
1/174
ANTARES
Corneal topographer
INSTRUCTIONS FOR USE
S4OPTIK, LLC
250 Cooper Ave., Suite 100
Tonawanda, NY, 14150
+1-888-224-6012
+1 800 313 8696
info@s4optik.com
0051


INSTRUCTIONS FOR USE
ANTARES | IFUANTARESEN00
This document is the property of
S4Optik.
Any reproduction, even partial, it is prohibited.
1/48
1
INTRODUCTION ............................................................................. 3
1.1
SYMBOLS ..................................................................................................................... 3
1.1.1 Device symbols ..................................................................................................... 4
1.2
GENERAL WARNINGS .................................................................................................. 4
1.3
NORMATIVE REFERENCES ........................................................................................... 5
1.3.1 Community directives ........................................................................................... 5
1.3.2 Technical standards .............................................................................................. 5
1.3.3 Quality management systems standards.............................................................. 5
1.4
WARRANTY ................................................................................................................. 6
1.5
MANUFACTURER IDENTIFICATION .............................................................................. 7
2
SAFETY ........................................................................................... 8
2.1
SAFETY WARNINGS ...................................................................................................... 8
2.2
DEVICE IDENTIFICATION ............................................................................................ 10
2.2.1 Registration data in the Medical Devices List ..................................................... 10
2.2.2 Device data plate ................................................................................................ 10
2.2.3 Power supply data plate ..................................................................................... 11
2.3
INTENDED USE ........................................................................................................... 11
2.4
MEDICAL DEVICES CLASSIFICATION ........................................................................... 17
2.5
MEDICAL ELECTRICAL DEVICES CLASSIFICATION ....................................................... 17
2.6
ENVIRONMENTAL CONDITIONS ................................................................................. 18
2.7
DISPOSAL AT THE END THE USEFUL LIFE .................................................................... 19
2.8
MANUFACTURER DECLARATIONS ............................................................................. 21
2.8.1 Electromagnetic emissions ................................................................................. 21
3
DEVICE DESCRIPTION .................................................................. 24
3.1
PROVISION DESCRIPTION .......................................................................................... 24
3.1.1 Device ANTARES ................................................................................................. 26
3.1.2 Power supply ...................................................................................................... 27
3.1.3 Chin rest ............................................................................................................. 28
3.1.4 Electric table (optional) ...................................................................................... 29
3.2
TECHNICAL DATA ....................................................................................................... 29
4
DEVICE USE ................................................................................. 31
4.1
HOW TO INSTALL THE DEVICE ................................................................................... 31
4.2
HOW TO CONNECT THE DEVICE................................................................................. 33
4.3
HOW TO PLACE THE ELECTRIC CABLES ...................................................................... 35
4.4
HOW TO TURN ON THE DEVICE ................................................................................. 36
4.5
ADJUST THE CHIN REST .............................................................................................. 37
4.6
HOW TO CAPTURE THE IMAGE .................................................................................. 39
4.7
HOW TO CHANGE THE PAPER FOR CHIN CUP ............................................................ 41
4.8
HOW TO TURN OFF THE DEVICE ................................................................................ 42
5
ORDINARY MAINTENANCE .......................................................... 43
5.1
SAFETY WARNINGS .................................................................................................... 43
5.2
DEVICE CLEANING ...................................................................................................................... 43

INSTRUCTIONS FOR USE
ANTARES | IFUANTARESEN00
This document is the property of
S4Optik.
Any reproduction, even partial, it is prohibited.
2/48
5.3
SPARE PARTS AND ACCESSORIES LIST ......................................................................................... 44
5.4
TROUBLESHOOTING ................................................................................................................... 45

INSTRUCTIONS FOR USE
ANTARES | IFUANTARESEN00
This document is the property of
S4Optik.
Any reproduction, even partial, it is prohibited.
3/48
1 INTRODUCTION
The device is the result of a long research period, conducted by
experts to give the product technical innovation, quality and design.
The device can be easily used thanks to the guided manual capture
and the electronic control of all the functions of the device.
1.1 SYMBOLS
Within the instructions for use, on the package or on the device, there
can be the following symbols:
Symbol
Meaning
Caution
Warning, electricity
Read the instructions for use
General mandatory action sign
Note. Useful information for the user
General prohibition sign
Manufacturer
CE Marking (Directive 93/42/EEC) Identification number of the
notified body (IMQ)

INSTRUCTIONS FOR USE
ANTARES | IFUANTARESEN00
This document is the property of
S4Optik.
Any reproduction, even partial, it is prohibited.
4/48
Waste disposal in compliance with the Directive 2012/19/EU
(WEEE), and 2011/65/EU (RoHS II)
1.1.1 DEVICE SYMBOLS
1.2 GENERAL WARNINGS
THESE INSTRUCTIONS FOR USE REFER TO THE DEVICE ANTARES
("DEVICE" FROM NOW ON).
THE ORIGINAL TEXT IS IN ITALIAN.
Before using the device or if you don't use it since a long time, read
these instructions carefully. Read the instructions given in the
instructions manual and reported on the device?
Keep this manual close by for future consultation. If you should
decide to sell this appliance to other people, remember to also
include these instructions, complete and readable
Keep the original box and packaging, as the free-of-charge service
does not cover any damage resulting from inadequate packaging of
the product when this is sent back to an Authorized Service Center.
Verify the presence of damage signs on the device caused by the
transport/storage, before using the device.
Symbol
Meaning
Type B applied part
Class II equipment
INSTRUCTIONS FOR USE
ANTARES | IFUANTARESEN00
This document is the property of
S4Optik.
Any reproduction, even partial, it is prohibited.
5/48
It is forbidden to reproduce, totally or partially, texts or images
contained in these instructions for use without the written
authorization of the Manufacturer.
The Manufacturer reserves himself the right to modify the contents
of the instructions for use, without notice.
1.3 NORMATIVE REFERENCES
1.3.1
COMMUNITY DIRECTIVES
-
Directive 93/42/EEC and subsequent modifications and
integrations concerning medical devices
-
Directive 2012/19/EU on waste electrical and electronic equipment
(WEEE)
1.3.2
TECHNICAL STANDARDS
-
IEC 60601-1: 2005 + A1:2012 - Medical electrical equipment - Part
1: General requirements for basic safety and essential performance
-
EC 60601-1-2:2014 Edition 4 Collateral Standard: Electromagnetic
disturbances - Requirements and tests
-
UNI EN ISO 15004-1:2009 Ophthalmic Instruments. Fundamental
requirements and test methods - Part 1: General requirements
applicable to all ophthalmic instruments
-
UNI EN ISO 15004-2:2007 Ophthalmic Instruments. Fundamental
requirements and test methods - Part 2: Light hazard protection
-
UNI CEI EN ISO 14971:2012 Medical devices. Application of risk
management to medical devices.
-
UNI EN ISO 19980:2012 - Ophthalmic instruments - Corneal
topographers
1.3.3
QUALITY MANAGEMENT SYSTEMS STANDARDS
-
UNI CEI EN ISO 13845:2016 Medical devices. Quality management
systems - Requirements for regulatory purposes

