Rossmax MANDAUS II, GA102 Instruction Manual
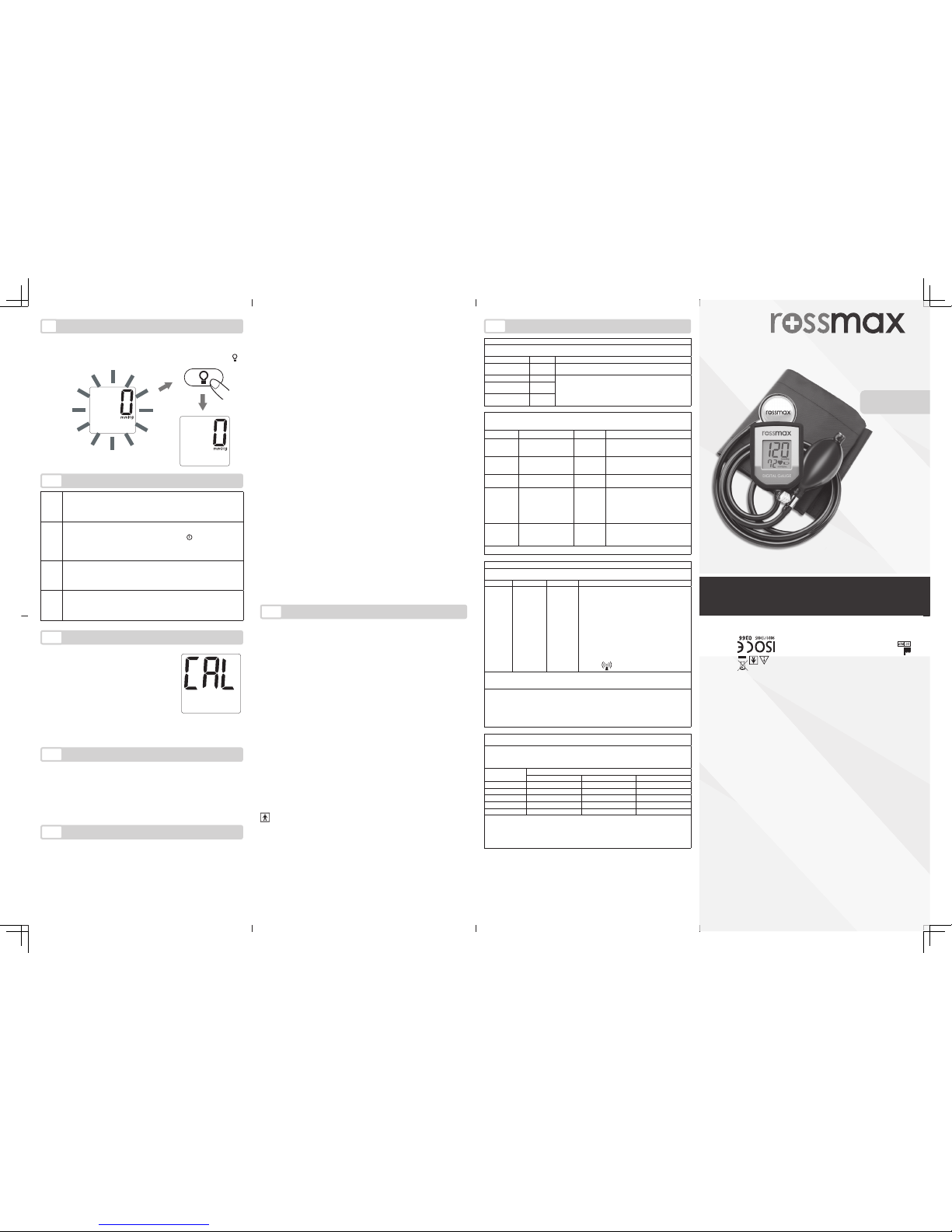
If the unit is used for over 10,000 times,
the calibration reminding message (CAL)
will appear every time the unit is switched
on. It is recommended that calibration be
completed every two years. Only the manufacturer or an authorized service techni-
cian is qualied for the calibration service.
