Rossmax NA100 Instruction Manual
or
15~20mins.
95~100°C
(203~212°F)
<40°C
(104°F)
1
2 3
4
5
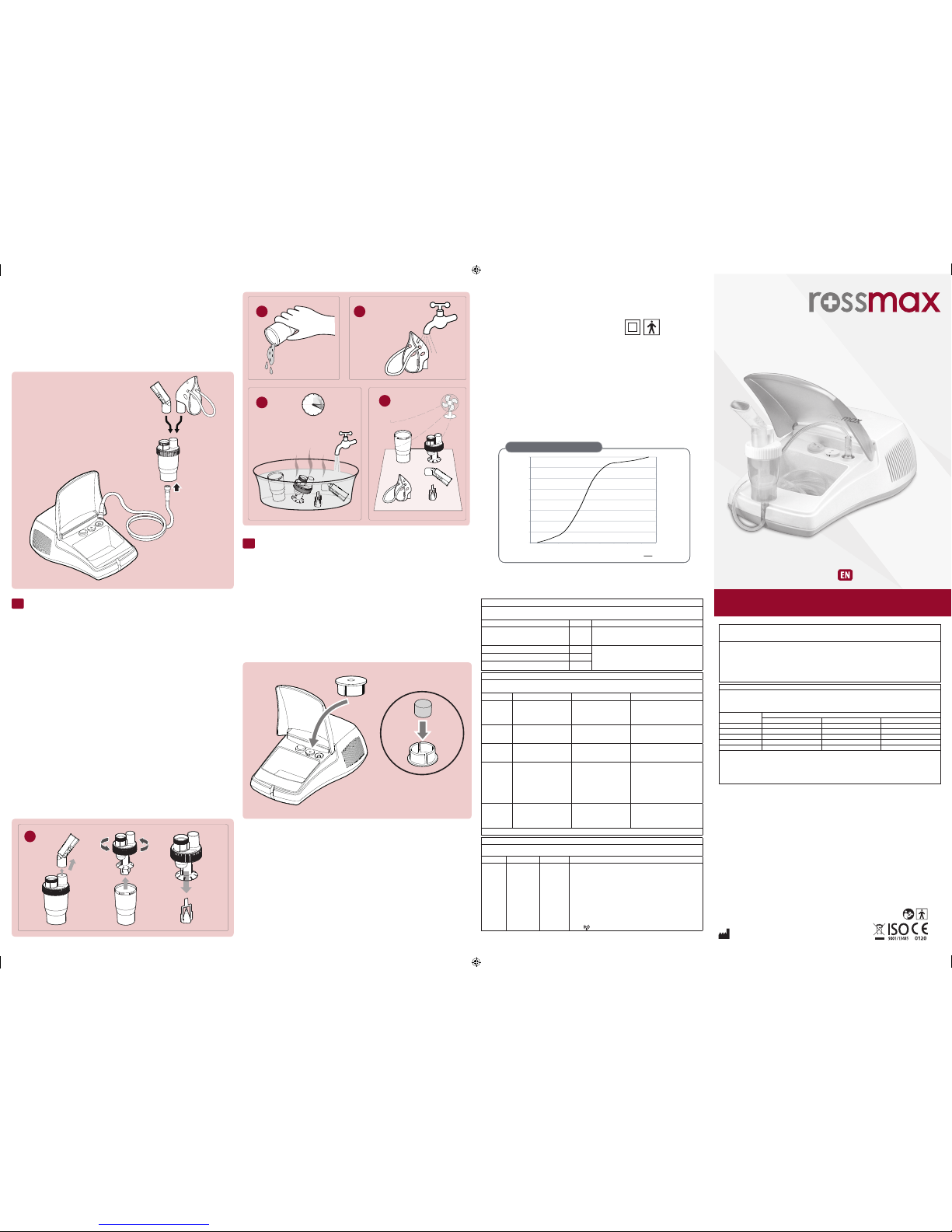
NA100 粒徑分佈圖 (MMAD ≦2.4)
Particle Size Distribution
Particle Diameter (μm)
Mass Frequency (%)
Cumulative Mass (%)
Cumulative Mass
35.00
40.00
30.00
25.00
20.00
15.00
10.00
5.00
0.00
00.520.931.553.5 69.8 14.8 21.3
100.00
90.00
80.00
70.00
60.00
50.00
40.00
30.00
20.00
10.00
0.00
Operating your nebulizer
After every use:
1. Unplug the unit from the power source.
2. Allow the unit to completely cool.
3. Carefully detach the air tubing from the nebulizer and pour
out any remaining medication.
