches that specified time, and the peaks
have not been detected, the software decreases the base-to-threshold value by 25% and rescans for peaks. It will repeat this with an additional 25% decrease in base-to-threshold if peaks
are still not detected. If peaks are finally detected, the run will proceed as usual. If peaks are still
not detected, the lane will switch to 'idle'. This will prevent sample loss. All other lanes will
proceed with the run. Users can then choose to manually elute their sample from that lane once
the run is complete. See section 12.4 and 12.5.
1.3 Collection Strategies
Collection timing (start, duration, and end) is based on the factory calibration
settings provided with a cassette definition file, and the user-input size range settings in
software.
Note: The agarose gel matrix does not contain intercalating dyes or gel stains, sample DNA
is not viewable or monitored
Size Selections can be undertaken using one of the following strategies:
• Labeled internal standards –Fluorescein labeled DNA internal standards are
added to samples, and run ahead of the input sample. The rates of migration of
the internal standards are used to determine the collection timing* within the
sample lane. Up to 12 samples may be run per cassette.
Note: The DNA sequence of internal standards may be found at www.sagescience.com/support
on the PippinHT resources page, under the Quick Guide download section.
Figure 1.3. A gel cassette during Elution. Electrophoretic flow if diverted through elution wells by
activating a second set of electrodes.

● ●
PippinHT Operations Manual 1-4
460005 Rev G
• Labeled external marker – The fluorescein labeled DNA marker is loaded in a
single dedicated sample lane during a run. The rates of migration of the markers
are used to determine the collection timing in the remaining sample lanes. At
least one DNA marker and up to 11 samples per cassette must be run per
cassette.
• Timed runs – The beginning and end time of elution is set by the user. Neither
internal standards or external markers are used.
1.4 Collection Types
The PippinHT can be programmed to size select either
targeted fractions or “High-Pass” collections. The fragment
size distributions are described below:
1. Minimum Size Distribution (“Tight” setting). The
cassette lane will collect the narrowest distribution of
fragments that is achievable given the resolution of
the agarose gel concentration within the cassette.
The distribution is Gaussian, and its range is typically
+/- 16% (CV 8%) of the median fragment size.
2. Range Size Distribution (“Range” setting). The
cassette lane will collect a distribution of fragments
between a starting base pair value and an ending
base pair value. The actual start and end fragment
sizes are typically within +/- 10% of the base pair
values input into the software.
3. High-Pass Size Distribution (uses “Range*”
setting). The cassette lane will collect all
fragments above a base pair threshold. The
method increases the average fragment length of
the collection depending on threshold value and
input DNA distribution profile. High-Pass protocols
require an external marker to be run in one lane.
* Although the cassette continues to collect until the end of the run,
an end base pair value will auto-fill in the software protocol field.
This value should be disregarded, and should not be changed by
users.
High Pass
Range
Tight

● ●
PippinHT Operations Manual 2-1
460005 Rev G
2 Keys to Successful Collections
It is recommended that users closely follow the instructions outlined in this manual until
they become thoroughly familiar with the system. The Quick Guide (included with
cassettes) provides protocol reminders, but is not intended for beginning users.
Keys to successful collections are outlined in the following sections:
• Purity of the input sample (Section 6)
• Optical Calibration (Section 8)
• Preparation of the cassettes (Section 9)
• Sample loading (Section 11)
In addition, extra steps should be taken to improve recovery of high molecular weight
collections using High-Pass protocols with 0.75% agarose cassettes. These
recommendations are outlined in section 2.3, below.
2.1 Common Misconceptions
Users should be aware of the following characteristics of the PippinHT system. These
are part of normal operation of the system, but may seem counterintuitive at first.
2.1.1 Narrowest is not always the best.
The PippinHT can produce very narrow size distributions from sheared genomic
DNA. However, narrower size distributions will necessarily mean that a smaller
fraction of the input DNA will be recovered. Users should broaden their collection
ranges if the default tight settings do not produce enough DNA for their
application.
Figure 2.1. A comparison of the relative sample yield between tight and broader range
size selections of DNA.