INSTRUCTIONS FOR USE
ANTARES | IFUANTARESEN00
This document is the property of
S4Optik.
Any reproduction, even partial, it is prohibited.
6/48
1.4 WARRANTY
The Manufacturer is responsible for the device conformity to the
Community directive 93/42/EEC as amended by the 2007/47/EC for:
-
features
-
safety and reliability
-
CE marking
The Manufacturer refuses any responsibility for:
-
installation and activation not activated in conformity to the
indications and the precautions reported in the instructions for use
-
use not in compliance with the instructions for use and precautions
reported in the instructions for use
-
use of accessories or spare parts not provided or suggested by the
Manufacturer
-
repairs and safety controls not effectuated by expert, qualified,
trained and personnel authorized by the Manufacturer
-
electrical system of the space where the device is installed not in
compliance with the technical standards, the laws and regulations
in effect in the country of installation of the device
-
direct or indirect consequences or damages to objects or persons,
originating from the improper use of the device or erroneous
clinical analysis originating from its use
The Manufacturer guarantees the device for 24 months after invoicing
The Warranty includes the substitution, at the Manufacturer's or an
Authorized Service Center, of components and materials and the
relative labor. The shipping and transport fees are to be paid by the
client.
The warranty does not cover:
-
reparations of faults originating from natural disasters, mechanical
shocks (fall, hit, etc), electrical system faults, negligence, improper
use, maintenance or reparations carried out with non-original
materials
-
any other improper use or not intended by the Manufacturer
-
damages caused by service lack or inefficiency, originating by
causes or circumstances out of the Manufacturers control

INSTRUCTIONS FOR USE
ANTARES | IFUANTARESEN00
This document is the property of
S4Optik.
Any reproduction, even partial, it is prohibited.
7/48
- the parts subject to usage and/or deterioration originating from
the normal use and those that might be broken because of an
improper use or maintenance carried out by personnel nonauthorized by the Manufacturer.
To ask maintenance interventions or to have technical information
about the device, address to an Authorized Service Center or directly
to the device Manufacturer.
The client will not be refunded for damages originating from the
device halt.
1.5 MANUFACTURER IDENTIFICATION
S4OPTIK, LLC
250 Cooper Ave., Suite 100
Tonawanda, NY, 14150
+1-888-224-6012
+1 800 313 8696
info@s4optik.com
https://s4optik.com/

INSTRUCTIONS FOR USE
ANTARES | IFUANTARESEN00
This document is the property of
S4Optik.
Any reproduction, even partial, it is prohibited.
8/48
2 SAFETY
2.1 SAFETY WARNINGS
DANGER
Electric shock danger. Do not let water fall on the device. Do not
immerse the device in water or other liquids.
DANGER
Electric shock danger. If the power cables are damaged they must
be replaced in an Authorized Service Center to prevent any risk.
DANGER
Electric shock danger. Unplug the power cable from the mains
socket before disinfecting the device and before any maintenance
operation.
CAUTION
Do not use the device if visibly damaged. Periodically inspect the
device and the connection cables to verify if there are damage
signs.
CAUTION
Always keep the device out of the reach of children.
CAUTION
Danger of device fall. Do not leave free cables which can represent
an obstacle or a danger for the patient or the operator.
CAUTION
Danger of stumbling and falling. Do not let the power cord or the
connection cables free in a place where people could walk.

INSTRUCTIONS FOR USE
ANTARES | IFUANTARESEN00
This document is the property of
S4Optik.
Any reproduction, even partial, it is prohibited.
9/48
CAUTION
Electric shock risk. Do not touch the power supply cables with wet
hands.
CAUTION
Electric shock risk. Do not leave the power supply cables in contact
with sharp corners or objects. Collect and attach always the power
supply cables.
CAUTION
If you notice a wired odor or smoke coming out of the device or if it
emanates heat, turn it off immediately. Do not keep using a
damaged product or a damaged part. Danger of injuries.
CAUTION
The electrical net must have a Residual-Current Circuit Breaker
(IΔn=30mA) Thermal-Magnetic Circuit Breaker (Vn=230V) to protect
the device. Place the device in such a way that the power socket is
easily accessible.
it is forbidden to carry out any technical operation on the device that
is not recalled or described in these instructions for use.
It is forbidden to place the device in humid, dusty places or
environments subject to sudden temperature and humidity
variations.
It is forbidden to use any extension cable not authorized by the
manufacturer.
It is forbidden to use the device outdoors.
INSTRUCTIONS FOR USE
ANTARES | IFUANTARESEN00
This document is the property of
S4Optik.
Any reproduction, even partial, it is prohibited.
10/48
The device does not generate and does not receive any
electromagnetic interference if it is placed near other electrical
appliances. No preventive or corrective actions are required.
2.2 DEVICE IDENTIFICATION
2.2.1
REGISTRATION DATA IN THE MEDICAL DEVICES LIST
CND (national medical devices classification)
Repertoire number (progressive system number attributed to the device)
Market release date
The device registration data can be verified on the Ministero della
Salute website on this page:
Ministero della Salute - Ricerca dispositivi
2.2.2
DEVICE DATA PLATE
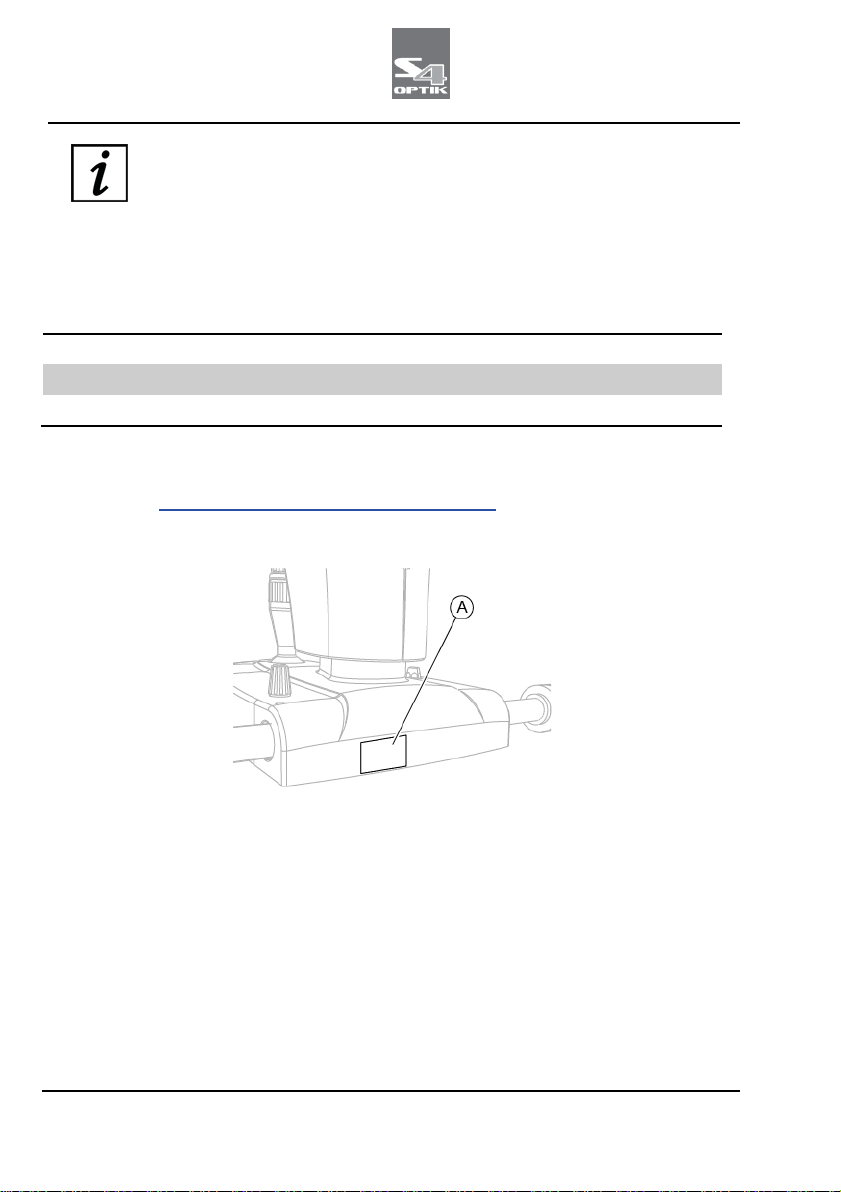
Fig 1 - Plates position
Pos
Description
A Device data plate