4. Follow the cleaning procedures provided in this guidebook.
E
Cleaning procedures
Rinsing technique (performed after each treatment or before
rst use).
1. Make sure that the power-switch has been turned to the
“OFF” position and the unit has been disconnected from the
power source.
2. Disconnect the air tube from the nebulizer device.
3. Gently twist and pull up the cover of the nebulizer kit to open
and separate.
4. Rinse the nebulizer kit and components with hot tap water.
5. Dry with clean towels or completely air dry.
6. Reassemble the nebulizer kit.
NOTE: For the rst time cleaning or after the unit has been
stored for an extended period of time, thoroughly clean all
components, except the air tube. The nebulizer kit is dishwasher
safe.
Cleaning the compressor
Wipe the compressor daily using a soft cloth.
NOTE: Any other form of cleaning or cleaning agents may
damage the nish of the unit.
F
Changing the air lter
It is important to change the air lter approximately when the
air lter turns gray. It is recommended to change air lter every 2
months.
1. Remove the air lter cover by gently pulling forward.
2. Discard the gray lter.
3. Replace with a new, clean air lter.
4. Securely re-attach the air lter cover to the unit.
NOTE: Air lters cannot be cleaned or washed. Only NA100 air
lters can be used. Do not substitute alternate material
such as cotton. Do not operate without an air lter.
• Check the air lter and replace if necessary.
Protection against electric shock:
• Class II equipment .
Type BF applied parts:
• Mouthpiece and mask .
Protection against harmful ingress of water and particulate
matter:
• IP21
Degree of safety in the presence of ammable anesthetics
or oxygen:
• No AP/APG (not suitable for use in the presence of ammable
anesthetics or oxygen).
Troubleshooting
If any abnormality occurs during use, please check and correct the
following:
1. Unit does not operate when power switch is pressed: Check the
AC connection to the outlet.
2. No misting or low rate of misting:
• Check that there is medication in the nebulizer cup.
• Check the main unit if there is any physical damage.
• Check the position of the nozzle inside the nebulizer.
• Make sure that air tube and other components are properly
attached.
Model: NA100
Piston Nebulizer
www.rossmax.com
Instruction Manual
Rossmax Swiss GmbH,
Tramstrasse 16, CH-9442 Berneck, Switzerland
INXXXXXXXXXXXXXXX
S&S_IB_NA100_EN_SW_ver1550
EMC guidance and manufacturer’s declaration
Guidance and manufacturer’s declaration-electromagnetic emissions
The NA100 is intended for use in the electromagnetic environment specied below.
The customer or the user of the NA100 should assure that it is used in such an environment.
Emission test Compliance Electromagnetic environment-guidance
RF emissions CISPR 11 Group 1
The NA100 uses RF energy only for its internal function. Therefore,
its RF emissions are very low and are not likely to cause any interference in nearby electronic equipment.
RF emissions CISPR 11 Class B The NA100 is suitable for use in all establishments, including do-
mestic establishments and those directly connected to the public
low-voltage power supply network that supplies buildings used for
domestic purposes.
Harmonic emissions IEC 61000-3-2 Class A
Voltage uctuations/icker emissions IEC 61000-3-3 Compliance
Guidance and manufacturer’s declaration-electromagnetic immunity
The NA100 is intended for use in the electromagnetic environment specied below.
The customer or the user of the NA100 should assure that it is used in such an environment.
Immunity test IEC 60601 test level Compliance level Electromagnetic environment-guidance
Electrostatic
discharge (ESD)
IEC 61000-4-2
± 6 kV contact
± 8 kV air
± 6 kV contact
± 8 kV air
Floors should be wood, concrete or ceramic
tile. If oors are covered with synthetic
material, the relative humidity should be at
least 30%
Electrical fast
transient/burst IEC
61000-4-4
± 2kV for power
supply lines
± 1kV for input/output lines
+ 2kV for power
supply lines
Not applicable
Mains power quality should be that of a
typical commercial or hospital environment.