Please contact the manufacturer, Rossmax
International Ltd. or your local distributors. (The PC Link socket
for calibration purpose is inside the battery compartment.)
IN0GA1020000000XX
The default is to have the back light on once the pressure is inated over 30 mmHg, and throughout the measurement process. To switch the back light off at any time, press the “ ”
key.
1. Rossmax Mandaus II are exclusively designed for blood pressure measurement at the upper arm or thigh on healthy skin.
2. Please do not inate to more than 300 mmHg.
3. Do not take your blood pressure for more than 3 minutes.
4. Wait for at least 2 minutes before repeated measurements.
12. Safety Information
1. The unit contains high-precision assemblies. Therefore, avoid ex-
treme temperatures, humidity, and direct sunlight. Avoid dropping or strongly shocking the main unit, and protect it from
dust.
2. Clean the blood pressure monitor body and the cuff carefully
with a slightly damp, soft cloth. Do not press. Do not wash the
cuff or use chemical cleaner on it. Never use thinner, alcohol or
petrol (gasoline) as cleaner.
13. Cautionary Notes
14. Specications
15.
EMC guidance and manufacturer’s declaration
Guidance and manufacturer’s declaration-electromagnetic emissions
The Mandaus II is intended for use in the electromagnetic environment specied below.
The customer or the user of the Mandaus II should assure that it is used in such an environment.
Emission test Compliance Electromagnetic environment-guidance
RF emissions CISPR 11 Group 1 The Mandaus II uses RF energy only for its internal function. Therefore, its RF emissions
are very low and are not likely to cause any interference in nearby electronic equipment.
RF emissions CISPR 11 Class B The Mandaus II is suitable for use in all establishments, including domestic establish-
ments and those directly connected to the public low-voltage power supply network that
supplies buildings used for domestic purposes.
Harmonic emissions
IEC 61000-3-2
Not applicable
Voltage uctuations/icker
emissions IEC 61000-3-3
Not applicable
Guidance and manufacturer’s declaration-electromagnetic immunity
The Mandaus II is intended for use in the electromagnetic environment specied below.
The customer or the user of the Mandaus II should assure that it is used in such an environment.
Immunity test IEC 60601 test level Compliance level Electromagnetic environment-guidance
Electrostatic discharge (ESD) IEC
61000-4-2
± 6 kV contact
± 8 kV air
± 6 kV contact
± 8 kV air
Floors should be wood, concrete or ceramic tile. If
oors are covered with synthetic material, the relative
humidity should be at least 30%
Electrical fast
transient/burst IEC
61000-4-4
± 2kV for power supply lines
± 1kV for input/output lines
Not applicable
Not applicable
Mains power quality should be that of a typical commercial or hospital environment.
Surge IEC
61000-4-5
± 1kV line(s) to line(s)
± 2kV line(s) to earth
Not applicable
Not applicable
Mains power quality should be that of a typical commercial or hospital environment.
Voltage Dips, short
interruptions and
voltage variations
on power supply
input lines IEC
61000-4-11
<5% UT(>95% dip in UT) for 0,5
cycle
40% UT(60% dip in UT) for 5
cycles
70% UT(30% dip in UT) for 25
cycles
<5% UT(>95% dip in UT) for 5 s
Not applicable
Not applicable
Not applicable
Not applicable
Mains power quality should be that of a typical
commercial or hospital environment. If the user of
the Mandaus II requires continued operation during
power mains interruptions, it is recommended that
the Mandaus II be powered from an uninterruptible
power supply or a battery.
Power frequency
(50/60 Hz) magnetic eld IEC
61000-4-8
3 A/m 3 A/m Power frequency magnetic elds should be at levels
characteristics of a typical location in a typical com-
mercial or hospital environment.
