Rossmax SB200 Instructions For Use Manual
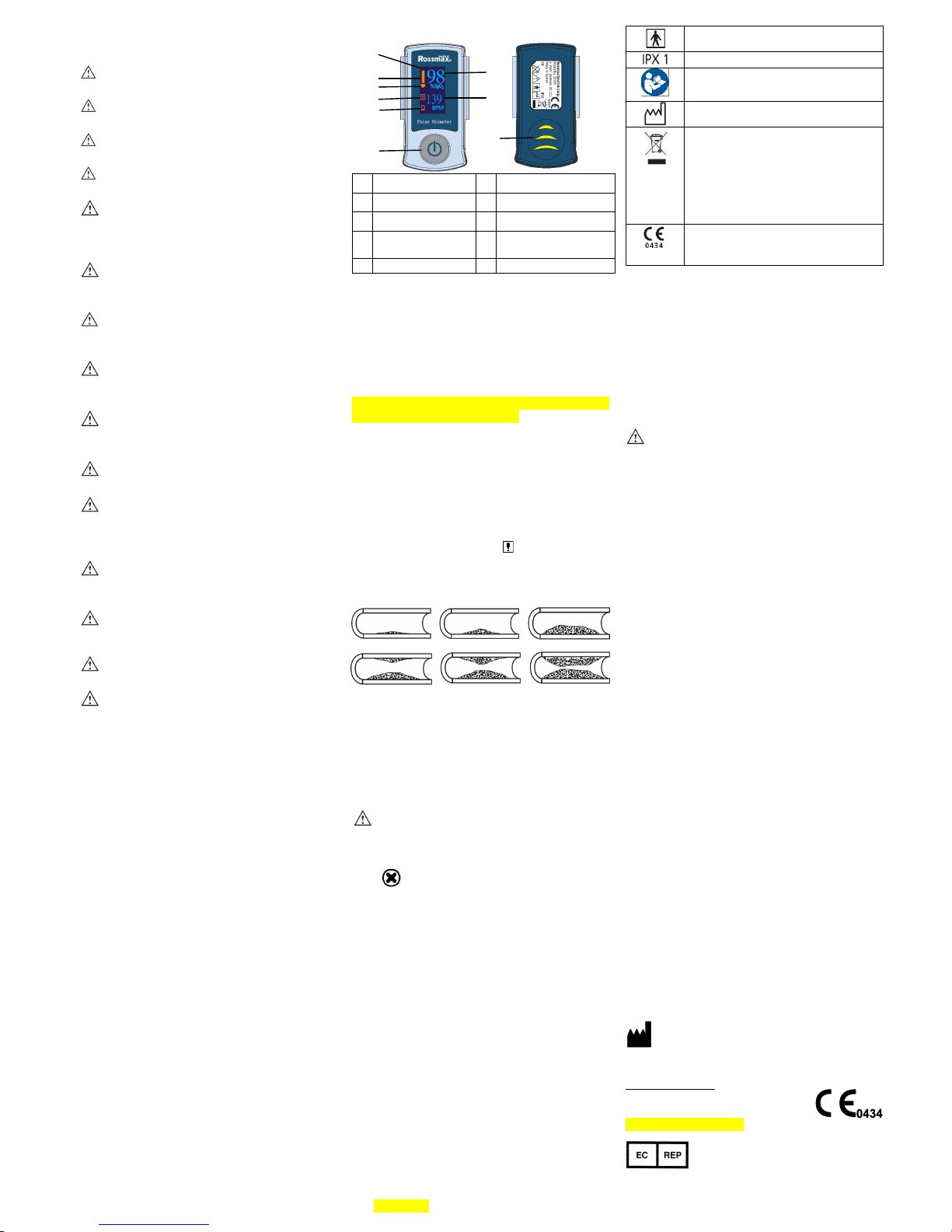
SB200 Fingertip Pulse Oximeter
Instruction for Use
1. Safety information
WARNING: The SpO2 device is to be operated by
trained personnel only.
WARNING: Do not use the SpO2 device in the presence
of flammable anesthetics or gas to prevent explosion hazard.
WARNING: Do not use the SpO2 device in the
Magnetic Resonance Imaging (MRI) ambience.
WARNING: The SpO
2
readings and pulse signals can be
affected by the conditions of ambience and patient.
WARNING: Do not open up the SpO2 device except for
the battery cover. The SpO2 device is without any
user-serviceable part inside and only qualified service
personnel can perform maintenance service.