INSTRUCTIONS FOR USE
ANTARES | IFUANTARESEN00
This document is the property of
S4Optik.
Any reproduction, even partial, it is prohibited.
11/48
Fig 2 - Device data plate
2.2.3
POWER SUPPLY DATA PLATE
Fig 3 - Power supply PSP2402 data plate
2.3 INTENDED USE
ANTARES Corneal topographer is an electro-medical device used to
perform the corneal topography and for the diagnostic of the lacrimal
dysfunction.
The device has been designed for the capture and the elaboration of
an image of the cornea in the ophthalmic procedure.
Thanks to the high resolution color video camera, you can film "live"
the corneal surface and visualize it on the computer screen.

INSTRUCTIONS FOR USE
ANTARES | IFUANTARESEN00
This document is the property of
S4Optik.
Any reproduction, even partial, it is prohibited.
12/48
The device provides information on the curvature, the elevation and
the refractive power, and a great number of concise parameters for
the diagnostic and the follow-up of the corneal surface.
Corneal topography
The device provides information on elevation, curvature and dioptric
power of the anterior corneal surfaces over a diameter of 10 mm.
In addiction to the diagnostic of the corneal anterior surface, the most
common application fields are the simulation and the application of
corneal contact lenses, the analysis of the tear film dysfunctions, of the
meibomian glands, and the keratokonus screening.
Pupillography
The pupillography module is completely integrated with the
topography and allows to:
-
Perform the pupillometry measurement in scotopic light condition
in order to evaluate the maximal pupil extension and the optic
zone dimension that has to be set for a treatment.
-
Perform the pupillometry measurement in scotopic light condition
(0.04 lux).
-
Perform the pupillometry measurement in mesopic light condition
(4 lux).
-
Perform the pupillometry measurement in photopic light condition
(50 lux).
-
Perform the dynamic pupillometry measurement, starting form
400 lux and turning off the luminous source so that the pupil
dilates to its maximal extension.
-
Evaluate the pupillary decentralization with respect to the corneal
vertex for each of the conditions described above and the pupillary
center deviation during the dilatation.

INSTRUCTIONS FOR USE
ANTARES | IFUANTARESEN00
This document is the property of
S4Optik.
Any reproduction, even partial, it is prohibited.
13/48
Meibography
The device allows to analyze the Meibomian glands in a non-invasive
method. The miebography is performed through the infrared
illumination which enhance the contrast, magnifying the anatomic
structure of the glands without causing any discomfort to the patient.
Analysis of the tear film
The Placido's disk allows the advanced analysis of the tear film and the
evaluation of the NI-BUT (Non Invasive Break-up Time).
Videokeratoscopy
The device is provided with a luminous source with white light for the
capture of color images and videos.
The luminous source with cobalt blue light allows the analysis of the
clearance of rigid contact lenses in fluorescein.
Moreover, the device allows the change of magnification for the
capture of images with wide field of the tear meniscus and redness of
the eye.
Keratoconus screening
An efficient keratoconous screening system, clinically validated, based
on a self-learning system, provides suggestions on the risk underlining
the cases which have a greater possibility of complications.
Module for contact lenses application.
The module for the application of contact lenses application allows to
perform the simulation of rigid contact lenses thanks to a wide
database of models and international manufacturers.
More features of the device with the application software
The device, with the application software allows:
-
Guided manual capture
-
Management of the patients data and possibility to effectuate
personalized researches and statistics