● ●
PippinHT Operations Manual 2-2
460005 Rev G
2.1.2 DNA undergoing elution is smaller than DNA at the detector.
The branch point between the separation and elution channels is downstream
from the detector position. Figure 2.2 shows a cassette channel to illustrate.
During normal operation, the leading edge of the DNA fraction scheduled for
elution passes the detector before the start of elution (by up to several minutes).
This offset can give rise to the impression that sample elution is late, even in runs
that are functioning properly.
Figure 2.2. An illustration of the time and base pair difference between the detector
and branch point.
2.1.3 The rate of electrophoresis is faster in the separation channel than in
the elution channel. This may cause misconceptions with regard to the timing
of broad size selections. For instance, if one sample is programmed to select
from 400 – 600bp, and a second sample is programmed to select from 200 – 600
bp, the narrower range will finish eluting before the broader one, even though both
elutions complete collection at 600 bp. The collections of large DNA fragments,
especially with pulsed –field protocols, can also appear counter-intuitive with
respect to timing.
Figure 2.3. An illustration comparing the relative rate of electrophoresis in the separation
and elution channels

● ●
PippinHT Operations Manual 2-3
460005 Rev G
2.2 Improving Sample Yield
Users should expect an intrinsic yield of between 50-80% of the sample input. This is
based on product validation studies in which restriction fragments have been run and
collected, and the respective DNA amounts compared.
Yield can be affected by DNA becoming lightly bound to the low molecular weight cutoff membrane in the elution well. At the end of the run, the PippinHT will briefly
reverse field (for 5 seconds) in every elution channel. This improves sample yield by
up to 20%.
Note: The reverse field feature may be disabled in the Systems Option screen.
2.3 Improving Sample Yield for HMW DNA (High-Pass
Protocols)
Recovering high molecular weight DNA from High-Pass protocols tend to have lower
yields than low molecular weight size selection. The following additional steps are
highly recommended to improve product yield:
1. Wait at least 45 min after termination of the run before removing eluted DNA (it is
OK to leave samples in instrument for hours, i.e. for an overnight run).
2. Rinse the elution well with Tween solution. Tween solution (0.1% Tween 20 in
electrophoresis buffer) is provided by Sage Science with cassette kits used with
HMW collections.
After removing the initial eluted volume:
• Remove the eluted sample from elution well at the end of the run
• Add 30l of Tween solution (supplied with the gel cassettes) to the elution well
• Wait 1 minute
• Remove solution and pool with the original extracted sample, or process
separately
.

● ●
PippinHT Operations Manual 3-1
460005 Rev G
3 Safety and Precautions
Icons Used
In this manual, the following icons will be used to provide the user with information
pertinent to the use of the PippinHT.
Caution! Warns the user that injury or instrument damage may occur if the
contents of the warning are not properly followed.
High Voltage! Warns of the risk of electrical shock if the contents of the
warning are not properly followed.
Important! Provide important information about the proper use of the system
that may influence the quality of the result.
Note. Provides additional information regarding the function of the system or
applications for which is used.
Caution! Class I invisible Laser Radiation Present. Avoid long-term
viewing of laser.
Safe Use Guidelines
The PippinHT system is designed to operate under the following environmental
conditions:
• Pollution Degree 2
• Installation category 2
• Altitude 2000m
• Indoor use
• Ambient temperature 17-22oC
• Humidity 10-80%, non-condensing
Standard laboratory precautions should be taken when handling PippinHT Gel cassettes
and operating the PippinHT:
• Wear a lab coat, safety glasses, and gloves.
• Use in proximity of an eye wash station and/or running water.
Caution! The PippinHT was designed to be operated on a flat surface. Do not
operate on a tilted surface or tilt during operation.

● ●
PippinHT Operations Manual 4-1
460005 Rev G
4 Unpacking and Installation
4.1 Unpacking the PippinHT
The PippinHT instrumentation is shipped in two boxes. One will contain the PippinHT
and accessories. The second box will contain the computer monitor in the
manufacturer’s original packaging. With the boxes in the upright position, open and
confirm that the following items are enclosed:
Monitor
• LCD computer monitor (1366 x 768)
• Video cable
• Power cord
PippinHT
• PippinHT Instrument
• Accessory box
o Computer keyboard, USB
o Computer mouse, USB
o Power supply
o Power cord
o Mouse Pad
o Operations Manual
o USB Memory Stick
o Two rinse cassettes (for maintenance of electrodes)
o Two calibration fixtures (for setting optical baseline before each run)
4.2 Setting up the PippinHT
1. Remove the accessory box located just inside the instrument packaging from a
cardboard shelf atop the instrument. Remove the keyboard, mouse, and instrument
power supply from their packaging.
2. Remove the cardboard shelf from the box.
3. Remove the foam insert at the top of the instrument.
4. Reach into the box and grab the instrument from the bottom. The PippinHT weighs
approximately 20 lbs. Place the instrument onto a table or bench top.
5. Return the foam inserts and cardboard shelf to the box. If feasible, place the box in
storage.