WARNING: Do not expose the SpO2 device to
extreme moisture (such as rain) to ensure accurate
performance and device safety.
WARNING: If the accuracy of measurement by
the SpO2 device is uncertain, check the patient’s vital
signs by alternate means.
Warning: This device is intended only as an
adjunct in patient assessment. It must be used in
conjunction with clinical signs and symptoms.
WARNING: Reposition the device at least once
every 4 hours to allow the patient’s skin to breath and
to check patient’s condition regularly.
WARNING: The SpO2 device is not intended for
the use of neonatal.
WARNING: Do not use the SpO2 device with
other devices (such as, the cuff of blood pressure
monitor) that may interfere with blood flow and cause
inaccurate measurement.
Warning: The SpO2 device will be affected by
electromagnetic interference or strong ambient light
source during operation.
WARNING: User should stay calm and position finger
stably. The accuracy of measurement take n right after exercise
or during hand shaking could be compromised.
WARNING: SpO2 should not be applied to a body part
other than the finger or to a wounded body part.
Warning: Please remove the batteries from the
battery compartment if the device will not be used for
a long period of time.
1.
Introduction
The Fingertip pulse oximeter is to spot-check oxygen
saturation in blood (SpO2) and pulse rate. The pulse
oximeter is used on adults and pediatric at hospital,
clinics, and/or home.
The device contains a dual light source (Red LED and
Infrared red LED) and a photodetector. Bone, tissue,
pigmentation, and venous vessels normally absorb a
constant amount of light over time. The arteriolar bed
normally pulsates and absorbs variable amounts of
light during the pulsations of blood flow. The ratio of
light absorbed is translated in an oxygen saturation
measurement (SpO2). Because a measurement of SpO2
is dependent on light from the device, excessive
ambient light can interfere with this measurement.
The wavelength of red LED is 660nm and Infrared
LED is 905/880nm with maximum optical output
power of 4mW.
2. Features
Measure and display reliable SpO2 value and heart
rate and vascular age analysis.
Single turn-on key for easy operation.
Bright Organic LED display.
Light, compact, and portable.
Battery power is for a continuing use of 16 hours.
Two “AAA” Alkaline batteries for power supply.
Device will be off automatically after 8 seconds in
idling.
Visual alarm and audio alarm.
3. Product introduction
Top View Bottom View
1 OLED display 6 Power On/Off Button
2 Pulse strength 7 Battery compartment
3 Pulse search icon 8 Pulse rate icon
4 Alarm icon or
vascular age
9 SpO2 icon
5 Battery indicator
4. Operation
Open up battery compartment cover carefully and then
install two “AAA” Alkaline batteries according to the
(+/-) polarity.
Press the “power switch” key for 1 second to activate
the device. Information of version appears on the
screen. The device is then in the “self-test” mode with
the software version shown. The measurement starts at
the completion of the “self-test.”
Insert your finger into the device. For best results use
the middle finger on your left hand.
The pulse bar with “-“icon on the screen up and down
means the measurement result will be ready soon.
The readings of oxygen saturation, pulse rate, and
pulse strength will appear on screen in 8 seconds
average. The readings and icon of vascular age will
appear on the screen in 30 seconds or maximum 2
minutes. Pressing “power switch” is to change the
viewing direction. While SpO2 exceeds the Min.
threshold (90); the device will sound the alarm with
two beeps and alarm icon flickering. If the
measurement fails, the icon “- -“will appear on screen.
Reading flashing while SpO2 is low. While LED or
sensor is malfunctioning, displaying “Er” signal.
Vascular age is classified into 6 levels as follows:
Level 1: Artery and blood circulation in excellent condition
Level 2: Artery and blood circulation in good condition
Level 3: Artery and blood circulation in above average
Condition
Level 4: Artery and blood circulation in average condition
Level 5: Artery and blood circulation in below average
condition
Level 6: Artery and blood circulation in poor condition
Warnings: The classification of artery and blood
circulation condition is for reference only. Please consult your
physician for further advice.
When the vessel elasticity cannot be measured, it will
show
The device will turn itself off automatically after
8-second idling. While the battery power is low, the
battery indicator icon will flash twice per second.
Please replace the batteries as soon as possible or the
device will be off automatically in 30 seconds.
