Rossmax AC1000 f Instruction Manual

Model:
AC1000 f
EN
Instruction Manual
www.rossmax.com

Table of Contents:
Introduction .................................................................................... 2
Cautionary Notes ............................................................................. 3
Notes on Safety ................................................................................ 4
Name/Function of Each Part ............................................................. 6
Monitor Installation - Trolley (Optional) ............................................. 7
Real Fuzzy Measuring Technology .................................................... 8
Preliminary Remarks ......................................................................... 8
Display Explanations ......................................................................... 9
How to Use the POWER Source ...................................................... 10
Applying the Cuff .......................................................................... 14
Measurement Procedures ............................................................... 17
Setting Deation Rate ..................................................................... 18
Recalling Values from Memory ....................................................... 19
Clearing Values from Memory ........................................................ 19
How to clean the unit after use ...................................................... 19
Troubleshooting ............................................................................. 20
Specications ................................................................................. 21
EMC guidance and manufacturer’s declaration ................................ 22
Introduction
Blood pressure measurements determined with AC1000f are equivalent
to those obtained by a trained observer using cuff/stethoscope auscultation method, within the limits prescribed by the American National
Standard, Electronic or Automated Sphygmomanometers. This unit is to
be used by adult consumers in physicians’ ofces, hospitals, clinics and
other medical facilities. Do not use this device on infants or neonates.
AC1000f is protected against manufacturing defects by an established
International Warranty Program. For warranty information, you can contact the manufacturer, Rossmax International Ltd.
Attention: Consult the accompanying documents. Please read this
manual carefully before use. For specic information on
your own blood pressure, contact your physician. Please be
sure to keep this manual.
2
Cautionary Notes
1. The unit contains high-precision assemblies. Therefore, avoid extreme temperatures, humidity, and direct sunlight. Avoid dropping or
strongly shocking the main unit, and protect it from dust.
2. Leaky batteries can damage the unit. Remove the batteries when the
unit is not used for a long time.
3. The unit should not be operated by children so to avoid hazardous
situations.
4. If the unit is stored near freezing, allow it to acclimate at room temperature before use.
5. This unit is not eld serviceable. You should not use any tool to open
the device nor should you attempt to adjust anything inside the device. If you have any problems, please contact the store or the doctor from whom you purchased this unit or please contact Rossmax
International Ltd.
6. As a common issue for all blood pressure monitors using the oscillometric measurement function, the device may have difculty in determining the proper blood pressure for users diagnosed with common
arrhythmia (atrial or ventricular premature beats or atrial brillation),
diabetes, poor circulation of blood, kidney problems, or for users suffered from stroke, or for unconscious users.
7. To stop operation at any time, press the START/STOP and ON/OFF key,
and the air in the cuff will be rapidly exhausted.
8. Once the ination reaches 300 mmHg, the unit will start deating
rapidly for safety reasons.
9. Electromagnetic interference: The device contains sensitive electronic
components. Avoid strong electrical or electromagnetic elds in the
direct vicinity of the device (e.g. mobile telephones, microwave ovens). These may lead to temporary impairment of measurement accuracy.
10. Dispose of device, batteries, components and accessories according
to local regulations.
11. This monitor may not meet its performance specication if stored or
used outside temperature and humidity ranges specied in Speci-
cations.
3
Notes on Safety
Warning:
• Self diagnosis of measured results or treatment is dangerous.
Please follow the instruction of the doctor or healthcare provider.
• If cuff ination does not stop, remove the cuff or pull out the air
tube from the main unit.
• If battery uid gets into your eye or comes in contact with skin,
wash the effected area with water repeatedly. Immediately consult a doctor for treatment.
• Do not wrap the cuff over an arm to which intravenous injection
or transfusion is being conducted, or when otherwise contraindicated.
• Do not connect the air tube or the cuff to other equipment
which is connected to an intra corporeal organ. Air embolisms
may result.
• Do not use this unit in the presence of ammable gas or anesthetics or in a high pressure oxygen room or oxygen tent.
• Do not use the battery pack for devices other than for this unit.
• Do not disassemble the battery.
• Do not touch the AC adaptor with wet hands.
• Do not use any cuff other than the models exclusive for this unit.
• Do not use this unit on infants.
• Do not use this unit on patients using a pump oxygenator.
• Do not use an AC adaptor or battery pack no specied for this
unit.
• Do not use a cellular pone near this unit.
• Do not use this unit in a vehicle.
• Do not install the parts and/or instruments not specied for this
unit.
• Do not use a broken power cord or AC adaptor.
• Do not install or store this unit where it may come in contact
with water or liquid medication.
• This is a Class II device with double insulation.
4
General advice:
• Do not place or put anything on this unit.
• Do not drop this unit.