INSTRUCTIONS FOR USE
ANTARES | IFUANTARESEN00
This document is the property of
S4Optik.
Any reproduction, even partial, it is prohibited.
14/48
-
Advanced editing system of the rings which allows to modify the
position of the edges in order to provide a proper reconstruction
even on distorted surfaces.
-
Availability of the following maps: sagittal curvature map,
tangential curvature map, elevation, refractive power, Gaussian
curvature map, corneal thickness.
-
Screens and summaries which allow to personalize the device
depending on the user:
-
Four maps summary
-
Single map screen
-
Keratoconus summary
-
Six maps summary
-
Advanced altimetry and Zernike summary
-
Corneal wavefront analysis with setting of the pupil, it
includes the maps of the most common aberrations
-
Corneal wavefront analysis with summary of visual quality
referred tho the anterior corneal face with PSF, Spot
Diagram, MTF and vision simulation for the analyzed
wavefront
-
Tools for the follow-up control with differential maps with 2 or 3
elements
-
Tools for the follow-up control with comparison between 4
different maps
-
A wide series of concise descriptors of the features of the cornea,
such as:
-
Autofit for the research of the best contact lens based on
the altimetric elevation measure of the cornea, on a
database of more than 50.000 lenses
-
Possibility to personalize the contact lens and to simulate its
application
-
Sim-K to simulate the measurement of an ophtalmoscope
with fixed targets (for the anterior surface)
-
Principal corneal meridians in the zones of 3 mm, 5 mm and
7 mm
-
Flatter and steeper hemimeridians in the zones of 3 mm, 5
mm and 7 mm

INSTRUCTIONS FOR USE
ANTARES | IFUANTARESEN00
This document is the property of
S4Optik.
Any reproduction, even partial, it is prohibited.
15/48
-
Peripheral degrees
-
Pupil decentralization, pupil diameter, and corneal diameter
size
-
Keratorefractive indices calculated in the pupil area for an
evaluation of the patient's visual quality
-
Keratoconus screening index for diagnosis and follow-up
The device must be used only by practitioners, within the limits of the
law and the regulations for the exercise of the profession.
The device must be used in combination with a PC and the application
software Phoenix.
System minimum requirements
-
PC: 4 GB RAM - Video Card 1 GB RAM (not shared) resolution 1024
x 768 pixels
-
Operating system: Windows XP, Windows 7 and Windows 10
(32/64 bit).
Read the instructions for use of the application software.
The PC must be compliant to the norm IEC 60950-1 Information
technology equipment - Safety - Part 1: General requirements.
If the PC is installed in the patient area it is necessary to install an
isolation electrical supply compliant with the directive IEC 606011:2005 + A1:2012- "Medical electrical equipment - Part 1: General
requirements for basic safety and essential performance".
It is possible to connect other accessories to the PC (printer, modem,
scanner, etc) through the analogical or digital interfaces.
The accessories (printer, modem, scanner, etc) must be installed
outside the patient area.

INSTRUCTIONS FOR USE
ANTARES | IFUANTARESEN00
This document is the property of
S4Optik.
Any reproduction, even partial, it is prohibited.
16/48
The accessories must be compliant to the norm IEC 60950-1
Information technology equipment - Safety - Part 1: General
requirements.
If the accessories are installed in the patient area it is necessary to
install an isolation electrical supply compliant with the directive IEC
60601-1:2005 + A1:2102 - "Medical electrical equipment - Part 1:
General requirements for basic safety and essential performance".
Patient area: any volume in which intentional or unintentional
contact can occur between patient and parts of the system or
between patient and other persons touching parts of the system.
Fig 4 - Patient area

INSTRUCTIONS FOR USE
ANTARES | IFUANTARESEN00
This document is the property of
S4Optik.
Any reproduction, even partial, it is prohibited.
17/48
2.4 MEDICAL DEVICES CLASSIFICATION
Technical data
Value
Classification in compliance with the
attached IX to the Directive 93/42/EEC
and subsequent modifications
Class Im
2.5 MEDICAL ELECTRICAL DEVICES CLASSIFICATION
Classification in compliance with the technical specification EN 606011:2005 + A1:2012
Technical data
Value
Type of protection against the direct
and indirect contacts
Class II
Applied parts
Type B
Protection degree against humidity
IP20 (no protection against liquid
infiltration)
Sterilization or disinfection method
This device can be disinfected
Protection degree in presence of
anesthetics or inflammable detergents
No protection
Electrical connection degree between
device and patient
Appliances with part applied on the
patient
Use conditions
Continuous functioning

INSTRUCTIONS FOR USE
ANTARES | IFUANTARESEN00
This document is the property of
S4Optik.
Any reproduction, even partial, it is prohibited.
18/48
2.6 ENVIRONMENTAL CONDITIONS
Phase
Technical data
Min
Max
Transport
Temperature
-40°C
+70°C
Atmospheric pressure
500 hPa
1060 hPa
Relative humidity
10%
95%
Storage
Temperature
-10°C
+55°C
Atmospheric pressure
700 hPa
1060 hPa Relative humidity
10%
95%
Use
Temperature
+10°C
+35°C Atmospheric pressure
800 hPa
1060 hPa Relative humidity
30%
90%
Phase
Technical data
Min
Vibration
Sinusoidal
10 Hz to 500 Hz, 0.5g
Shock
30g duration 6ms
Bumb
10g duration 6ms
CAUTION
Danger of device damages. During transport and storage, the
device can be exposed to the environmental conditions for a
maximum period of 15 weeks, only if kept in the original packaging.

INSTRUCTIONS FOR USE
ANTARES | IFUANTARESEN00
This document is the property of
S4Optik.
Any reproduction, even partial, it is prohibited.
19/48
2.7 DISPOSAL AT THE END THE USEFUL LIFE
Instruction for disposal of product correctly according to European
Directive 2012/19/EU, and 2011/65/EU about the reduction of use
of dangerous substances in the electrical and electronic
equipments, and waste disposal.
At the end of its useful life, the device must not be disposed of as
urban waste. The device can be delivered to the appropriate
separate waste collection centers set up by municipal
administrations or to retailers that offer this service. Separately
disposing an electrical device prevents possible negative
consequences for the environment and health caused by its
improper disposal, and lets the materials it is made of to be
recycled so as to achieve a significant savings of energy and
resources. On the label of the device there is the symbol of the of
the crossed-out wheeled bin. The graphic symbol of the crossed-out
wheeled bin, indicates the obligation to collect and dispose
separately the electrical and electronic equipment at the end of
their useful life.
The user has to consider the effects potentially dangerous for the
environment and the human health originating from an improper
disposal of the whole device or its parts.
In case the user wishes to dispose of the device used at the end of its
useful life, the Manufacturer facilitates the possibility of its reuse and
the recovery and recycling of the materials contained therein. This to
prevent the release of hazardous substances into the environment and
to promote conservation of natural resources. Before disposing the
device, it is necessary to take into consideration the European and
national regulations that order what follows:
- not to dispose as urban waste but collect it separately and address
to a firm specialized in the disposal of electrical and electronic
equipment or to the local administration in charge for waste
collection.