NOTE: UT is the a.c. mains voltage prior to application of the test level.
Guidance and manufacturer’s declaration-electromagnetic immunity
The Mandaus II is intended for use in the electromagnetic environment specied below.
The customer or the user of the Mandaus II should assure that is used in such and environment.
Immunity test IEC 60601 test level Compliance level Electromagnetic environment-guidance
Conducted RF
IEC 61000-4-6
Radiated RF
IEC 61000-4-3
3 Vrms
150 KHz to 80 MHz
3 V/m
80MHz to 2,5 GHz
Not applicable
3 V/m
Portable and mobile RF communications equipment should be used
no closer to any part of the Mandaus II, including cables, than the
recommended separation distance calculated from the equation
applicable to the frequency of the transmitter.
Recommended separation distance:
d = 1,2 √P
d = 1,2 √P 80MHz to 800 MHz
d = 2,3 √P 800MHz to 2,5 GHz Where P is the maximum output power
rating of the transmitter in watts (W) according to the transmitter manufacturer and d is the recommended separation distance in metres (m).
Field strengths from xed RF transmitters, as determined by an electromagnetic site survey, a should be less than the compliance level in each
frequency range.
b
Interference may occur in the vicinity of equipment marked with the following symbol:
NOTE1: At 80 MHz and 800 MHz, the higher frequency range applies.
NOTE2: These guidelines may not apply in all situations. Electromagnetic propagation is affected by absorption and reection from
structures, objects and people.
a. Field strengths from xed transmitters, such as base stations for radio (cellular/cordless) telephones and land mobile radios, ama-
teur radio, AM and FM radio broadcast and TV broadcast cannot be predicted theoretically with accuracy. To assess the electromagnetic environment due to xed RF transmitters, an electromagnetic site survey should be considered. If the measured eld strength in
the location in which the Mandaus II is used exceeds the applicable RF compliance level above, the Mandaus II should be observed
to verify normal operation. If abnormal performance is observed, additional measures may be necessary, such as re-orienting or
relocating the Mandaus II.
b. Over the frequency range 150 kHz to 80 MHz, eld strengths should be less than 3 V/m.
Recommended separation distance between
portable and mobile RF communications equipment and the Mandaus II
The Mandaus II is intended for use in an electromagnetic environment in which radiated RF disturbances are controlled. The customer
or the user of the Mandaus II can help prevent electromagnetic interference by maintaining a minimum distance between portable and
mobile RF communications equipment (transmitters) and the Mandaus II as recommended below, according to the maximum output
power of the communications equipment.
Rated maximum output
power of transmitter / W
Separation distance according to frequency of transmitter / m
150 kHz to 80 MHz / d = 1,2 √P 80 MHz to 800 MHz / d = 1,2 √P 800 MHz to 2,5 GHz / d = 2,3 √P
0,01 0,12 0,12 0,23
0,1 0,38 0,38 0,73
1 1,2
1,2 2,3
10 3,8 3,8 7,3
100 12 12 23
For transmitters rated at a maximum output power not listed above, the recommended separation distance d in meters (m) can be estimated using the equation applicable to the frequency of the transmitter, where P is the maximum output power rating of the transmitter
in watts (W) according to the transmitter manufacturer.
NOTE1: At 80 MHz and 800 MHz, the separation distance for the higher frequency range applies.
NOTE2 These guidelines may not apply in all situations. Electromagnetic propagation is affected by absorption and reection from
structures, objects and people.