● ●
PippinHT Operations Manual 4-2
460005 Rev G
6. Remove the computer monitor from the manufacturer’s box and unpack the items.
7. Using the video cable provided by the manufacturer, connect the monitor (VGA port,
under the monitor screen) to PippinHT (DVI port, on the rear panel, see figure 4.1).
8. Using the power cord and power supply provided by the manufacturer, plug the monitor
into an electrical outlet.
9. Turn on the monitor.
Important! Make sure to plug in and turn the monitor on before powering on the instrument.
This way the proper screen resolution will be set automatically when the PippinHT software is
launched. Otherwise, the PippinHT will need to be powered down and restarted to set the correct
screen resolution.
10. Connect the computer keyboard to any USB port located in the back of the Instrument.
11. Connect the computer mouse to the PippinHT into a second USB port at back of
instrument.
12. Connect PippinHT instrument to an electrical outlet using the power cord and power
supply provided with the PippinHT. The power entry port is located is on the rear panel
of the instrument (figure 4.1).
13. When instrument is powered up or power cycled, the blue LEDs on the instrument nests
will be ON (figure 4.2).
14. Press power switch located on the rear of the instrument. The blue light on the front
panel will turn on (figure 4.2).
15. Wait for software to launch, this will take 30 seconds - 1 minute. The PippinHT is ready
for use.
Power Entry Port
Power Switch
Figure 4.1. Rear Panel Ports
USB Ports (4)
DVI monitor port

● ●
PippinHT Operations Manual 4-3
460005 Rev G
4.3 Unpacking Gel Cassette Kits
Gel cassettes are shipped in boxes with the following items. Ensure boxes are in the
upright position and confirm that following contents are present:
o 10 or 4 foil-sealed gel cassettes (store at R.T.)
o 1 package of adhesive tape for sealing elution wells
o User Quick Guide
o Reagent kits (divide volumes by half for 4-cassette kits) (store at 4ºC)
3%, 2% or 1.5% agarose cassettes:
Package 1
• 1200 μl fluorescein-labeled DNA internal standards/ loading
solution mix (5 μl/sample)
• 2 x 60 ml of Electrophoresis Buffer
Package 2
• 2 x 600 μl of loading solution (packaged separately) – for size
selection using an external marker
0.75% agarose cassettes (uses external markers only)
▪ 500 μl fluorescein-labeled marker solution (25ul per lane)
▪ 2 x 60 ml of Electrophoresis Buffer
▪ 4 X 800l of 0.1% Tween20 in running buffer. Tween solution
is used to improve recovery of large DNA fragments.
Important! Fluorescein labels will degrade at room temperature – minimize time at RT for labeled
standards and markers
Nest LED lights turn ON
when the instrument is
powered up.
When running, a
green light is
also indicated on
the front panel.
After the power
button is
pressed, the blue
front panel LED
will light, and the
software will
launch.
Figure 4.2. Instrument indicator lights and optical array.

● ●
PippinHT Operations Manual 4-4
460005 Rev G
4.3.1 Storage Conditions for Cassettes and Reagents
The following storage requirements should be observed:
• Gel Cassettes- Store at room temperature in the foil bags until ready for use.
• DNA Internal standards / loading solution mix– Store at 4ºC. Equilibrate to
room temperature before use. Return to 4ºC, immediately after use.
• Electrophoresis buffer – may be stored at room temperature or 4ºC. Equilibrate
to room temperature before use.
• Loading solution – store at 4ºC. Equilibrate to room temperature before use.
Figure 4.3. Gel Cassette Kit