INSTRUCTIONS FOR USE
ANTARES | IFUANTARESEN00
This document is the property of
S4Optik.
Any reproduction, even partial, it is prohibited.
20/48
-
in the event that a new device is purchased from the same
Manufacturer to replace an old one placed on the market before
13 August 2005, equivalent and with the same functions of the new
device, the Distributor or Manufacturer are legally required to
collect the old device.
-
if the user decides to dispose a used device, put on the market
after the 13th August 2005, the Distributor or the Manufacturer
have to collect it.
-
the Manufacturer takes care, by joining a consortium for the
technological devices waste, of the treatment and the recycling of
the used device by paying its costs.
The Manufacturer is available to give the user all the information
about the dangerous substances contained in the device, and on the
recycling modalities of those substances and about the possibility of
a reuse of the used device.
Strict sanctions for transgressors are provided for by law.
For specific information about the disposal in other countries than
Italy, contact the local Dealer.

INSTRUCTIONS FOR USE
ANTARES | IFUANTARESEN00
This document is the property of
S4Optik.
Any reproduction, even partial, it is prohibited.
21/48
2.8 MANUFACTURER DECLARATIONS
2.8.1
ELECTROMAGNETIC EMISSIONS
The device is designed to be used in a room with the following
electromagnetic characteristics:
Emission test
Compliance
Electromagnetic environment
Radio frequency emission.
CISPR 11
Group 1
The device uses radio frequency energy
only for its inner functioning. The radio
frequency emissions of the device are
very low and should not cause
interferences with the near appliances.
Radio frequency emission.
CISPR 11
Class B
The device can be used in all the
environments, included the domestic
environment. The device can be
connected directly to a low tension
power supply net as there is in the
housing units.
Harmonic emissions. IEC
61000-3-2
Class A
The device can be used in all the
environments, included the domestic
environment. The device can be
connected directly to a low tension
power supply net as there is in the
housing units.
Limitation of voltage
changes, voltage
fluctuations and flicker.
IEC 61000-3-3
Compliant
The device can be used in all the
environments, included the domestic
environment. The device can be
connected directly to a low tension
power supply net as there is in the
housing units.

INSTRUCTIONS FOR USE
ANTARES | IFUANTARESEN00
This document is the property of
S4Optik.
Any reproduction, even partial, it is prohibited.
22/48
Immunity test
IEC 60601-1-2
test level
Conformity
level
Electromagnetic environment
Electrostatic
discharge. IEC
61000-4-2
±6 kV contact.
±8 kV air
±6 kV contact.
±8 kV air
Floors should be wood, concrete
or ceramic tile. If the floors are
covered with synthetic material
the relative humidity should be
at least 30%.
Electrical fast
transient/burst.
IEC 61000-4-4
±2 kV for power
supply lines. ±1
kV for
input/output
lines
±2 kV for
power supply
lines. Nonapplicable
Mains power quality shall be
that of a typical commercial or
hospital environment.
Surge IEC 610004-5
±1 kV
differential
mode. ±2 kV
common mode
±1 kV
differential
mode. ±2 kV
common
mode
Mains power quality shall be
that of a typical commercial or
hospital environment.
Voltage dips.
Short
interruptions and
voltage variations
on power supply
input lines. IEC
61000-4-11
<5% Un for 0.5
cycles. 40% Un
for 5 cycles.
70% Un for 25
cycles. <5% Un
for 5 s
<5% Un for
0.5 cycles.
40% Un for 5
cycles. 70%
Un for 25
cycles. <5%
Un for 5 s
Mains power quality shall be
that of a typical commercial or
hospital environment. If the user
of the device requires continued
operation during power mains
interruptions, it is
recommended that the device is
powered from an uninterrupted
power supply or battery.
Power frequency
(50/60Hz)
magnetic fields.
IEC 61000-4-8
3 A/m
3 A/m
Power frequency of the
magnetic fields should be that of
a typical commercial or hospital
environment.

This document is the property of
S4Optik.
Any reproduction, even partial, it is prohibited.
23/48
INSTRUCTIONS FOR USE
ANTARES | IFUANTARESEN00
Immunity test
IEC 60601-1-2
test level
Conformity
level
Electromagnetic environment
RF conduced IEC
61000-4-6
RF conduced IEC
61000-4-3
3 Vrms
from 150 kHz to
80 MHz
3 V/m
from 80 MHz to
2.5 GHz
3 Vrms
3 V/m
(1)
(1) Portable and mobile RF communication equipment should be used
no closer to any part of the device, including cables, than the
recommended separation distance calculated from the equation
applicable to the frequency of the transmitter.
d=1,167*sqrt (P)
d=1,167*sqrt (P) 80 MHz to 800 MHz
d=2,333*sqrt (P) 800 MHz to 2,5 GHz
P: is the maximum output power rating of the transmitter in watts (W)
according to the transmitter Manufacturer.
d: is the recommended distance in metres (m) at which the portable
radio frequency (RF) appliances can be used.
Field strengths from fixed RF transmitters, as determined by an
electromagnetic site survey, should be less than the compliance level
in each frequency range. Interference may occur in the vicinity of
equipment marked with the following symbol:
(Un) is the AC mains voltage prior to application of the test level.
At 80 MHz and 800 MHz, the higher frequency range applies. These
guidelines may not apply in all situations. Electromagnetic
propagation is affected by absorption and reflection from structures,
objects and people.

This document is the property of
S4Optik.
Any reproduction, even partial, it is prohibited.
24/48
INSTRUCTIONS FOR USE
ANTARES | IFUANTARESEN00
3 DEVICE DESCRIPTION
3.1 PROVISION DESCRIPTION
Fig 5 - Provision description