Power Source : DC 3V Two AAA Batteries
Measurement Range : Pressure: 0-299 mmHg;
Pulse: 30-240 beats/min
Accuracy : Pressure:
0°C~17°C, 34°C~46°C: ±6 mmHg
18°C~33°C: ±3 mmHg
Pulse: ±5% of reading
Pressure Sensor : Semi-conductor
Ination : Manual Ination
Deation : Manually operated deation valves
Auto-shut-off : 1 min. after last key operation
Back light auto-shut-off : 15 sec. after last key operation
Operation environment : 0°C~46°C (32°F~115°F);
85% RH max
Storage environment : -34°C~65°C (-30°F~149°F);
85% RH max
Dimensions : 78(L) x 63(W) x 48(H) mm
Weight : 90g (Mandaus II)
267g [Mandaus II+Rossmax Gauge
Cuff (M)]
Limited users : Adult users
: Type BF
Device and cuff are designed to pro-
vide special protection against electrical shocks.
*Specications are subject to change without notice.
1
2
4
3
5 6
5 6
9. Back light Control
Err
Heartbeat rate detection error. The cuff is deflated
too rapidly. Please keep the deflation rate among 2-3
mmHg per second.
EE
A pressure of over 15 mmHg remaining for more than 3
minutes is detected. Please press the “ ” key to switch
the unit off, or the unit will automatically switch off
after 60 seconds.
E3
Data Error. Please remove and reload the batteries. If
error keeps occurring, return the device to the local
distributor or service center.
01
The cuff is inflated to a pressure over 299 mmHg. Please
open the valve immediately to rapidly exhaust the air in
the cuff.
10. Error Messages
11. Maintenance
3. Leaky batteries can damage the unit. Remove the batteries when
the unit is not used for a long time.
4. The unit should not be operated by children so to avoid hazardous situations.
5. If the unit is stored near freezing, allow it to acclimate at room
temperature before use.
6. Mandaus II is not eld serviceable. You should not use any tool
to open the device nor should you attempt to adjust anything
inside the device. If you have any problems, please contact who
you purchased Mandaus II from or please contact Rossmax International Ltd.
7. To stop operation at any time, open the air release valve, and the
air in the cuff will be rapidly exhausted.
8. Please note that this is a home healthcare product only and it is
not intended to serve as a substitute for the advice of a physician
or medical professional.
9. Do not use this device for diagnosis or treatment of any health
problem or disease. Measurement results are for reference only.
Consult a healthcare professional for interpretation of pressure
measurements. Contact your physician if you have or suspect any
medical problem. Do not change your medications without the
advice of your physician or healthcare professional.
10. Electromagnetic interference: The device contains sensitive elec-
tronic components. Avoid strong electrical or electromagnetic
elds in the direct vicinity of the device (e.g. mobile telephones,
microwave ovens). These may lead to temporary impairment of
measurement accuracy.
11. Dispose of device, batteries, components and accessories accord-
ing to local regulations.
12. This monitor may not meet its performance specication if stored
or used outside temperature and humidity ranges specied in
Specications.
www.rossmaxhealth.com
Instruction Manual
Model: GA102
MANDAUS II
EN
Edition: V-3-PA01-3310
Issue Date: 2011/05/13
Rossmax International Ltd.
12F., No. 189, Kang Chien Rd., Taipei, 114, Taiwan.