5. Specifications
6.1 Performance
Scope of measurement: SpO2: 35% - 99%
Pulse rate: 30-250 bpm (beats per minute)
Accuracy: SpO2:70%-99%:±2%,35%-69%:
unspecified
Pulse rate: 30-250 ± 3 bpm
6.2
Electrical specifications
Battery (2 “AAA” Alkaline batteries)
Battery capability: Can be used for 16 hours
continuously depending on the type of battery used
.
6.3
Environmental conditions
Operating temperature 5℃ - 40℃ (41℉ – 104℉)
Storage temperature: -20℃ - 70℃ (-4℉ – 158℉)
Relative humidity: 15% -95% (no condensing)
6.4
Physical characteristics
Weight: 37g (excluding battery) Size: 63.5 x 34 x 35 mm
6.5 Standards
IEC60601-1-2, Class B, IEC60601-1, Type BF,
ISO 80601-2-61
6.6
Markings
Type BF (Body Floating)
Drip proof
ATTENTION
Read instructions for use before use.
Date of manufacture
Used batteries should not be disposed of
in the household rubbish. Batteries
should be deposited at a collection point
for used batteries. At the end of its life,
the appliance should not be disposed of
in household rubbish. Enquire about the
options for environment-friendly and
appropriate disposal. Take into account
local regulations.
Complies with the European Medical
Device Directive 93/42/EEC as amended
by 2007/47/EC
7. Problem shooting and maintenance
7.1 Dysfunction and resolution
Low battery-Please replace the battery
Switch On failure-
Check the power of battery
Check the placement of battery
Return to manufacturer for calibration
7.2
Cleaning
Surface cleanings is by using a soft cloth dampened
with either a commercial, non-abrasive cleaner or a
solution of 70% isopropyl alcohol in water, and lightly
wiping the surfaces of the oximeter.
Please switch off pulse oximeter before cleaning.
Clean the LED and photo-sensor with moist cloth or
cotton ball and alcohol gently.
The aforementioned general cleaning process is not
for infection prevention. Please contact the specialist
for the process of contagious infection.
7.3
Disposal
Used batteries should not be disposed of in the
household rubbish. Batteries should be deposited at a
collection point for used batteries.
At the end of its life, the appliance should not be
disposed of in household rubbish. Enquire about the
options for environment-friendly and appropriate
disposal. Take into account local regulations.
7.4 Electromagnetic interference
Caution: This device has been tested and found to
comply with the limits for medical devices to the IEC
60601-1-2 and MDD 93/42/EEC as amended by
2007/47/EC. These limits are designed to provide
reasonable protection against harmful interference in a
typical medical installation. However, because of the
proliferation of radio-frequency transmitting
equipment and other sources of electrical noise in
healthcare environments (for example, electrosurgical
units, cellular phones, mobile two-way radios,
electrical appliances, and high-definition television), it
is possible that high levels of such interference due to
close proximity or strength of a source may result in
disruption of performance of this device.
This Fingertip pulse oximeter is not designed for use
in environments in which the pulse can be obscured by
electromagnetic interference. During such
interference, measurements may seem inappropriate or
the monitor may not seem to operate correctly.
6. Warranty
The company warrants pulse oximeter at the time of
its original purchase and for the subsequence time
period of one year.
The warranty does not cover the followings:
The device series number label is torn off or
cannot be recognized.
Damage to the device resulting from
misconnection with other devices.
Damage to the device resulting from accidents.
Changes performed by users without the prior
written authorization of the company.
Rossmax InnoTek Corp.
12F, No. 189, Kang Chien Rd., Taipei 11494, Taiwan
Tel: +886-2-2659-7888 Fax: +886-2-2659-7666
www.rossmax.com
The text is subject to change without
further notice.
Version 11; Jan. 20, 2016
1
9
8
7
2
3
5
6
4
APG
APG
APG
APG
APG
APG
IBP innovative business promotion
GmbH
Botzstrasse 6, D-07743 Jena, Germany
Declaration of Conformity for EN 60601-1-2
Recommended separation distances between
portable and mobile RF communications equipment and the ME equipment
The Finger
-
tip pulse oximeter
is intended
for use in an electromagnetic environment in which radiated RF disturbances
are controlled. The customer or the user of the Finger-tip pulse oximeter
can help prevent electromagnetic interference
by maintaining a minimum distance between portable and mobile RF communications equipment (transmitters) and the
Finger-
tip pulse oximeter as recommended below, according to the maximum output power of the communications
equipment.