This document is the property of
S4Optik.
Any reproduction, even partial, it is prohibited.
25/48
INSTRUCTIONS FOR USE
ANTARES | IFUANTARESEN00
Pos Denomination
Description
A Chin rest
Adjustable height Adjustable distance
between chin and forehead
B Protection carter
Protection against accidental hand
crushing.
C Power supply
D Device ANTARES
Composed by a camera unit equipped
with one or several micro video
cameras for capturing the images. USB
cable for connection between device
and computer.
E Application
software
Application software for image capture
and device management.
F Protective cover
optional
Place on the device when it is not in
use to protect it from dust.
G Set of hex keys
with screws
optional
H Package of paper
for chin cup
I Electric table
optional
Adjustable electric support surface
with one or two columns. Drawer and
auxiliary sockets with fairlead.
J Calibration tool
K 2 fuses
L Isolation
transformer
optional
230V/230V for the use of the non
electro-medical appliances in the
patient area.
For the list of accessories and available models contact the
Manufacturer or the local Distributor.
This document is the property of
S4Optik.
Any reproduction, even partial, it is prohibited.
26/48
INSTRUCTIONS FOR USE
ANTARES | IFUANTARESEN00
3.1.1
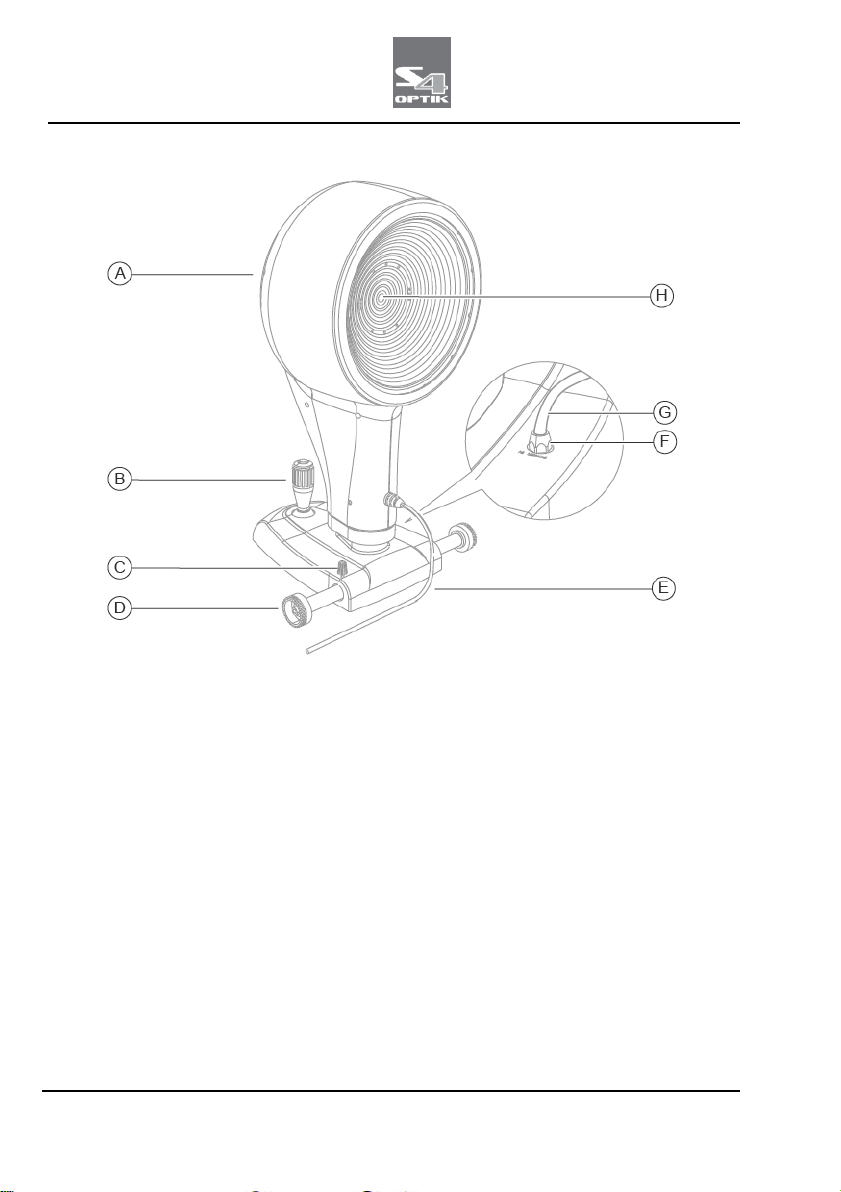
DEVICE ANTARES
Fig 6 - Device ANTARES
Pos
Description
A Device ANTARES
B Joystick
C Device blocking knob
D Cogged wheels
E USB connection cable between device and computer
F Power supply connector
G Device power supply cable
H Shooting channel

This document is the property of
S4Optik.
Any reproduction, even partial, it is prohibited.
27/48
INSTRUCTIONS FOR USE
ANTARES | IFUANTARESEN00
3.1.2
POWER SUPPLY
Fig 7 - Power supply
Pos
Description
A Data plate
B Power supply
C Power ON control light
D ON/OFF switch
E Power supply out connector
F Power supply mains connector
G Power supply cable
H Device power supply cable

This document is the property of
S4Optik.
Any reproduction, even partial, it is prohibited.
28/48
INSTRUCTIONS FOR USE
ANTARES | IFUANTARESEN00
3.1.3
CHIN REST
Fig 8 - Chin rest
Pos
Description
A Chin rest support
B Handle
C Knob
D Chin cup
E Forehead rest
F Chin rest structure

This document is the property of
S4Optik.
Any reproduction, even partial, it is prohibited.
29/48
INSTRUCTIONS FOR USE
ANTARES | IFUANTARESEN00
3.1.4
ELECTRIC TABLE (OPTIONAL)
Different table models are available accordingly to the client's choice.
The electric table is composed by a support surface on which are
installed the cogged guides for the device housing. The table has one
or two telescopic columns, motorized, that allow to adjust the height
of the support plane.
Fig 9 - One column table
Fig 10 - Two columns table
Read the instructions for use of the electric table.
3.2 TECHNICAL DATA
Technical data
Value
Data transfer
USB3
Mains power
External power supply 24 VCC. In: 100240Vac - 50/60Hz - 0.9-05A Out: 24Vdc 40W
Net cable
with C14 socket
Dimensions (Height x Length x Depth)
515 x 315 x 255 mm
Weight
6.5 kg
Chin rest stroke
70 mm ±1

This document is the property of
S4Optik.
Any reproduction, even partial, it is prohibited.
30/48
INSTRUCTIONS FOR USE
ANTARES | IFUANTARESEN00
Technical data
Value
Minimum height of the chin cup from
the working plan
24 cm
Base movement (x, y, z)
105 x 110 x 30 mm
Working distance
74 mm
Light sources
Technical data
Value
Placido
Led @450-650 nm
Fluorescein stimulation
Led @470 nm
Pupillography and meibography
Led @875 nm
Topography
Technical data
Value
Placido's disk
24
Measured points
6144
Topographic covering (in 43D)
10 mm
Dioptric range
from 1D to 100D
Accuracy
Class A according to UNI EN ISO 199802012

This document is the property of
S4Optik.
Any reproduction, even partial, it is prohibited.
31/48
INSTRUCTIONS FOR USE
ANTARES | IFUANTARESEN00
4 DEVICE USE
4.1 HOW TO INSTALL THE DEVICE
CAUTION
Danger of device fall. The electric table must be installed on a
horizontal and stable surface.
1 Place the electric table in the room. The electric table must be
lifted by two people.
2 If present, block the table wheels. Lower the lever of the
brake.
3 Place the power supply under the support plane. Screw the
screws in the four holes.
Fig 11 - Table placement
Fig 12 - Power supply placement
4 Place the device on the support surface on the cogged guides.
5 Block the two protection carters to the cogged wheels on the
support plane.