• Turn off power to the unit and unplug the AC adaptor from the
electric outlet before moving the unit.
• Read the instruction manual of the other devices to be used at
the same time with this unit, to understand and be aware of the
interaction between the devices.
• When using the unit:
- Do not inate the cuff without being wrapped over the arm.
- Do not use a damaged cuff.
- Be sure that patients do not touch the buttons of this unit.
• After using the unit:
- Do not disinfect this unit by autoclave or gas sterilization (EtO,
glutaraldehyde, or high concentration ozone).
• Do not install or store this unit in the following places.
- Under the direct sunlight.
- Dusty or salty environment.
- Places having slope or where combustible gas may be generated.
- Under high temperature and high humidity.
5
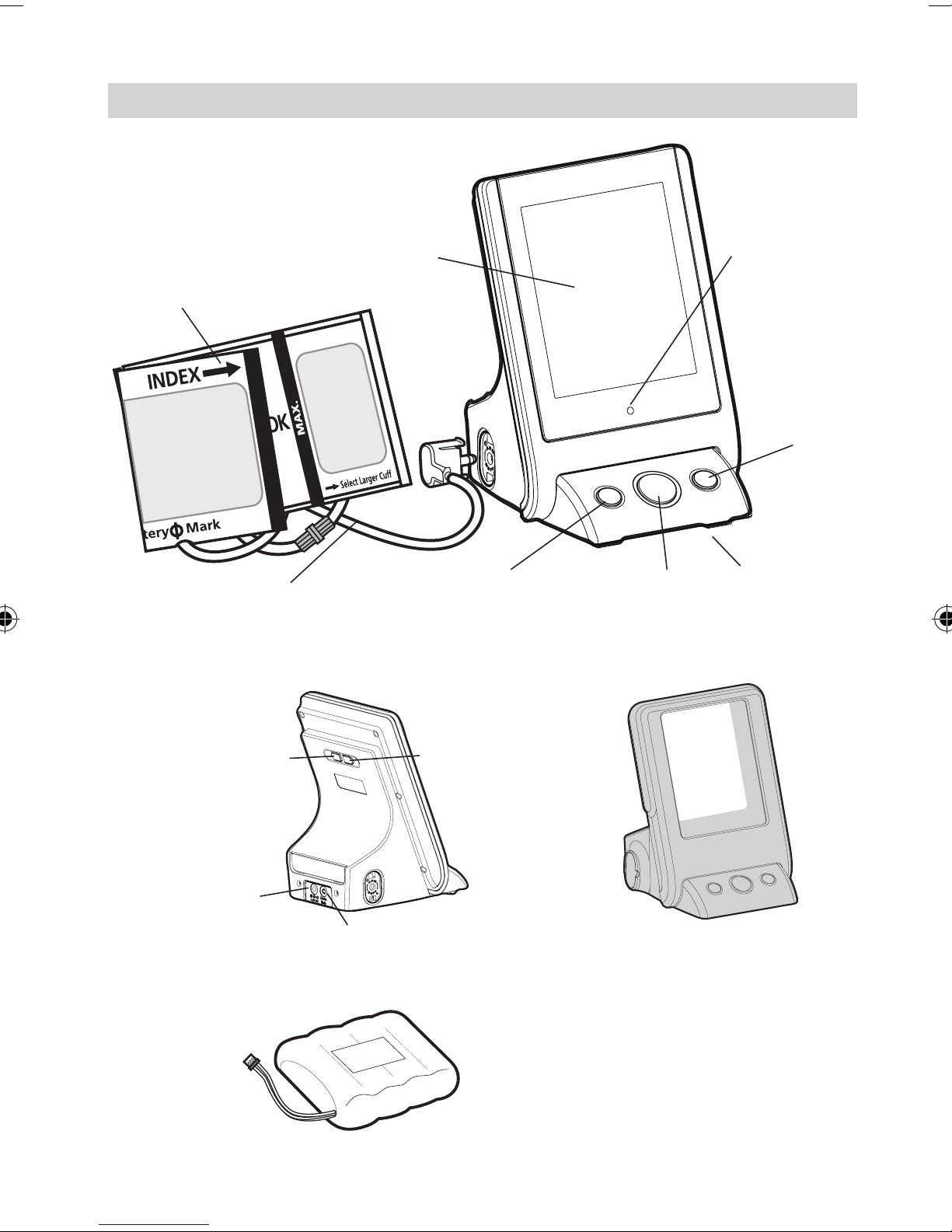
Name/Function of Each Part
LCD Display
Arm Cuff
Air Tube and
Connector
Memory/
Marker key
ON/OFF key
Charging
LED
START/
STOP key
Battery Cover
(Located on
back of unit)
Auto/Manual
Switching Key
AC Adaptor
Jack
Deation Rate
setting key