Rated maximum output
power of transmitter
W
Separation distance according to frequ
ency of transmitter
m
150 kHz to 80 MHz
d=
1
5,3
V
P
80 MHz to 800 MHz
d=
1
5,3
E
P
800 MHz to 2.5 GHz
d=
1
7
E
P
0.01 0.12 0.12 0.23
0.1 0.37 0.37 0.74
1 1.17 1.17 2.33
10 3.7 3.7 7.37
100 11.67 11.67 23.33
Declaration – electromagnetic emissions and immunity – for EQUIPMENT and SYSTEMS that are not LIFE-SUPPORTING and
are specified for use only in a shielded location
The Finger
-
tip pulse oximete
r declaration
– electromagnetic immunity
The
Finger
-
tip pulse oximeter
system is intended for use in the electromagnetic environment specified below.
The customer or the user of the
Finger
-
tip pulse oximeter
system should assure that it is used in such a
n environment.
Immunity test IEC 60601 test level Compliance level Electromagnetic environment - guidance
Conducted RF
IEC 61000-4-6
3 Vrms
150 kHz to 80 MHz
3V
Portable and mobile RF communications equipment should be
used no closer to any part of the E
QUIPMENT or SYSTEM
including cables, than the recommended separation distance
calculated from the equation applicable to the frequency of the
transmitter.
Interference may occur in the vicinity of equipment marked
with the following symbol.
Radiated RF
IEC 61000-4-3
3 V/m
80 MHz to 2.5 GHz
3V/m
Declaration – electromagnetic immunity
The Finger
-
tip pulse oximeter system is intended for use in the electromagnetic environment specified below.
The customer or the user of the Finger-tip pulse oximeter system should assure that it is used in such an environment.
Immunity test IEC 60601 test level Compliance level Electromagnetic environment - guidance
Electrostatic
discharge (ESD)
IEC 61000-4-2
±6 kV contact
±8 kV air
±6 kV contact
±8 kV air
Floors should
be wood, concrete or ceramic
tile. If floors are covered with synthetic
material, the relative humidity should be at
least 30 %.
Electrical fast
transient/burst
IEC 61000
-4-4
±2 kV for power supply lines
±1 kV for input/output lines
±2 kV for power supply lines
Mains power quality should be that of a
typical commercial or hospital environment.
Surge
IEC 61000
-4-5
±1 kV differential mode
±2 kV common mode
±1 kV differential mode
±2 kV common mode
Mains power quality should be that of a
typical commercial o
r hospital environment.
Voltage dips, short
interruptions and
voltage variations
on power supply
input lines
IEC 61000-4-11
<<<<
5 % UT
(>>>>95 % dip in UT) for 0.5 cycle
40 % UT
(60 % dip in UT) for 5 cycles
70 % UT
(30 % dip in UT) for 25 cycles
<<<<5 % UT
(>>>>95 % dip in UT) for 5 sec
<<<<
5 % UT
(>>>>95 % dip in UT) for 0.5 cycle
40 % UT
(60 % dip in UT) for 5 cycles
70 % UT
(30 % dip in UT) for 25 cycles
<<<<5 % UT
(>>>>95 % dip in UT) for 5 sec
Mains power quality should be that of a
typical commercial or hospital environment.
If the user of the EQUIPMENT or SYSTEM
requires continued operation during power
mains interruptions, it is recommended th
at
the EQUIPMENT or SYSTEM be powered
from an uninterruptible power supply or a
battery.
Power frequency
(50/60 Hz) magnetic
field IEC 61000
-4-8
3 A/m 3 A/m
Power frequency magnetic fields should be at
levels characteristic of a typical location in a
typi
cal commercial or hospital environment.
Declaration – electromagnetic emissions
The
Finger
-
tip pulse oximeter
is intended for use in the electromagnetic environment specified below. The customer or the user of
the Finger-tip pulse oximeter should assure that it is used in such an environment.
Emissions test
Compliance
Electromagnetic environment - guidance
CE emissions
CISPR11
Group 1
The
Finger
-
tip pulse oximeter
uses RF energy only for its internal function. Therefore, its
RF emissions are very low and are not likely to cause any interference in nearby
electronic equipment.
RE emissions
CISPR11
Class B
The
Finger
-
tip pulse oximeter
is suitable for use in all establishments, including domestic
establishments and those directly connected to the public low-voltage power supply
network that supplies buildings used for domestic purposes.
Harmonic emissions
IEC 61000
-3-2
Class A
Voltage fluctuations/
Flicker emissions
IEC 61000
-3-3
Complies
 Loading...
Loading...