This document is the property of
S4Optik.
Any reproduction, even partial, it is prohibited.
32/48
INSTRUCTIONS FOR USE
ANTARES | IFUANTARESEN00
Fig 13 - How to place the device
Fig 14 - Protection carters placement
6 Install the chin rest. Under the support plane, there are two
screws to block the chin rest support to the support plane.
7 Carry out the electrical connection between the several
components.
Fig 15 - Chin rest placement

This document is the property of
S4Optik.
Any reproduction, even partial, it is prohibited.
33/48
INSTRUCTIONS FOR USE
ANTARES | IFUANTARESEN00
4.2 HOW TO CONNECT THE DEVICE
Fig 16 - Device connection

This document is the property of
S4Optik.
Any reproduction, even partial, it is prohibited.
34/48
INSTRUCTIONS FOR USE
ANTARES | IFUANTARESEN00
Pos Denomination
A USB connection cable between device and PC
B Power cable for the connection of the electric table with the power
supply
C Power cable for the connection of the electric table with the power
supply
D Power cable for the connection between the power supply and the device

This document is the property of
S4Optik.
Any reproduction, even partial, it is prohibited.
35/48
INSTRUCTIONS FOR USE
ANTARES | IFUANTARESEN00
4.3 HOW TO PLACE THE ELECTRIC CABLES
CAUTION
Danger of device fall. Do not leave free cables which can represent
an obstacle or a danger for the patient or the operator.
CAUTION
Danger of stumbling and falling. Do not let the power cord or the
connection cables free in a place where people could walk.
CAUTION
Electric shock risk. Do not leave the power supply cables in contact
with sharp corners or objects. Collect and attach always the power
supply cables.
It is forbidden to use any extension cable not authorized by the
manufacturer.
Fig 17 - Power sockets position
Fig 18 - Wireway

This document is the property of
S4Optik.
Any reproduction, even partial, it is prohibited.
36/48
INSTRUCTIONS FOR USE
ANTARES | IFUANTARESEN00
The power socket is on the column of the electric table, below, and it
has to be used for the connection with the mains power. One of the
power sockets on the column of the electric table, on top, is
dedicated to the device power supply. Block the cables under the
support surface with the cable rivets. If you have it, place the cables
in the wireway under the support plane.
4.4 HOW TO TURN ON THE DEVICE
1 Turn on the PC.
2 Push the activation switch of the power supply on ON.
3 Launch the application software Phoenix.
4 Wait until the main screen of the application software is
shown.
5 Click on NEW PATIENT and enter his personal data. If the
patient is already present in the database, you can
automatically search the surname in the surname command
line.
6 A new examination will be created automatically.
7 Select the tool to use.
8 The image capture screen will open. Now it will be possible to
capture the image.
Creation of a new exam
-
Click on the button NEW EXAMINATION.
-
Select the tool to use.
-
The image capture screen will open. Now it will be possible to
capture the image.

This document is the property of
S4Optik.
Any reproduction, even partial, it is prohibited.
37/48
INSTRUCTIONS FOR USE
ANTARES | IFUANTARESEN00
4.5 ADJUST THE CHIN REST
1 Tell the patient to take a seat.
2 Ask the patient to put the chin on the chin cup and the
forehead against the forehead rest.
3 Verify the correct eyes position respectively to the shooting
channel.
Fig 19 - Patient position on the chin rest

This document is the property of
S4Optik.
Any reproduction, even partial, it is prohibited.
38/48
INSTRUCTIONS FOR USE
ANTARES | IFUANTARESEN00
4 Lift or lower the chin cup as needed.
Fig 20 - Knob rotation
Fig 21 - Chin cup placement

This document is the property of
S4Optik.
Any reproduction, even partial, it is prohibited.
39/48
INSTRUCTIONS FOR USE
ANTARES | IFUANTARESEN00
4.6 HOW TO CAPTURE THE IMAGE
1 Turn the joystick and align the device with the patient's eye.
2 Move towards the eye with the tool. Keep centered the
reflection of the corneal vertex.
3 Push the button on the joystick. Perform some micro
movements back and forward with the joystick and keep the
best image alignment. The image will be automatically
captured.
Fig 22 - How to place the device
Fig 23 - Distance from the patient
4 Double click on the captured image to process and visualize
the elaboration on the computer screen.
Fig 24 - Image capture

This document is the property of
S4Optik.
Any reproduction, even partial, it is prohibited.
40/48
INSTRUCTIONS FOR USE
ANTARES | IFUANTARESEN00
Refer to the application software instructions for the image
managing in the database.

This document is the property of
S4Optik.
Any reproduction, even partial, it is prohibited.
41/48
INSTRUCTIONS FOR USE
ANTARES | IFUANTARESEN00
4.7 HOW TO CHANGE THE PAPER FOR CHIN CUP
At the end of each exam remove the paper for chin cup in order to
always have a new and hygienic one for the next patient.
This device is provided with a package of paper for chin cup. When you
use the last paper change the package.
1 Extract the two plastic rivets
2 Place the new package of paper for chin cup
3 Insert the plastic rivets in the holes of the package and in the
holes of the chin cup.
Fig 25 - How to change the papers for chin cup
To order the spare package see the code in the "Spare parts list page
44"

This document is the property of
S4Optik.
Any reproduction, even partial, it is prohibited.
42/48
INSTRUCTIONS FOR USE
ANTARES | IFUANTARESEN00
4.8 HOW TO TURN OFF THE DEVICE
CAUTION
Do not turn off the computer and do not disconnect the connection
cable between the computer and the device when the program is in
use.
1 Immobilize the device. Turn the locking knob.
2 Exit the images management systems program. Turn off the
computer
3 Push the activation switch of the power supply on OFF.
4 Place the protective cover on the device to prevent dust to fall
on the device.
Fig 26 - Device blocking

This document is the property of
S4Optik.
Any reproduction, even partial, it is prohibited.
43/48
INSTRUCTIONS FOR USE
ANTARES | IFUANTARESEN00
5 ORDINARY MAINTENANCE
5.1 SAFETY WARNINGS
DANGER
Electric shock danger. Unplug the power cable from the mains
socket before disinfecting the device and before any maintenance
operation.
CAUTION
The device does not contain any piece that requires the user's
intervention. Do not dismantle any part of the device.
it is forbidden to carry out any maintenance operation on the device
that is not recalled in instructions for use.
In case of operational faults or malfunctions or for every operation
not mentioned in the instructions for use, there is the obligation to
address an authorized technical assistance center of the device
Manufacturer.
5.2 DEVICE CLEANING
Clean the external parts of the device using a damp non-abrasive cloth
to avoid damaging the material.
CAUTION
Danger of material damages. Do not use solvents or diluent to clean
the device.