Rossmax Swiss GmbH, Tramstrasse 16 CH-9442 Berneck
Switzerland
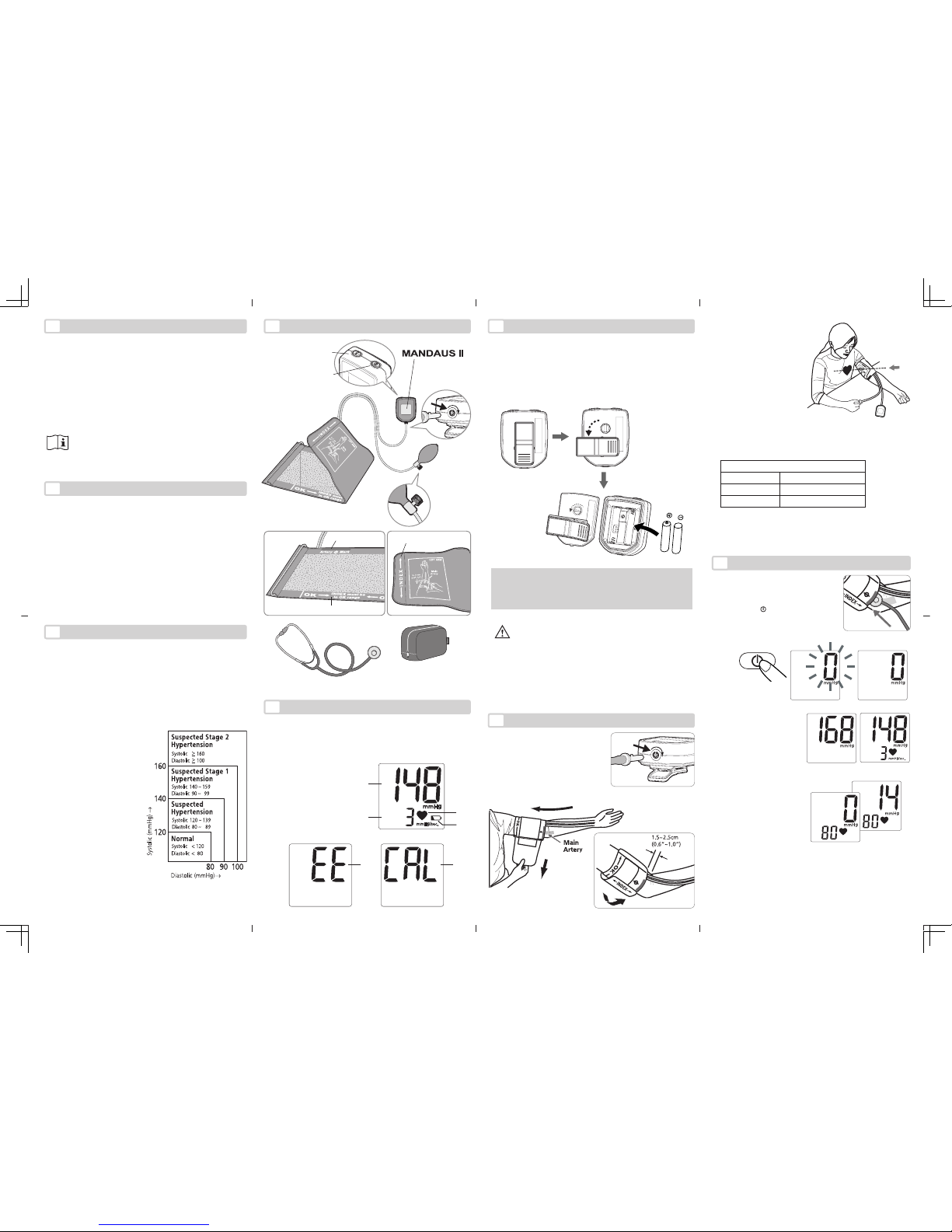
3. With the valve closed,
press the bulb and continue pumping to a val-
ue 30-40 mmHg above
your normal blood pres-
sure. (Fig. 3)
4. Open the valve to deflate the cuff gradually
at a rate of 2-3 mmHg
per second. (The deflation rate is displayed on
LCD.) (Fig. 4)
5. Record the onset of Ko-
rotkoff sound as the systolic pressure, and the
disappearance of these sounds as diastolic pressure.
6. After measurement is completed, fully open the valve to
release any remaining air in the cuff. The heartbeat rate is
displayed on the LCD. (Fig. 5)
4. Using the correct cuff size is important for an accurate
reading.
5. Display Explanations
7. Applying the Cuff
3. Blood Pressure Standard
1. Introduction
4. Name/Function of Each Part
2. Preliminary Remarks
Attention: Consult the accompanying documents.
Please read this manual carefully before
use. Please be sure to keep this manual.
Mandaus II complies with the European regulations and bears
the CE mark "CE 0366". The quality of the device has been
verified and conforms to the provisions of the EC council directive 93/42/EEC (Medical Device Directive), Annex I essential
requirements and applied harmonized standards.