Surge IEC 610004-5
± 1kV line(s) to line(s)
± 2kV line(s) to earth
+ 1kV dierential mode
Not applicable
Mains power quality should be that of a
typical commercial or hospital environment.
Voltage Dips, short
interruptions and
voltage variations
on power supply
input lines IEC
61000-4-11
<5% UT(>95% dip in UT) for 0,5
cycle
40% UT(60% dip in UT) for 5
cycles
70% UT(30% dip in UT) for 25
cycles
<5% UT(>95% dip in UT) for 5 s
<5% UT(>95% dip in UT) for 0,5
cycle
40% UT(60% dip in UT) for 5
cycles
70% UT(30% dip in UT) for 25
cycles
<5% UT(>95% dip in UT) for 5 s
Mains power quality should be that of a
typical commercial or hospital environment. If the user of the NA100 requires
continued operation during power mains
interruptions, it is recommended that the
NA100 be powered from an uninterruptible
power supply or a battery.
Power frequency
(50/60 Hz)
magnetic eld IEC
61000-4-8
3 A/m 3 A/m The NA100 power frequency magnetic
elds should be at levels characteristic of a
typical location in a typical commercial or
hospital environment.
NOTE: UT is the a.c. mains voltage prior to application of the test level.
Guidance and manufacturer’s declaration-electromagnetic immunity
The NA100 is intended for use in the electromagnetic environment specied below.
The customer or the user of the NA100 should assure that is used in such and environment.
Immunity test IEC 60601 test level Compliance level Electromagnetic environment-guidance
Conducted RF
IEC 610004-6
Radiated RF
IEC 610004-3
3 Vrms
150 KHz to 80 MHz
3 V/m
80MHz to 2,5 GHz
3 Vrms
3 V/m
Portable and mobile RF communications equipment should be used no closer to
any part of the NA100 including cables, than the recommended separation distance calculated from the equation applicable to the frequency of the transmitter.
Recommended separation distance:
d = 1,2 √P, d = 1,2 √P 80MHz to 800 MHz, d = 2,3 √P 800MHz to 2,5 GHz
Where P is the maximum output power rating of the transmitter in watts (W)
according to the transmitter manufacturer and d is the recommended separation
distance in metres (m).
Field strengths from xed RF transmitters, as determined by an electromagnetic
site survey, a should be less than the compliance level in each frequency range. b
Interference may occur in the vicinity of equipment marked with the following
symbol:
NOTE1: At 80 MHz and 800 MHz, the higher frequency range applies.
NOTE2: These guidelines may not apply in all situations. Electromagnetic propagation is aected by absorption and reection from
structures, objects and people.
a: Field strengths from xed transmitters, such as base stations for radio (cellular/cordless) telephones and land mobile radios, amateur
radio, AM and FM radio broadcast and TV broadcast cannot be predicted theoretically with accuracy. To assess the electromagnetic
environment due to xed RF transmitters, an electromagnetic site survey should be considered. If the measured eld strength in
the location in which the NA100 is used exceeds the applicable RF compliance level above, the NA100 should be observed to verify
normal operation. If abnormal performance is observed, additional measures my be necessary, such as re-orienting or relocating the
NA100.
b: Over the frequency range 150 kHz to 80 MHz, eld strengths should be less than 3 V/m.
Recommended separation distance between portable and mobile RF communications equipment and the NA100
The NA100 is intended for use in an electromagnetic environment in which radiated RF disturbances are controlled. The customer or the
user of the NA100 can help prevent electromagnetic interference by maintaining a minimum distance between portable and mobile RF
communications equipment (transmitters) and the NA100 as recommended below, according to the maximum output power of the
communications equipment.
Rated maximum output
power of transmitter (W)
Separation distance according to frequency of transmitter (m)
150kHz to 80MHz / d=1,2√P 80MHz to 800MHz / d=1,2√P 800MHz to 2,5GHz / d=2,3√P
0,01 0,12 0,12 0,23
0,1 0,38 0,38 0,73
1 1,2 1,2 2,3
10 3,8 3,8 7,3
100 12 12 23
For transmitters rated at a maximum output power not listed above, the recommended separation distance d in metres (m) can be estimated using the equation applicable to the frequency of the transmitter, where p is the maximum output power rating of the transmitter
in watts (W) according to the transmitter manufacturer.