This document is the property of
S4Optik.
Any reproduction, even partial, it is prohibited.
44/48
INSTRUCTIONS FOR USE
ANTARES | IFUANTARESEN00
5.3 SPARE PARTS AND ACCESSORIES LIST
Code
Description
30010071D3F
Power supply cable
10101300
Isolation transformer 230V/230V. Power supply cable
800 VA (maximum charge)
4014020
Package of paper for chin cup (50 pieces)
4013090
Protective cover
10070524
Table top 45x90 mm
10070144
Electric support plane with one column (230 V, 50 Hz)
33071095
Power supply cable for electric support (95 cm)
103103900
PSP2402 input 100-240 V AC 50/60 Hz max 0,9 A
output 24 VDC 2 A
300409135
Power supply cable 1,5 m
300409136
Power supply cable 5 m
100104210
Concave calibration tools for contact lenses.
100104250
Calibration set for corneal maps
100104700
Chin rest for the table top
3020150
USB 3.0 Cable 5 m

This document is the property of
S4Optik.
Any reproduction, even partial, it is prohibited.
45/48
INSTRUCTIONS FOR USE
ANTARES | IFUANTARESEN00
5.4 TROUBLESHOOTING
Inconvenient
Cause
Solution
Note
The device does
not switch on
Power cable not
connected with the
power supply
Connect the power
cable of the device
to the power supply
Press the switch on
of the device.
If the device is
powered trough the
auxiliary power
supply of the table,
check the
connection of the
table to the power
line. Check the
functioning of the
table fuses.
The PC does not
start
Power cable not
connected with the
power supply
Connect the power
cable to the power
supply. Push the
button of the
power supply on
ON. Replace the PC.
Make sure the
power outlet of the
room works
properly.
PC Operating
system does not
start
Hard Disk failure.
Spoiled operating
system
Replace the Hard
Disk. Reinstall the
operating system.
Replace the PC.
Make sure the New
PC features are
equivalent to those
required by the
device.
The application
software Phoenix
does not start
Hard Disk failure.
The anti virus
software impedes
the starting of the
application
software Phoenix.
Spoiled operating
system The
application
software Phoenix
does not work
properly.
Replace the Hard
Disk. Check the
settings of the anti
virus software.
Reinstall the
operating system.
Reinstall the
application
software Phoenix.
Contact the
Customers Service.
The installation of
the application
software Phoenix
needs the
administrator
privileges.

This document is the property of
S4Optik.
Any reproduction, even partial, it is prohibited.
46/48
INSTRUCTIONS FOR USE
ANTARES | IFUANTARESEN00
Inconvenient
Cause
Solution
Note
The application
software Phoenix
does not work
properly.
The connection
cable between
device and PC does
not work properly.
The anti virus
software interferes
with the drivers of
the application
software Phoenix.
The application
software Phoenix is
installed as local
user
Unplug and plug in
again the
connection cable
between device and
PC. Replace the
connection cable
between device and
PC. Uninstall the
anti virus software.
Reinstall the
application
software Phoenix.
The installation of
the application
software Phoenix
needs the
administrator
privileges.
The mouse of the
PC does not work
Connection cable
with the PC
disconnected.
Mouse switch in
position OFF. The
mouse batteries are
down (only for
wireless mouse)
Check that the
mouse connection
cable properly fit in
USB port. Switch
the mouse button
in position ON.
Replace mouse
batteries (only for
wireless mouse).
From the control
panel of the PC,
check that there are
no devices conflicts.

This document is the property of
S4Optik.
Any reproduction, even partial, it is prohibited.
47/48
INSTRUCTIONS FOR USE
ANTARES | IFUANTARESEN00
Inconvenient
Cause
Solution
Note
Check that the
Connection cable
keyboard
with the PC
connection cable
The keyboard of
the PC does not
work
disconnected.
Keyboard switch in
position OFF. The
keyboard batteries
properly fit in USB
port. Switch the
keyboard button in
position ON.
From the control
panel of the PC,
check that there are
no devices conflicts.
are down (only for
Replace keyboard
wireless keyboard)
batteries (only for
wireless keyboard).
Verify that in the
configuration
screen of the
The images can't be
saved in the
database
The database is not
connected with the
application
software Phoenix.
Power connection
absent. The USB
cable does not work
database is
specified the
correct path to the
“phoenix.mdb” file.
Restore the
connection to the
database file. Check
the functioning of
Regularly verify the
connections with
the data net.
the net connection.
Replace the USB
cable.
Ask the patient to
Failed image
capture
The patient moved
or closed the eyes
during the capture
keep the eyes open,
look the fixation
light and not to
move the eyes.

This document is the property of
S4Optik.
Any reproduction, even partial, it is prohibited.
48/48
INSTRUCTIONS FOR USE
ANTARES | IFUANTARESEN00
Inconvenient
Cause
Solution
Note
Poor image quality
from Placido's disk
The tear film is not
well distributed on
the cornea surface
(dry eye)
Ask the patient to
close and open the
eyes.
Failed image focus
from Placido's disk
Presence of dust of
grease on the
optical parts of the
device.
Clean the surface of
optical parts with a
soft cloth.
Make sure the
patient does not
touch the optical
parts.
Missing installation
of black sticker
below the base of
the device. Fault of
positioning
detector
Some colors and
material of the
table top may not
Missing
reflect the infrared
acknowledgment
Install the black
light. Move a white
of eye position left
sticker below the
paper below the
/ right position by
base of the device.
base of the device
the device
to check the
functioning of the
positioning
detector.
The joystick plastic
Before starting the
exam check that
the device blocking
knob is loosened.
protection has not
Remove the joystick
Device movement
been removed from
plastic protection
difficulties (ahead,
the base during the
from the base.
back, left, right)
installation. Device
Loosen the device
blocking knob is
blocking knob.
fastened


4/174
S4OPTIK, LLC
250 Cooper Ave., Suite 100
Tonawanda, NY
14150
+1-888-224-6012
+1 800 313 8696
info@s4optik.com
ANTARES | IFUANTARESEN00 - 07/2018
 Loading...
Loading...