● ●
PippinHT Operations Manual 5-5
460005 Rev G
5 Workflow Summary
Prepare samples
add loading solution/standard mix to samples
(max. 12 samples/cassette)
Use internal standard
or external marker?
internal standard
High-Pass protocol?*
Yes
No
Prepare samples
add loading solution to samples
prepare external marker (3,2,&1.5% cassettes)
or bring external marker solution to RT
(0.75%/High-Pass cassettes)
(max.11 samples/cassette)
external marker
Program a Size Selection Protocol
Select “USE INTERNAL STANDARDS”
Program a Size Selection Protocol
Enter reference lane No. for the external marker
Select “APPLY TO ALL LANES”
Calibrate the Optics
Prepare the Cassette(s)
Run the Continuity Test on the Cassette(s)
Load Samples onto the Cassette(s)
Run the PippinHT
* 0.75% agarose cassettes
are used for High-Pass
collections only.

● ●
PippinHT Operations Manual 6-1
460005 Rev G
6 Sample Preparation
6.1 Input Sample Characteristics
When running the PippinHT™, characteristics of input DNA can affect separation
resolution and efficiency of product recovery. The following general guidelines should be
followed:
• Ionic strength: The ionic strength of the sample should be lower than the
ionic strength of the buffer (80mM monovalent ions). High salt concentrations can
result in unpredictable DNA mobility.
• Protein in the sample: DNA-binding proteins such as ligases or polymerases
can affect the mobility of fragments during separation. Proteins can also reduce
DNA recovery from the elution module by increasing the binding of DNA to the
ultrafiltration membrane at the back of the elution module. For best results,
samples should be de-proteinized prior to loading whenever possible.
• Input DNA size distribution: A knowledge of the input size distribution is
obviously important to program accurate size selection settings. PippinHT
cassettes are calibrated using the Agilent Bioanalyzer to evaluate input and
product sizes, and so, for best results, input size distributions should be
evaluated using the Bioanalyzer. For low concentration samples, the Agilent HS
chip is very useful .
6.2 Preparing DNA Samples for the PippinHT
The marker provided with the PippinHT reagent kit may be used as either an internal or
external marker. The method of sample preparation is dependent on the type of marker
used.
6.2.1 Sample preparation for use with an internal standard
1. Bring the DNA sample up to 20 µl with TE.
2. Bring the Standard/Loading-solution mix to room temperature (return to 4°C
immediately after use).
3. For each sample, combine 20 µl of sample with 5 µl of Standard/Loading-solution
mix.
4. Mix thoroughly by vortexing and centrifuge briefly.

● ●
PippinHT Operations Manual 6-2
460005 Rev G
6.2.2 Sample preparation for use with an external marker
Prepare the DNA samples
1. Bring the DNA sample up to 20 µl with TE.
2. Bring the loading solution to room temperature.
3. For each sample, combine 20 µl of sample with 5 µl of loading solution.
4. Mix thoroughly by vortexing and centrifuge briefly.
5. Prepare external marker as described below.
Prepare the external marker
A. For 3%, 2%, and 1.5% agarose gel cassettes:
1. Bring DNA standard/loading solution mix to room temperature (return to 4°C
immediately after use).
2. For each external marker, combine 5 µl DNA standard/loading solution mix with
20 µl of TE.
3. Mix thoroughly by vortexing and centrifuge briefly.
B. For 0.75% agarose gel cassettes (High-Pass size selection)
1. Bring the external marker solution to room temperature.
6.3 Recommended sample Load Guidelines
Maximum Load: 1.5 g sheared genomic DNA
Minimum Load: 15 ng sheared genomic DNA

● ●
PippinHT Operations Manual 7-1
460005 Rev G
7 Programming a Protocol
The default screen on the PippinHT has a tabbed format. The Protocol Editor is the
second tab.
7.1 Overview
The Protocol Editor tab is the screen with which users create new protocols or edit
existing protocols for DNA size selection. A protocol is created for a cassette type (within
the applicable target range) and is saved as a named protocol file with a “.phprot” file
extension. When running a cassette, the size selection protocol file is selected and
applied to the run.
Protocol
Editor Tab
Protocol File Selector
Protocol
Parameters
File
Commands
Warnings Text Box
DNA Marker/
Standard Lane
Assignment
Tabs
Toggle
between
Cassette
1 & 2
Copy
parameters
Cassette Type Selector