EN 1060-1: 1995/A1: 2002 Non-invasive sphygmomanometers
- Part 1 - General requirements
EN 1060-3: 1997/A2: 2009 Non-invasive sphygmomanometers
- Part 3 - Supplementary requirements for electro-mechanical
blood pressure measuring systems
8. Measurement Procedures
1. Place the stethoscope head over the
main artery, underneath the artery
mark of the cuff. (Fig. 1)
2. Press the “ ” key. After zeroing,
Mandaus II is ready to measure. (Fig.
2)
Thank you for choosing Rossmax Mandaus II, the shock and
splash resistant backlighted digital sphygmomanometer. Blood
pressure measurements determined with Mandaus II are equivalent to those obtained by a trained observer using cuff/stethoscope auscultation method.
Mandaus II is protected against manufacturing defects by an
established International Warranty Program. For warranty information, you can contact the manufacturer, Rossmax International Ltd. or your local distributors.
The National High Blood Pressure Education Program
Coordinating Committee has developed a blood pressure
standard, classifying blood pressure ranges into 4 stages.
(Ref. The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure-Complete Report
JNC-7, 2004).
This blood pressure classification are based on historical data,
and may not be directly applicable to any particular patient.
It is important that you consult with your physician regularly.
Your physician will tell you your
normal blood pressure range
as well as the point at which
you will be considered at risk
before making any healthcare
decision based on the device's
readings.
For reliable monitoring and
reference of blood pressure,
keeping long- term records is
recommended. Please download the blood pressure log
(chart for recording and tracking crucial info: date, time,
blood pressure, pulse rate and
body condition) at www.rossmaxhealth.com.
Arm Cuff
Air Tube and
Connector
Inflating
Bulb
Air
Release
Valve
Back light key
On/Off/Zeroing key
Carrying Case
Stethoscope
Index Line
Artery Mark
Arm Circumference Indicator
1. Blood Pressure
2. Deflation Rate (During Measurement)/
Heartbeat Rate (After Measurement)
3. Heartbeat Mark
4. Weak Battery Mark
5. Error Mark
6. Calibration Reminder
1
2
4
3
5 6
5
6
6. Installing and Replacing the Batteries
1. Rotate the clip through 90 °.
2. Loosen the screw on the battery cover.
3. Install the batteries with correct polarities.
4. Replace the battery cover and tighten the screw.
5. Rotate the clip back.
You need to replace the batteries when:
1. low battery icon appears on display.
2. the On/Off/Zeroing key is pressed and nothing appears on
display.
Caution: 1. Batteries are hazardous waste. Do not dispose them
together with the household garbage.
2. There are no user serviceable parts inside. Batteries
or damage from old batteries are not covered by
warranty.
3. Use exclusively brand batteries. Always replace with
new batteries together. Use batteries of the same
brand and same type.
6
1. Plug the connector into the gauge
and twist clockwise to secure the
connection.
2. Place the cuff over the bare upper
arm, wrap it with the tube pointing your palm, and the artery mark
over your main artery.
Applicable cuff circumferences
Rossmax Gauge Cuffs
Young Adult (S) 18 - 26 cm (7.0”-10.3”)
Adult (M) 24 - 36 cm (9.5” – 14.2”)
Large Adult (L) 34 - 46 cm (13.4”-18.1”)
3. The edge of the cuff
should be at approximate-
ly 1.5 to 2.5 cm above the
inner side of the elbow
joint. If the index line falls
within the range of the
arm circumference indicator, the cuff circumference
is suitable, otherwise you
may need a cuff with a different circumference.
5. To stop operation at any time, open the air release valve,
and the air in the cuff will be rapidly exhausted.
4
3
5 6
Fig. 1
Fig. 2
Fig. 3 Fig. 4
5 6
Fig. 5
4
3
5 6
5 6
 Loading...
Loading...