NOTE1: At 80 MHz and 800 MHz, the separation distance for the higher frequency range applies.
NOTE2: These guidelines may not apply in all situations. Electromagnetic propagation is aected by absorption and reection from struc-
tures, objects and people.
1
2
3
4
7
5
6
8
9
10
11
12
English
Introduction
Thank you for purchasing a NA100 Compressor Nebulizer.
With proper care and use, your nebulizer will provide you
with many years of reliable treatments. This unit operates on
standard AC power. Treatments are delivered quickly, safely and
conveniently making this unit ideal for all ages. We encourage
you to thoroughly read this guidebook to learn about the
features of your nebulizer. Your compressor nebulizer should
be used under the supervision of a licensed physician and/or
a respiratory therapist. Together with your physician and/or
respiratory therapist, you can feel comfortable and condent
knowing that you are obtaining the most eective inhalation
treatments for your respiratory condition.
NOTE: Your nebulizer is intended for use in treatment of asthma,
COPD and other respiratory ailments in which an aerosolized
medication is required during therapy. Please consult with your
physician and/or pharmacist to determine if your prescription
medication is approved for use with this nebulizer. For type,
dose, and regime of medication follow the instructions of your
doctor or respiratory therapist.
This device fulls the provision of the EC directive 93/42/EEC
(Medical Device Directive) and the European Standard EN
13544-1:2007+A1:2009 Respiratory therapy equipment - Part 1:
Nebulizing systems and their components.
Please read this manual carefully before use and be sure to
keep this manual.
Cautions
Please use general safety precautions when operating your
nebulizer. This unit should be used only for its intended purpose
as described in this guidebook and with medications only under
the supervision and instruction of your physician. Do not use the
device in anesthetic or ventilator breathing circuits.
Product cautions
READ THE FOLLOWING BEFORE USING
• To avoid electrical shock: keep unit away from water.
• Do not handle the unit of power cord with wet hands.
• Do not immerse the unit in liquid.
• Do not use while bathing.
• Do not reach for a unit that has fallen into water immediately
unplug the unit.
• Do not use the unit if it has any damaged parts (including plug),
if it has been submersed in water or dropped. Promptly send
the unit for examination and repair.
• The unit should not be used where ammable gas, oxygen or
aerosol spray products are being used.
• Keep the air vents open. Do not place the unit on a soft surface
where the openings can be blocked.
• If the medication cup is empty, do not attempt to operate the
unit.
• If any abnormality occurs, discontinue to use until the unit has
been examined and repaired.
• The unit should not be left unattended while plugged in.
• Do not tilt or shake the unit when in operation.
• Disconnect the unit from the electrical outlet before cleaning,
lling and after each use.
• Do not use attachments unless recommended by the
manufacturer.
• Do not disassemble or attempt to repair the unit.
• Do not use the device in anesthetic or ventilator breathing
circuits.
Operating cautions
• Close adult supervision is highly recommended when the unit is
used by children or invalids.
• Keep your eyes away from the output of medication mist.
• The maximum capacity of the medication cup is 5 ml and should
not be overlled.
• Do not use this unit while operating a vehicle.
• If any discomfort or abnormality occurs, stop using the unit
immediately.
• Do not use the device if the air tube is bent.
• Pentamidine is not an approved medication for use with this
device.
Storage cautions
• Do not store the unit in direct sunlight, high temperature or
humidity.
• Keep the unit out of reach of small children.
• Always keep the unit unplugged while not in use
Cleaning cautions
• Check air lter, nebulizer, mouthpiece and any other optional
component before each use. Dirty or worn parts should be
replaced.
• Do not immerse the unit in water. It may damage the unit.
• Disconnect the unit from the electrical outlet before cleaning.
• Clean all necessary parts after each use as instructed in this
guidebook.
• Always dispose of any remaining medication in the medication
cup after each use. Use fresh medication each time you use the
device.
• Do not store the air tube with moisture or medication remaining
in the air tube. This could result in infection as a result of bacteria.
MEDICAL DISCLAIMER:
This manual and product are not meant to be a substitute for
advice provided by your doctor or other medical professionals.
Don’t use the information contained herein or this product
for diagnosing or treating a health problem or prescribing any
medication. If you have or suspect that you have a medical
problem, promptly consult your doctor.