● ●
PippinHT Operations Manual 7-2
460005 Rev G
7.1.1 Protocol Parameters Detail
The protocol parameters section is shown below. Size selection must are entered for
lane pairs and different values may be entered for each lane pair. The odd-numbered
lanes are the lanes which are optically detected.
The function of the columns or fields are provided below. From left-to-right:
Programming Modes
The user selects which of the three size selection modes that will be used. When this is
selected, the editable fields will appear in the size selection parameters:
• Tight - collects the narrowest fragment distribution that can be collected by the
system.
The user enters a target base-pair value (the median for the distribution) in the size
selection parameters.
• Range – collects a fragment range. The user enters a start and end base-pair
value in the size selection parameters.
Note: Range mode is used for programming High-Pass protocols.
• Time – collects fragments base on a starting elution time (time after the
beginning of a run) and ending elution time.
Note: Programming modes may be independently applied to individual sample lanes
Programming
Modes
Size selection
parameters
(in base pairs)
Time-mode
parameters
Cassette 2 Tab

● ●
PippinHT Operations Manual 7-3
460005 Rev G
Ref Lane
This indicates which lane is used for an internal standard or external marker. The
default is off. Internal standard runs will display the same lane number as the parameter
field. For external marker runs, each field will display the lane number into which the
marker was designated to be loaded.
Size Selection Parameters
Users enter the size selection parameters in these fields, in base-pairs, based on the
programming mode that was selected. The “Pause” field pauses the instrument to allow
users to collect two serial collections. The value in the Pause field must be a value
within the size range selected (i.e. if a 200-400 bp range is selected, the Pause value
must be between 201 and 399).
Time Mode Parameters
Users enter the size selection parameters, as time (hh:mm:ss) after the start of a run.
The Pause value must be within the start time and end time that were entered (T Start
and T End).
Sample ID Template
Users can optionally enter Sample ID Template text. These fields are stored with the
saved protocols and appear in the Sample ID field whenever the protocol is used. If
sample information is entered or imported into the Sample ID field in the Main screen,
then the Sample ID Template names will prefix the Sample ID names.
LED On
The LED On button allows users to ensure the LED detector remains on in the
designated lane-pair for the entirety of the run. This feature is for advance users and
non-standard runs. Under normal operation, LED are automatically turned off after the
internal standards or external marker has been detected by the instrument.
Range Flag
The Range Flag is an visual indicator of type of size selection. Tight collection display
green and range collections above the minimum distributions indicate an orange color
and the word “ Broad”. If a range distribution is selected that is below the achievable
minimum distribution, the display will indicate a yellow color and the word “Narrow”.
Timed mode selections do not indicate a range flag.
Note: Size selections can be entered that are below the minimum possible. In these instances,
the range flag will indicate a yellow color and the word “narrow”. These collections will not have
a narrower fragment distribution, but the specified minimum with a lower yield:
Pause On
The Pause On displays a light gray color if the pause feature has been enabled for that
lane-pair.

● ●
PippinHT Operations Manual 7-4
460005 Rev G
7.2 Writing a Size Selection Protocol
The procedure below describes programming using internal standards. Users
should also follow this procedure if using external markers, and then read section 7.3
for specific information about using external markers.
1. Click the “Protocol Editor” tab along top of the screen.
2. Click the Protocol folder icon. A pop-up screen will appear that lists previously saved
protocols. Select “NEW”, to create a new protocol. This will clear all of the fields in
the screen to the default state.
Note: Previously save protocols may be selected and opened from the protocol pop-up window.
Theses may be edited and saved or resaved with a new file name. Any changes to a protocol or
the default screen will initiate a flashing yellow light in the lower right hand corner under a
“Protocol Changes NOT Applied” message. This will remain flashing until the protocol is saved or
resaved:
3. If necessary, click on the tab for Cassette 1 (Cassette 2 may also be selected if it is
the only cassette to run with the protocol). It is important to keep track of which
cassette is being programmed.
Note: Parameters programmed on Cassette 1 may be copied to Cassette 2, but not vice versa. If
creating a 2-cassette protocol, starting with cassette 1 is recommended.
Protocol folder
NEW
 Loading...
Loading...