Data Link Socket Protection Jelly
Battery Pack:
4.8V, 1700mAh
NIMH Battery
6
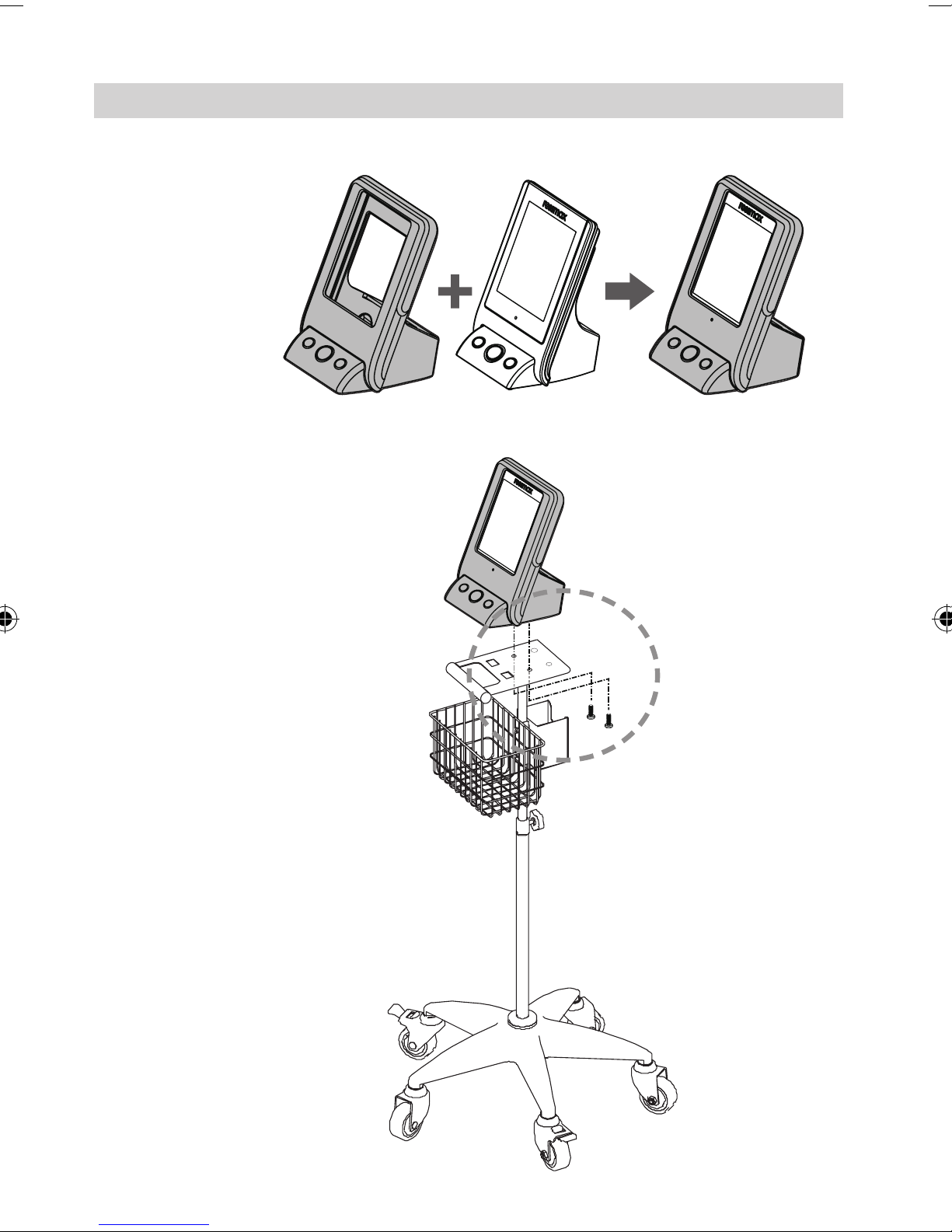
Monitor Installation - Trolley (Optional)
Placing Protection Jelly
onto the Monitor
Installing Monitor
on the Trolley
7
Real Fuzzy Measuring Technology
This unit uses the oscillometric method to detect your blood pressure.
Before the cuff starts inating, the device will establish a baseline cuff
pressure equivalent to the air pressure. This unit will determine the ap-
propriate ination level based on pressure oscillations, followed by cuff
deation.
During the deation, the device will detect the amplitude and slope
of the pressure oscillations and thereby determine for you the systolic
blood pressure, diastolic blood pressure, and pulse.
Preliminary Remarks
This Blood Pressure Monitor complies with the European regulations
and bears the CE mark "CE 0120". The quality of the device has been
veried and conforms to the provisions of the EC council directive
93/42/EEC (Medical Device Directive), Annex I essential requirements
and applied harmonized standards.