This instrument is covered by a 1 year guarantee from the purchase
date. The guarantee is valid only on presentation of the guarantee
card completed by the dealer conrming purchase date or the
receipt. Nebulizer Components are not included. Opening or
altering the instrument invalidates the guarantee. The guarantee
does not cover damage, accidents or non-compliance with the
instruction manual. Please contact Rossmax Service or distributors.
Product specications
Power AC 230V/50Hz or AC 220V/60Hz or
AC 110V/60Hz
Power Consumption ≤ 130W
Sound Level ≤ 55 dBA (1 meter away from
NA100)
Compressor Pressure Range > 30 psi (207 kPa)
Operating Flow Range ≥ 4.0 lpm
Operating Temperature
Range
10ºC to 40ºC (50ºF to 104ºF)
Operating Humidity Range 10 ~ 90% RH
Operating Atmospheric
Pressure Range
700-1060 hPa
Storage Temperature Range -20ºC to 60ºC (-4ºF to 140ºF)
Storage Humidity Range 10 ~ 90% RH
Dimension (L x W x H) 280mmx190mmx100mm
(11.02x7.48x3.93 inches)
Weight 1750g (without accessories)
Medication Capacity 5ml(cc)
Particle Size (MMAD) ≤ 2.4μm
Dv50 (Spraytec) ≤ 4.4μm
Average Nebulization Rate Fully open Valve ≥ 0.4ml/min
(0.9% Saline Solution)
Closed Valve ≥ 0.22ml/min
(0.9% Saline Solution)
Standard Accessories Nebulizer Kit, Air Tube, Mouth-
piece, Filters, Adult and child
masks, Service manual
*Subject to technical modication without prior notice.
* Performance may vary with drugs such as suspensions or high
viscosity, See drug supplier’s data sheet for further details.
A
Product identication
1. Nebulizer Kit 2. Nozzle 3. Angled Mouthpiece
4. Air Filter 5. Child Mask 6. Adult Mask
7. Air Tube 8. Power Switch 9. Air Filter Slot
10. Air Output 11. Air Intake 12. Power Cord
The nebulization rates can be adjusted by the user in a very easy
way without exchanging parts. Higher nebulization rate/fully
open is for higher viscosity medications and higher breathing
capacity user while lower nebulization rate with closed valve
will be more appropriate for kids / infants with lower breathing
capacity.
C
Assembling your nebulizer kit
Follow the cleaning instructions in this guidebook under
“Cleaning technique” prior to using your nebulizer for the rst
time or after it has been stored for an extended period of time.
REMEMBER: Always unplug the compressor and make sure the
power-switch is turned to the “OFF” position before cleaning,
assembling and before or after each use.
1. Place the compressor on a at, stable surface within reach.
2. Gently twist and pull straight up on the lid of the nebulizer to
separate into two parts (medication cup and cover).
3. Make sure that the nozzle is properly installed on the upper
cover. The stem inside the medication cup inserts into the
tube of the nozzle.
4. Add the prescribed amount of medication to the medication
cup.
5. Reassemble the nebulizer by carefully twisting the medication
cup and cover together. Make sure that the two parts t
securely.
WARNING: The symbol on this product means that it’s an
electronic product and following the European directive
2012/19/EU the electronic products have to be disposed
on your local recycling centre for safe treatment.
B
Valve Adjustable Technology
The proprietary adjustable valve is able to deliver medications of
dierent viscosity level according to every user’s conditions and
needs at ease. Our VA technology allows users to adjust dierent
levels of nebulization rate ranging from 0.22 – 0.4 ml/min at
consistent particle size.
D
Operating your nebulizer
The nebulizer is operable at up to a 45° angle. If the angle is
greater than 45°, no aerosol will be generated.
1. Attach one end of the air tube connector to the air output.
2. Carefully attach the opposite end of the air tubing connector
to the stem at the base of the nebulizer kit.
3. Attach the angled mouthpiece or mask to the top of the
nebulizer.
4. The capacity of the medication cup is 2~5 ml.
NOTE: A 30-minute interval is recommended after each use. The
compressor will automatically shut o if it becomes overheated.
If / when this happens, immediately:
1. Press the power-switch to the “OFF” position.
2. Unplug the power cord from the outlet.
3. Allow the motor to cool for 30 minutes.
Before restarting the unit, make sure that the air vents are not
obstructed.
 Loading...
Loading...