EN 1060-1: 1995/A2: 2009 Non-invasive sphygmomanometers - Part
1 - General requirements
EN 1060-3: 1997/A2: 2009 Non-invasive sphygmomanometers - Part 3
- Supplementary requirements for electro-mechanical blood pressure
measuring systems
EN 1060-4: 2004 Non-invasive sphygmomanometers - Part 4: Test Procedures to determine the overall system accuracy of automated noninvasive sphygmomanometers.
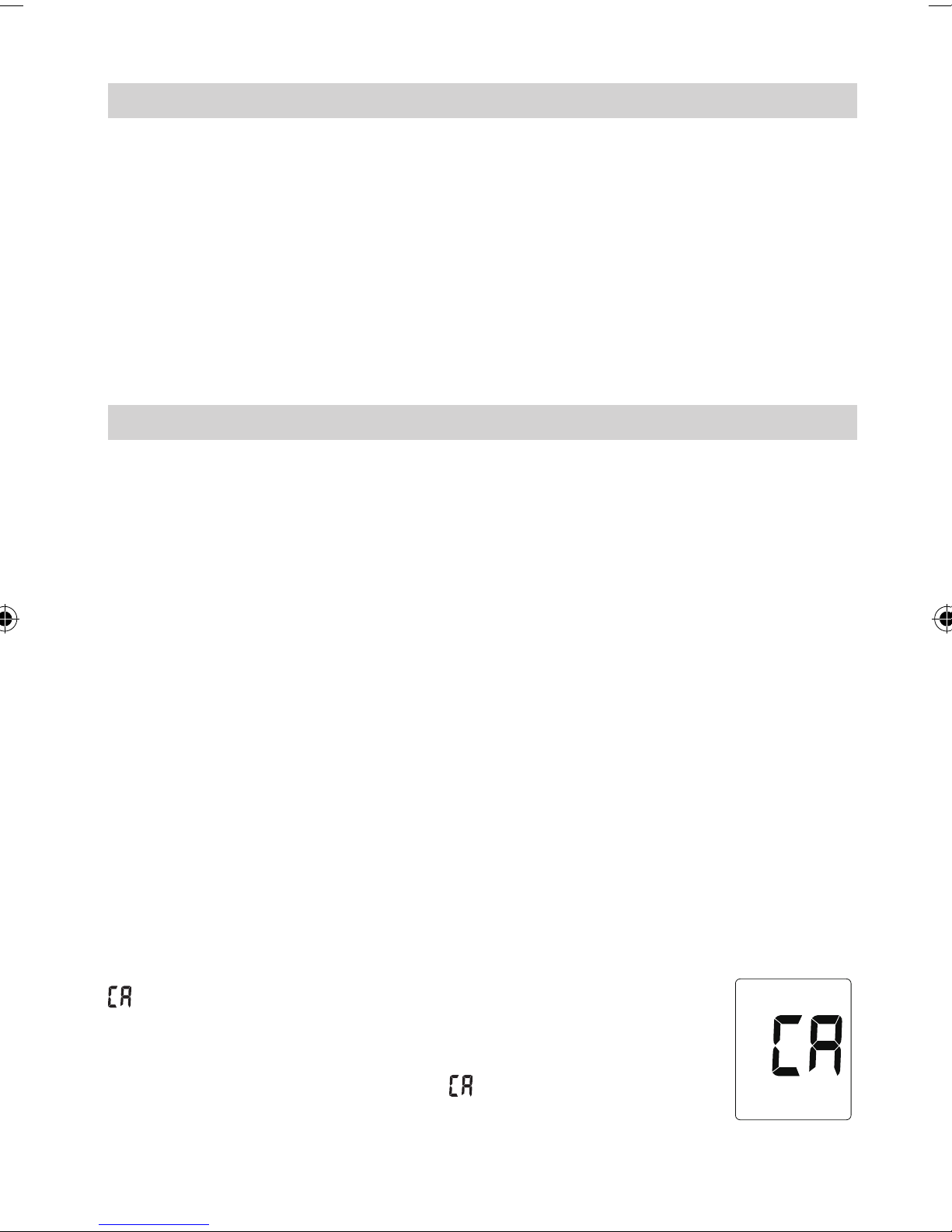
This blood pressure monitor was designed for long service time. To
ensure continued accuracy, it's recommended that all digital blood pressure monitors require re-calibration. The monitor does not require recalibration for 2 years, at which time your monitor displays
. The unit should also be re-calibrated if the monitor
sustains damage due to blunt force (such as dropping) or
exposure to uids and / or extreme hot or cold temperature / humidity changes. When appears, simply return
to your nearest dealer for re-calibration service.
8
 Loading...
Loading...